Phosphate and phytate adsorption and precipitation on ferrihydrite surfaces†
Abstract
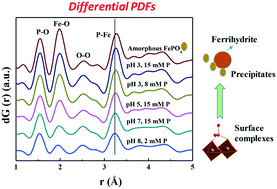
Phosphorous (P) sorption on mineral surfaces largely controls P mobility and bioavailability, hence its pollution potential, but the sorption speciation and mechanism remain poorly understood. We have identified and quantified the speciation of both phosphate and phytate sorbed on ferrihydrite with various P loadings at pH 3–8 using differential atomic pair distribution function (d-PDF) analysis, synchrotron-based X-ray diffraction (XRD), and P and Fe K-edge X-ray absorption near edge structure (XANES) and attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy. With increasing P sorption loading for both phosphate and phytate, the sorption mechanism transits from bidentate-binuclear surface complexation to unidentified ternary complexation and to precipitation of amorphous FePO4 and amorphous Fe-phytate. At a given P sorption loading, phosphate precipitates more readily than phytate. Both phosphate and phytate promote ferrihydrite dissolution with phytate more intensively, but the dissolved FeIII concentration in the bulk solution is low because the majority of the released FeIII precipitate with the anions. Results also show that amorphous FePO4 and amorphous Fe-phytate have similar PO4 local coordination environment. These new insights into the P surface complexation and precipitation, and the ligand-promoted dissolution behavior improve our understanding of P fate in soils, aquatic environment and water treatment systems as mediated by mineral-water interfacial reactions.


 Please wait while we load your content...
Please wait while we load your content...