Research highlights: applications of life-cycle assessment as a tool for characterizing environmental impacts of engineered nanomaterials
Miranda J.
Gallagher
*a,
Caley
Allen
a,
Joseph T.
Buchman
b,
Tian A.
Qiu
b,
Peter L.
Clement
b,
Miriam O. P.
Krause
c and
Leanne M.
Gilbertson
d
aDepartment of Chemistry, Johns Hopkins University, Baltimore, MD 21218, USA. E-mail: mgallagher@jhu.edu
bDepartment of Chemistry, University of Minnesota, Minneapolis, MN 55455, USA
cCenter for Sustainable Nanotechnology, Department of Chemistry, University of Minnesota, Minneapolis, MN 55455, USA
dDepartment of Civil and Environmental Engineering, University of Pittsburgh, Pittsburgh, PA 15261, USA
First published on 6th February 2017
Abstract
The upstream and downstream environmental impacts of engineered nanomaterials (ENMs) are increasingly realized, and have motivated research to advance promising applications while precluding adverse impacts. Life-cycle assessment (LCA) is a comprehensive tool that considers the entire lifetime of a material, product or process—from raw material acquisition to end-of-life—and can be used to characterize these impacts as various environmental and human health categories. The motivation for this highlight stems from the curiosity of experimentalists and theorists researching the environmental and biological impacts that could result from widespread implementation of nanotechnology. In particular, we are motivated to identify how our research on the nano–bio interface can liaise with the nano-LCA community to advance nano-LCA in a safe and sustainable manner. As such, this highlight focuses on four recent nano-LCA publications that survey across several system levels and address the topics of: (i) upstream impacts from nanoparticle synthesis, (ii) extended lifetimes through the incorporation of ENMs in paints, (iii) integration of nano-specific data into existing life-cycle models, and (iv) the establishment of a nano-specific LCA framework.
Introduction
The exciting discoveries and applications of engineered nanomaterials (ENMs) result from the fact that the same chemical can have very different properties when fabricated on the macro- and nano-scales. For example, a silver dollar cut into 2.1 quintillion pieces transforms into an effective antibacterial agent due to increased surface area that facilitates rapid dissolution. These unique properties realized at the nano-scale have motivated a growing multibillion-dollar market of nano-enabled products, offering tangible benefits (https://www.nano.gov/nanotech-101/nanotechnology-facts). As with any emerging technology, there remain questions surrounding the embedded and imposed impacts on the environment and human health. Life-cycle assessment (LCA) is a comprehensive tool that can be used to characterize such impacts. While LCA is an established methodology, challenges arise when it is applied to nanomaterials. Four publications are highlighted here for their contributions to advancing nano-LCA, each addressing a unique challenge. The first details the importance of upstream impacts associated with silver nanoparticles by comparing seven synthesis methods. The second describes the influence of extended lifetime on the impacts of nano-enabled paints revealing the importance of user behavior, namely repainting frequency, in realizing a net benefit from nano-enabling. The third proposes a streamlined approach to the challenge of integrating data from the ENM manufacturing stage into the existing USEtox® model, which was originally designed for chemicals. The final publication details a new LCA tool, RedNano, which uniquely tracks nanoparticle impacts based on user identified geographic areas and demonstrates its applicability through the case study of post-consumer fate of nanoparticles in the Los Angeles area.Comparative life-cycle assessment of silver nanoparticle synthesis routes
In this unique investigation, Leila Pourzahedi and Matthew J. Eckelman (Pourzahedi & Eckelman, Environ. Sci.: Nano, 2015, 2, 361–369, DOI: 10.1039/c5en00075k) detail a cradle-to-gate (i.e., from raw material acquisition through manufacture) impact comparison of seven silver nanoparticle (AgNP) synthesis pathways. Determining resource intensity using impact assessment is a critical component of minimizing the impacts of nanoparticles because the experimental procedures could require excessive amounts of energy and materials, use harmful or toxic chemicals, and/or produce low yields. There were two general categories of synthesis considered: wet chemistry techniques and physical techniques. The wet chemistry techniques considered include five different chemical reductions utilizing silver solutions to produce the desired metallic nanoparticles. Specifically, chemical reduction (CR) of silver nitrate was achieved with trisodium citrate (CR-TSC), ethylene glycol (CR-EG), sodium borohydride (CR-SB), or soluble starch from potatoes (CR-starch). The physical chemistry routes investigated were reactive magnetron sputtering with an argon and nitrogen gas mixture (RMS-AR-N), flame spray pyrolysis (FSP), and arc plasma (AP). The relative environmental impacts, such as ozone depletion, acidification, and ecotoxicity (Fig. 1) were determined and compared across these seven synthesis methods.A mass-based comparison of the synthetic routes with a functional unit of 1 kg of AgNPs was determined using the TRACI 2.1 model from the EPA (Fig. 1a). Due to low AgNP yield and high electricity consumption, the flame spray pyrolysis synthesis resulted in the highest impacts across all categories except fossil fuel depletion. Interestingly, the results suggest that the bio-based chemical reduction using starch had relatively high impacts per kg of synthesized particles. When these results are rescaled with respect to their size-dependent toxicity (Fig. 1b), reduction through trisodium citrate resulted in the largest impact, with flame spray pyrolysis and the bio-based reduction with starch being the second most impactful methods. The trend in relative impacts is correlated with the particle size produced from the different syntheses. Trisodium citrate produces AgNPs with larger diameters and therefore smaller surface-to-volume ratios. Therefore, a higher concentration of particles is required to perform the same antimicrobial functionality as other particles in this study.
AgNP annual production volumes are on the order of hundreds of tons and they are used in a wide variety of high-tech and consumer products. As such, analyzing and comparing LCA results is a critical step towards reducing the upstream impacts of the AgNPs. Yet it is also important to consider impacts on more than a mass-basis as demonstrated here through the function-based comparison of the seven synthesis approaches. In doing so, the relative impacts across all impact categories shifted (Fig. 1a and b).
Effect of nanomaterial inclusion on the life-cycle impact of façade coatings
While ENMs have demonstrated promise in improving construction materials, the environmental impacts of nano-enabled products should be explored before embracing these materials. Hischier et al. (Hischier et al., J. Nanopart. Res., 2015, 17, 68, DOI: 10.1007/s11051-015-2881-0) use the ReCiPe methodology and USEtox® model to determine the ecological impact of including nanoparticles in paints. The authors look at three different nano-enabled paints that incorporate nano-TiO2, nano-Ag, or nano-SiO2 either as an additive to or a replacement for bulk ingredients, and compare these models to paints with no nanoparticles. Nano-TiO2 and nano-SiO2 are of interest industrially because they can extend the lifetime of the paint via photocatalytic cleaning and scratch resistance, respectively. Nano-Ag is of interest as an interior paint additive intended to improve hygiene due to its antimicrobial properties. The authors perform a comprehensive cradle-to-grave LCA (i.e., from raw material acquisition through disposal) to identify primary contributing factors to the environmental and human health impacts of these paint alternatives.A primary benefit realized through the inclusion of nano-TiO2 and nano-SiO2 in façades is the extension of the paint lifetime. For an average building with a lifetime of 80 years, paints with 20- or 27-year lifetimes will need to be replaced four and three times, respectively. Thus, the extended lifetime of these nano-enabled paints has the potential to significantly reduce the ecological impacts associated with upstream ‘embedded’ resources as well as waste associated with the more frequent re-application. While nano-Ag imparts antimicrobial properties, there is no known impact on the interior paint lifetime, which yields higher impacts than non-nano-Ag paints.
Another primary factor in determining the change of ecological impact over the life-cycle of façade coatings is whether the nanoparticle replaces an existing component or is an addition to the paint recipe. For nano-TiO2, the nanoparticle replaces bulk TiO2, while nano-SiO2 and nano-Ag are additives to the paint mixtures. When replacing a bulk component, the balance in production impacts of the bulk versus nanomaterial is considered. For additive materials, the production of the nanomaterial imparts an added impact compared to the conventional paint alternative.
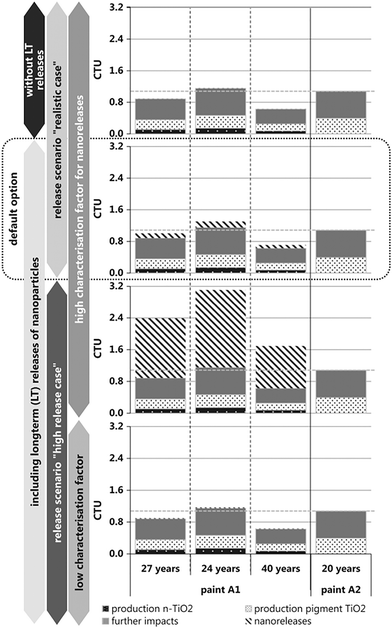
The authors address a number of questions surrounding the release of incorporated nanoparticles both during use and from landfills at end-of-life. In evaluating the ecological impact of release, there is a need for knowledge about the released nanomaterials and nano-specific characterization factors (CFs), a conversion unit to determine the impact of a given LCA result. For example, the CF (1 kg ammonia output = 0.35 kg PO43−) is used to characterize eutrophication (EU) potential with units of kg PO43− equivalents (H. Baumann & A.-M. Tillman, The Hitch Hiker's Guide to LCA, Studentlitteratur, Lund, 2004). As the authors note, the relevant data on nano-TiO2, nano-SiO2, and nano-Ag is sparse and is an area that requires attention. Their calculations for ecological impact, shown in Fig. 2 for paint with (A1) and without (A2) nano-TiO2, vary significantly depending on assumptions about release rates and CFs. The lower CF value used (CFw = 0.28 Potentially Affected Fraction of aquatic species (PAF) day m3 kg−1) had a lower particle concentration, and an attachment efficiency (α) of 1. The higher CF value used (CFw = 32.1 PAF day m3 kg−1) had a higher particle concentration with an α = 0.001 (B. Salieri et al., Sci. Total Environ., 2015, 505, 494–502, DOI: 10.1016/j.scitotenv.2014.09.107). This demonstrates that when there is no nano-TiO2 release or when using the lower CF value, the effects of increased lifetime dominate and the impact is approximately the same as from the paint without nano-TiO2. However, the ecological impact of the realistic/high release scenario indicates a moderate increase in ecological impact and using the higher CF value results in highest ecological impact when compared to the paint with conventional pigment-grade TiO2 (A2).
In this highlighted paper, Hischier et al. present a constructive approach for industry to share the rationale for dynamic changes to existing products without disclosing proprietary information. Decreasing the environmental impact of paints containing ENMs is best achieved when (i) the ENM replaces a component of the modified paint, (ii) the consumption of paint is decreased, either by extending lifetime or decreasing the paint needed to cover a given area, and (iii) the release of the nano-additive is minimized. Until questions can be answered about the specific effects of nano-TiO2, nano-SiO2, and nano-Ag in the environment, these three principles can provide basic guidelines for minimizing environmental impacts when using engineered nanoparticle components in coating materials.
Adapting a conventional LCA model for nanoparticle-specific properties
Unique properties of nanomaterials such as size- and surface area-dependent reactivity require nano-specific amendments on traditional LCA approaches for molecular chemicals. The work by Walser et al. (Walser et al., J. Nanopart. Res., 2015, 17, 245, DOI: 10.1007/s11051-015-3053-y) presents a seven-step LCA framework for indoor NP emission and exposure, which adapts the UNEP-SETAC toxicity model, USEtox®, for the inclusion of NP-specific parameters.The seven steps in this framework include (1) estimating NP emission flow (EmF), (2) modeling indoor concentration using a one-box model, (3) estimating NP intake fraction (IF) via inhalation, (4) estimating NP retention in the human body, (5) estimating NP effect factor (EF), (6) calculating the CF of the specific scenario, and (7) uncertainty assessment (Fig. 3). NP-specific parameters are incorporated into several steps in this scheme. In step 2, since the one-box model in USEtox® for indoor exposure is designed for gases, particle-specific properties (including agglomeration and gravitational settling) are specifically evaluated in this work and amendments in calculation are made accordingly. In steps 3 and 4, unlike traditional chemicals, whose intake fraction is independent of lung physiology, the retention and deposition of small particles in the human body are taken into consideration to calculate an effective dose that is dependent on NP behaviors, such as size, shape, chemistry, and agglomeration.
The framework also includes a metric conversion tool to assist with the calculation of parameters in both exposure and toxicity. Primary data in nanoparticle fate and/or toxicity studies are presented in different formats: mass, number, volume, or surface area concentrations. As the USEtox® model was developed for mass-based emissions, unit conversion is necessary. However, such metric conversion needs to acknowledge the complexity of nanoparticle structure, including morphology and size distribution. The authors took into account the particle density, aerodynamic diameter, and geometric standard deviation in developing the metric conversion tool, while also pointing out that a stringent quality check of measured data and NP characterization needs to be done to correctly reflect the properties of nanoparticles. The authors also point out that mass concentration may not be the best descriptor for nanoparticle toxicity; thus, other more relevant metrics such as surface area concentration may be used in the future.
Overall, this framework provided guidance for estimating the technology- and activity-specific emission flow of nanoparticles as well as the CF, while taking into account NP-specific properties. Important contributions of this framework include identifying the importance of metric conversion and adapting NP-specific behavior like agglomeration into the unified USEtox® LCA model. While the framework has promise for improving risk assessment of nanoparticles in different scenarios, there is an emerging need for mining the large sets of raw data on nanoparticle behavior and toxicity, from this journal and others, to fully utilize the Walser et al. nanoparticle adaptation to the USEtox® model.
RedNano: a new simulation tool for investigating the environmental fate of nanomaterials
Liu et al. (Liu et al., Beilstein J. Nanotechnol., 2015, 6, 938–951, DOI: 10.3762/bjnano.6.97) from the University of California Center for Environmental Implications of Nanotechnology (UC CEIN) have combined the benefits of life-cycle inventory analysis approach (LearNano) and a modeling platform, MendNano, to create a simulation tool called RedNano to aid in nano-LCA. RedNano is packaged in a straightforward graphical user interface (GUI). A schematic of the approach is shown in Fig. 4. (1) The GUI enables the user to direct simulations through inputting specific research questions and selecting the tabulated and visualization output for the results (i.e., graphs, maps, and Sankey diagrams). (2) MendNano is used to determine the environmental distribution of the ENM of interest by compartmentalizing different environments and connecting them using intermedia transport processes. This tool can track ENM concentrations in the different compartments over time by using the inventory of rates for the different intermedia transport processes. Interestingly, different weather conditions can be simulated. Importantly, in these calculations, MendNano considers more than the average size of the nanoparticles and includes the particle size distribution. (3) The release rates of the ENM of interest are determined using LearNano. (4) Of particular note, this system uses primary data compiled from the literature for the regional ENM production rates throughout the world, which is found in a centralized parameter database. (5) Finally, RedNano has the ability to create a library to save the scenarios and simulations that have been investigated.The authors demonstrate the use of RedNano through some example case studies. A case study of TiO2 nanoparticles introduced into the environment in the greater Los Angeles area is used to demonstrate example air, water, and sediment outputs. The nano-TiO2 concentrations in air and water eventually reach a pseudo-steady state after 4 and 38 days, respectively. Rainfall influences the respective concentrations in the environmental compartments such that the nanoparticle concentration in air decreases with rainfall and the nano-TiO2 content of sediment/soils slowly increases over time.
While RedNano is equipped with robust parameter databases for information such as intermedia transport rates for ENMs in various locations or meteorological information, there is also a level of customizability in designing the scenario to study. Users can manually input these values or use submodels to determine them themselves. The user specifies the simulation time and the ENM release kinetics can be set to simulate seasonal and daily variability. In addition to graphical and tabulated results, outputs can be visualized as the expected release rates of ENM from different regions or as a breakdown of the compartment distribution based on its intended application. RedNano identifies the environmental fate compartments of ENMs. The intended use (e.g. paints, electronics) of the ENM is taken into account, as this will influence how much of the material is released and to which environmental compartments. The two key differences between this and other LCA frameworks is (i) the incorporation of the meteorological data and (ii) the beautiful output visualizations. LearNano and MendNano are easily accessible through a straightforward website (http://nanoinfo.org/); however registration is required for use.
Acknowledgements
This highlight was initiated from the literature discussion of a biweekly trainee (student and research scientist) group meeting in the Center for Sustainable Nanotechnology. The Center for Sustainable Nanotechnology is supported by a grant awarded to Prof. Bob Hamers by the National Science Foundation under grant number CHE-1503408.| This journal is © The Royal Society of Chemistry 2017 |