Application of nanotechnologies for removing pharmaceutically active compounds from water: development and future trends
Zhengqing
Cai
 a,
Amarendra Dhar
Dwivedi
a,
Amarendra Dhar
Dwivedi
 bc,
Wan-Ning
Lee
bc,
Wan-Ning
Lee
 d,
Xiao
Zhao
d,
Xiao
Zhao
 e,
Wen
Liu
e,
Wen
Liu
 *df,
Mika
Sillanpää
*df,
Mika
Sillanpää
 bg,
Dongye
Zhao
bg,
Dongye
Zhao
 e,
Ching-Hua
Huang
e,
Ching-Hua
Huang
 d and
Jie
Fu
d and
Jie
Fu
 *a
*a
aDepartment of Environmental Science and Engineering, Fudan University, Shanghai 200433, China. E-mail: jiefu@fudan.edu.cn; Fax: +86 21 6564 7707; Tel: +86 21 6564 7707
bLaboratory of Green Chemistry, School of Engineering Science, Lappeenranta University of Technology, Sammonkatu 12, 50130 Mikkeli, Finland
cSchool of Environmental Science and Engineering, POSTECH, Pohang 790-784, Republic of Korea
dSchool of Civil and Environmental Engineering, Georgia Institute of Technology, Atlanta, GA 30332, USA. E-mail: wen.liu@pku.edu.cn; Fax: +1 334 844 6290; Tel: +1 334 444 7129
eEnvironmental Engineering Program, Department of Civil Engineering, Auburn University, Auburn, AL 36849, USA
fCollege of Environmental Science and Engineering, Peking University, The Key Laboratory of Water and Sediment Sciences, Ministry of Education, Beijing 100871, China
gDepartment of Civil and Environmental Engineering, Florida International University, 10555, West Flagler Street, Miami, Florida 33174, USA
First published on 19th October 2017
Abstract
Pharmaceutically active compounds (PhACs) are widely detected emerging contaminants in water environments and possess high potential risks to human health and aquatic life; however, conventional water treatment processes cannot remove them sufficiently. The boom in nanoscience and nanotechnology offers opportunities to leapfrog on the back of these new technologies to develop innovative techniques in the field of water treatment. The extraordinary properties of nanomaterials, such as large surface area, quantum effect, electrochemical and magnetic properties, and other size-dependent physical and chemical properties, offer nanotechnologies great advantages over conventional technologies. To date, nanomaterials have been extensively applied or investigated in adsorption, photocatalysis, catalytic ozonation and filtration processes and have been shown to have many promising potential application prospects. Among the various nanomaterials, graphene and carbon nanotubes have shown a superior adsorption capacity for the removal of PhACs and possess great potential for modifying photocatalysts; moreover, they can also act as highly efficient catalysts for ozonation. The nano-sized photocatalysts, i.e. nano-TiO2, graphitic carbon nitride, MoS2 nanosheets, and ZnO, generally exhibit higher photocatalytic activity than bulk photocatalysts. The involvement of nanomaterials in a membrane can improve the permeability, selectivity, and anti-fouling properties of the membrane for improved filtration processes. However, some challenges, such as high cost, poor separation performance and environmental risks, are still impeding their engineering application. Aiming to provide readers with a comprehensive insight into the application of nanotechnologies for PhACs' remediation, the current review summarizes the recent advances and breakthroughs made in nanotechnology for PhACs' removal, highlights the modification methods for improving the effectiveness of treatment methods using nanomaterials, and proposes a number of possible further research directions.
Environmental significancePharmaceutically active compounds (PhACs) are found to be widely emerging contaminants in water environments and possess high potential risks to human health and eco-systems. However, conventional water treatment processes cannot completely remove these compounds, and thus advanced treatment techniques are necessary. Due to the blooming development of nanotechnology, there are great opportunities to efficiently remove PhACs from water. Due to their specific nanoscale properties, nanomaterials show great potential application for PhACs removal through the processes of adsorption, photocatalysis, enhanced advanced oxidation processes (AOPs) and filtration. This work gives an overview of the recent advances using nanotechnologies for PhACs remediation. |
1. Introduction
Pharmaceuticals are a large, diverse group of compounds designed to prevent, control, and cure diseases and improve health. The list of pharmaceuticals includes thousands of non-prescription and prescription drugs, i.e. antibiotics, antipyretics, antidepressants, analgesics, blood lipid regulators, contraceptive drugs, and chemotherapy agents.1,2 The most commonly used pharmaceuticals are listed in Table 1. The continuous growth in the global population, increasing investment needed for health-care, pervasive global market availability, and ageing societies in many countries have greatly led to an increased consumption of pharmaceuticals in the past few decades.3 Nowadays, pharmaceuticals are used in significant quantities throughout the world. For example, in the U.S., approximately 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 prescription pharmaceuticals and more than 100
000 prescription pharmaceuticals and more than 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 over-the-counter pharmaceuticals are distributed for human consumption.4 In China, the total usage of 36 frequently detected antibiotics was 92
000 over-the-counter pharmaceuticals are distributed for human consumption.4 In China, the total usage of 36 frequently detected antibiotics was 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 tons in 2013, of which ca. 53
700 tons in 2013, of which ca. 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 tons entered the receiving environment.5 Therefore, many pharmaceuticals have been widely detected in the effluents of wastewater treatment plants, in surface waters, in groundwater and even in some drinking waters, and pose a great threat to public health and aquatic ecosystems.6 In this introduction, we summarize the source and fate of pharmaceutical contaminants in the water environment and, most importantly, the corresponding treatment methods.
800 tons entered the receiving environment.5 Therefore, many pharmaceuticals have been widely detected in the effluents of wastewater treatment plants, in surface waters, in groundwater and even in some drinking waters, and pose a great threat to public health and aquatic ecosystems.6 In this introduction, we summarize the source and fate of pharmaceutical contaminants in the water environment and, most importantly, the corresponding treatment methods.
| Compound | Classification | MW (g mol−1) | Water solubilitya | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kowa Kowa |
|---|---|---|---|---|
a Water solubility (mg L−1, at 25 °C) and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow (octanol–water partition coefficient) values were obtained from the Syracuse Research Corporation (SRC) PhysProp database (http://www.syrres.com/esc/physdemo.htm) or PubChem database (http://pubchem.ncbi.nlm.nih.gov/). Kow (octanol–water partition coefficient) values were obtained from the Syracuse Research Corporation (SRC) PhysProp database (http://www.syrres.com/esc/physdemo.htm) or PubChem database (http://pubchem.ncbi.nlm.nih.gov/).
|
||||
| Acetaminophen | Antipyretic | 151 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.46 |
| Amoxicillin | Antibiotic | 365 | 3430 | 0.87 |
| Ampicillin | Antibiotic | 594.7 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
−1.13 |
| Aripiprazole | Atypical antipsychotic | 448.4 | 7.77 | 4.13 |
| Atenolol | Beta-blocker | 266.34 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
0.16 |
| Caffeine | Stimulant | 194 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
−0.07 |
| Carbadox | Antibiotic | 262 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
−1.37 |
| Carbamazepine | Antiepileptic/antidepressant | 236 | 17.7 | 2.45 |
| Ciprofloxacin | Antibiotic | 331.3 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
−1.74 |
| Crestor | Statin | 481.5 | 88.6 | 1.47 |
| Chloramphenicol | Antibiotic | 323.1 | 2500 | 1.14 |
| Diazepam | Anxiolytic | 284.8 | 50 | 2.82 |
| Diclofenac | Arthritis | 318.1 | 1113 | 0.70 |
| Dilantin (phenytoin) | Anti-convulsant | 252.3 | 32 | 2.47 |
| Ethynylestradiol | Contraceptive | 296.2 | 11.3 | 3.67 |
| Erythromycin–H2O | Antibiotic | 733.9 | 4.8 | 3.06 |
| Fluoxetine | Antidepressant | 309.3 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.1 |
| Furosemide | Loop diuretic | 330.7 | 102.6 | 2.03 |
| Gemfibrozil | Lipid regulator | 250 | 10.9 | 4.77 |
| Hydrochlorothiazide | Diuretic | 297.7 | 722 | −0.07 |
| Ibuprofen | Pain reliever | 206.1 | 21 | 3.97 |
| Ketoconazole | Antifungal | 531.4 | 0.29 | 4.34 |
| Meprobamate | Anxiolytic | 218.3 | 4700 | 0.70 |
| Naproxen | Analgesic | 230.1 | 15.9 | 3.18 |
| Ofloxacin | Antibiotic | 361.4 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
−0.3 |
| Pentoxifylline | Hemorrheologic | 278.1 | 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.29 |
| Penicillin G | Antibiotic | 356.4 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–100 000–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| Salbutamol | Treat bronchospasm | 239.3 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.4 |
| Salmeterol | β2 adrenergic receptor | 415.6 | 27.37 | 4.15 |
| Sulfachloropyridazine | Antibiotic | 285 | 7000 | 0.31 |
| Sulfamerazine | Antibiotic | 278 | 1500 | 0.89 |
| Sulfamethizole | Antibiotic | 270 | 1050 | 0.54 |
| Sulfamethoxazole | Antibiotic | 253 | 610 | 0.89 |
| Tetracycline | Antibiotic | 444.4 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
−2.56 |
| Triclosan | Antibiotic | 289.6 | 12 | 4.76 |
| Trimethoprim | Antibiotic | 290.1 | 400 | 0.91 |
1.1 Pharmaceutical pollution
Pharmaceutically active compounds (PhACs) are relatively stable, and cannot be fully assimilated by humans and animals during usage. Hence, they are only partially metabolized and some part is excreted in the urine and faeces and can enter wastewater treatment plants (WWTPs) or the water environment.8 Up to 90% of administered PhACs can be excreted unmetabolized in urine or stools and can enter domestic wastewater systems.9 Due to ineffective treatment, PhACs can enter the environment as parent compounds or active metabolites via the effluents from WWTPs. The other sources of PhACs are manufacturing processes, agricultural fields, concentrated animal feeding operations, landfill leachates, and urban run-off.1,2,6 Numerous studies on the occurrence of PhACs have been performed, and the concentration of PhACs in wastewater, surface water, and groundwater has been detected as ranging from the ng L−1 levels to more than μg L−1 levels.10,11 Wastewater treatment plants are considered as hotspots as a source of PhACs in the environment.12 In 1970s, Hignite and Azarnoff13 reported PhACs contaminants in wastewater effluents and surface waters in the U.S., but the PhACs did not cause much concern at that time. During 1999 and 2000, the U.S. Geological Survey (USGS) conducted the first U.S. nationwide reconnaissance of pharmaceuticals, hormones, and other organic wastewater contaminants (OWCs) in water resources from 139 susceptible streams across 30 states and found that 80% of the sampled streams were contaminated by OWCs with a high detected frequency for non-prescription drugs (about 80%) and antibiotics (about 50%).14 Thereafter, efforts have gradually shifted to study the source, fate, and treatment methods for PhACs.PhACs-contaminated surface water can enter drinking water sources, and consequently appear in our drinking water supplies. The most widely used drugs, i.e. caffeine, acetaminophen, and cotinine, have been detected in drinking water samples collected near Atlanta, Georgia.15 A study conducted by the USGS and the Center for Disease Control and Prevention documented that some prescription and non-prescription drugs and their metabolites have been frequently detected in drinking water,15 albeit the detected PhACs concentration in drinking water generally was low and did not exceed Federal drinking water standards or lifetime health advisories, although such standard or advisories have not been established for most of the PhACs.15
To date, PhACs have been widely detected in surface waters, groundwater, or tap waters in different countries, as shown in Fig. 1.16 More than 100 different PhACs were detected in the U.S. and several European countries in the aquatic environment with concentrations higher than the detection limit. More than 30 different PhACs have been found in Asia-Pacific, Caribbean States, Latin American, Eastern European and Western European countries.16
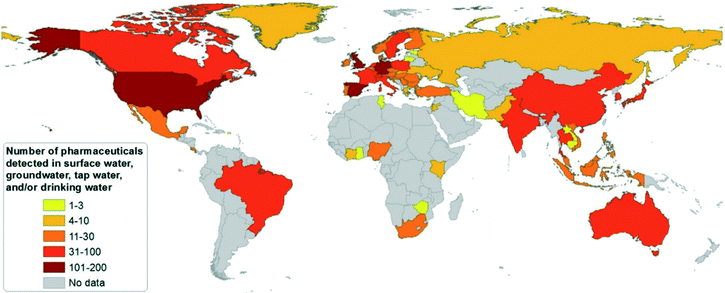 | ||
| Fig. 1 Country survey on the number of pharmaceutical substances detected in surface waters, groundwater or tap/drinking water (reproduced with permission from ref. 16, copyright 2016 John Wiley and Sons). | ||
Many pharmaceuticals are reported to have acute or chronic toxicity to fish and invertebrates (as shown in Fig. 2).17 Researchers and regulatory agencies have been scrutinizing the level of health risks associated with the exposure to PhACs in drinking water. Unfortunately, our knowledge on the chemical persistence and microbial resistance of PhACs remains insufficient. Moreover, little is known about the potential interactive effects (synergistic or antagonistic toxicity) of pharmaceuticals and other xenobiotic compounds.10
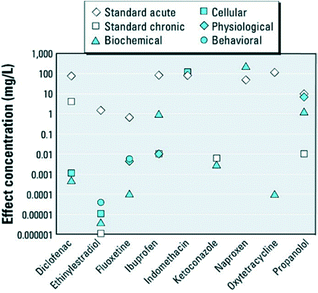 | ||
| Fig. 2 Standard acute and chronic and nonstandard endpoints of various pharmaceuticals in fish and invertebrates (reproduced with permission from ref. 17, copyright 2012 Environmental Health Perspectives). | ||
1.2 Conventional treatment methods
Conventional wastewater treatment techniques such as biological processes, activated carbon (AC) adsorption, ozonation, UV photolysis/photocatalysis, and wetlands treatment are commonly employed to remove organic pollutants from waters.18 However, these methods are limited due to shortcomings of low efficiency or high cost.It is widely reported that the most commonly applied biological processes cannot completely degrade recalcitrant PhACs.9,19,20 For instance, carbamazepine is nearly non-biodegradable, and some other commonly found PhACs (e.g. clofibric acid and bezafibrate) can only be partially removed (by 34–51% and 50–93%, respectively).9 In addition, the wastewater discharged by the pharmaceutical industry may include organic solvents, raw materials, reactants, intermediates, active pharmaceutical ingredients (APIs), and catalysts, making it very difficult to be treated.20 Therefore, alternative treatment methods are required to further eliminate the PhACs from wastewater.
Activated carbon, the most commonly applied adsorbent, is capable of removing many hydrophobic and charged PhACs from the aqueous phase. The non-specific dispersive interactions (e.g. van der Waals interactions) are the dominant adsorption mechanism and contribute most to the removal of most non-polar PhACs with a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow >2, while the electrostatic interactions between the ionic PhACs and charged activated carbon surface are responsible for the removal of polar PhACs.21,22 The adsorptive removal of PhACs by activated carbon is commonly applied as post-treatment after biological treatment or as an advanced treatment process for drinking water treatment. However, the total energy demand of activated carbon methods is considerably high, and the effectiveness is greatly affected by the natural dissolved organic matters (DOMs) in the wastewater matrix.
Kow >2, while the electrostatic interactions between the ionic PhACs and charged activated carbon surface are responsible for the removal of polar PhACs.21,22 The adsorptive removal of PhACs by activated carbon is commonly applied as post-treatment after biological treatment or as an advanced treatment process for drinking water treatment. However, the total energy demand of activated carbon methods is considerably high, and the effectiveness is greatly affected by the natural dissolved organic matters (DOMs) in the wastewater matrix.
Membrane filtration, i.e. nanofiltration and reverse osmosis (RO), have been shown to effectively remove PhACs with a low molecular weight.23 Membrane processes remove PhACs through the mechanisms of adsorption in the initial stages and rejection during the steady state, where the removal efficiency is dependent on the membrane characteristics (material, pore size, surface morphology, etc.), the physico-chemical properties of the PhACs (molecular weight, hydrophilicity/hydrophobicity, pKa, etc.), and solution parameters (ionic strength, pH, etc.).23 However, membrane fouling is still a challenge for the effective operation of membrane processes, and greatly restricts its engineering application, particularly for wastewater treatment.
Ozonation has been traditionally employed for treating organic contaminants in drinking water and is effective in removing PhACs from wastewater as a secondary treatment method.24 However, the ozonation of PhACs still has a few shortcomings, such as a high energy consumption and issues with the uncertain effects of the oxidation products/intermediates. Catalytic ozonation, as one of the most advanced oxidation processes (AOPs), is an alternative ozonation process with enhanced efficiency for organic pollutants removal. This method overcomes the limitations of ozonation processes, such as the formation of selective reactants and the incomplete mineralization of PhACs.25 The adsorption and diffusion of PhACs on catalysts are the rate limiting step for the catalytic ozonation process, and therefore, new photocatalysts are required to overcome these limits.
UV radiation is generally applied with photocatalysts (e.g. TiO2) to degrade PhACs in wastewater and shows a relatively high efficiency.26 Moreover, the photocatalytic degradation of PhACs under solar light has been considered as a sustainable method considering the low energy cost. However, the conventional photocatalysis methods still have a number of drawbacks, such as low solar light activity, a low quantum yield efficiency and high energy consumption. Therefore, new photocatalysts are needed to improve the quantum efficiency and extend the effective wavelength to the visible light region.
The development of conventional methods (such as adsorption, AOPs and biological technologies) for treating pharmaceuticals and personal care products (PPCPs) was critically reviewed by Xu et al.27 Many materials used in adsorption and AOPs were well discussed in this review, which provides readers with an overview of the merits and shortcomings of various technologies/materials for PPCPs removal. However, only limited novel materials, particularly nanomaterials, were mentioned. In recent years, novel approaches are being continuously proposed to supplement conventional water treatment processes and many of these can achieve a higher water quality with minimized costs.
1.3 The treatment methods associated with nanomaterials
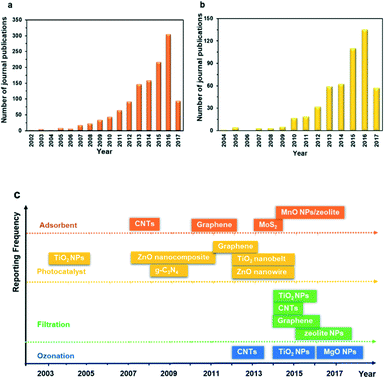
The recent development of nanotechnology offers leapfrogging opportunities for developing innovative technologies for the more efficient degradation of PhACs during water and wastewater treatment. Nanotechnology generally means the application of materials and structures with a nanoscale dimension of 1–100 nm.28,29 The basic structures of nanomaterials include nanoparticles or nanocrystals, nanolayers, and nanotubes.29 The extraordinary properties of nanomaterials, including large surface area, small size effect, quantum effect, photosensitivity, catalytic activity and electrochemical and magnetic properties, as well as tunable pore size and surface chemistry, are all promising features for treating the PhACs in wastewater, thus showing great application potential in adsorption, photocatalytic degradation, catalytic ozonation and modification of the membrane filtration process.30Fig. 3a and b reveal the number of publications on the application of nanomaterials as adsorbents and photocatalysts for treating PhACs; Fig. 3c summarizes the date of the initial application of selected nanomaterials for treating PhACs via various methods. It is obvious that the past decade has witnessed a surge of interest in the application of nanomaterials towards the removal of PhACs in water and in the wastewater treatment area. Based on the literature survey, it is worth noting that carbon nanotubes (CNTs) and graphene-associated nanomaterials offer exciting opportunities for treating PhACs-contaminated wastewater with high efficiency. These two carbon materials have been extensively investigated in recent years, which is one of the key reasons for the rapidly increased publications in this field. The exponential increase in the number of papers provides vast information for readers; however, to the best of our knowledge, there is a lack of comprehensive and high quality review articles focused on the removal of PhACs using nanomaterials, and only limited review papers about the removal of PhACs using nanotechnologies can be found. In 2013, Rivera-Utrilla et al.31 reviewed the removal of PhACs from water by using conventional treatment methods, but did not include the application of nanotechnologies. Jung et al.32 reviewed the application of CNTs to remove endocrine-disrupting compounds and PPCPs from water and proposed further research. Most recently, Cincinelli et al.33 summarized the nanotechnologies for the removal of pharmaceuticals and PPCPs from water and wastewater, but only highlighted CNTs, zeolites, nano-TiO2 and Fe3O4. Many other newly emerging nanomaterials, such as g-C3N4 and graphene, should be included, considering their promising application potential and the research efforts devoted to these. Moreover, it is worth noting that nanofiltration itself is not real nanotechnology,34 and the association of nanofiltration with nanotechnologies in numerous studies can lead to considerable confusion.
In this study, we present a thorough overview to summarize the recent developments and breakthroughs in the removal of PhACs using novel nanomaterials and nanotechnologies, including (1) the fundamental mechanisms of most commonly used methods combined with nanotechnologies, i.e. adsorption, photocatalysis, catalytic ozonation, and the membrane process; (2) the merits and limits of the nanotechnologies and barriers to their full-scale application; and (3) discussion of the current knowledge gaps and further evolution of nanotechnologies for the treatment of PhACs.
2. Adsorption
As briefly discussed in the introduction, adsorption is the most popular environmental remediation approach due to its flexibility in terms of design and operation, feasibility for retrofitting to current water treatment trains, and cost-effective and environmentally friendly nature.35 Conventional adsorbents, e.g., AC, zeolites, clays, polymeric adsorbents, and bentonite, have been extensively studied for removing water soluble PhACs including carbamazepine, diclofenac, ibuprofen, and ketoprofen.35 Unfortunately, these conventional adsorbents have a number of severe limitations, particularly their low adsorption capacity and rate. However, the booming development of many nanomaterials, such as CNTs, graphene, and nanoscale zeolites, sheds light on the enhanced adsorption of PhACs. These nano-adsorbents offer unique advantages, including large specific surface area, selective and abundant adsorption sites, short intraparticle diffusion distance, tunable pore size, and easy regeneration and reusability.30In this part, the recent advances, effectiveness and merits of the most widely studied nano-adsorbents, i.e. CNTs, graphene and nano-metal oxides, are summarized, and the limitations and challenges for their application are discussed.
2.1 Carbonaceous nanomaterials
The hybridization of carbon to different degrees results in the formation of a variety of allotropes, including CNTs,36 carbon nanofibers (CNFs),37 graphene,38,39 carbon nanoparticles, and carbon nanobeads (CNBs).40 Among these, sp2-hybridized carbon allotropes, i.e. CNTs and graphene, have attracted enormous attention as promising adsorbents due to their unique surface properties derived from their nanostructure, and they have been extensively studied for the adsorptive removal of PhACs from the aqueous phase.41,42Graphene is a two-dimensional (2D) graphite with a single atomic layer honeycomb network of sp2 hybridization. It was first obtained by Novoselov et al.43via the mechanical exfoliation of pyrolytic graphite in 2004. CNTs are rolled-up graphene sheets with a single-wall (SWCNTs) or multiwall (MWCNTs); they were discovered by Iijima in 1991 during the arc-evaporation synthesis of fullerene.44Fig. 4 shows the structures and images of graphene and CNTs. It has been predicted that the global market for graphene-based materials will increase at an annual growth rate of 58.7% and will reach $675 million in 2020.45,46 However, to the best of our knowledge, it was not until the years of 2008 and 2011 (ref. 47 and 48) that publications started to report the research on the adsorptive removal of PhACs by CNTs and graphene (Fig. 3).
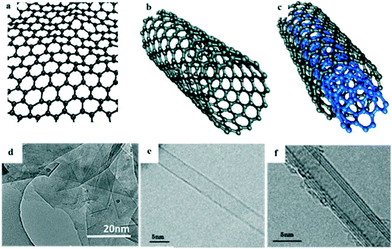 | ||
| Fig. 4 Structures and TEM images of: (a and d) graphene, (b and e) SWCNTs and (c and f) MWCNTs (reprint with permission from ref. 49–52, copyright 2009, 2013 American Chemical Society, 2011, 2015 Elsevier). | ||
The adsorption of PhACs on carbonaceous nanomaterials derive from five possible interactions, including hydrophobic effects, π–π stacking, hydrogen bonding and electrostatic and covalent interactions.53 The interaction types, strengths, and contribution to the overall adsorption are a function of the properties of both carbonaceous nanomaterials and the PhACs molecules. Therefore, for the adsorptive removal of PhACs by carbonaceous nanomaterials, the surface properties, such as surface area, functional groups on the adsorbent surface, the pores, determine its adsorption performance in the aqueous phase.
The large specific surface area of graphene (2630 m2 g−1 for single-layer graphene) and CNTs (∼650 m2 g−1 for SWCNTs and 420 m2 g−1 for MWCNTs) provide a large number of surface adsorption sites,45,54 which is believed to be the primary reason for their superior adsorption capacity. Under ideal conditions, four types of adsorption sites are available on CNTs, including the external surface area, inner cavities of tubes and the interstitial and groove areas between tubes. However, the inner cavities are generally inaccessible to PhACs molecules due to the blocking effect by impurities or amorphous carbon, while the interstitial areas between the CNTs bundles can be too small to fit the adsorbate molecules. Therefore, the external surface and groove area of CNTs are the primary effective surfaces for adsorption.55 While adsorption occurs on both sides of the nanosheets of graphene, the larger surface area and ratio of accessible surface provide them with an even higher potential as an excellent adsorbent. Besides their large surface area, the high hydrophobicity and extraordinary high mechanical properties are ideal properties to make graphene and CNTs excellent adsorbents.
To appreciate the role that CNTs and graphene can play in PhACs adsorption, it is useful to review the reported PhACs adsorptive removal performance by graphene and CNTs. Table 2 summarizes the PhACs removal efficiency and reaction conditions reported by some researchers. Notably, most of the reported CNTs and graphene exhibit high adsorption capacities for different PhACs.
| Adsorbents | PhACs | pH | M 0 | C 0 | t | R | Q e | k 2 |
|---|---|---|---|---|---|---|---|---|
| a M 0 (mg L−1) is the dosage of adsorbents; C0 (mg L−1) is the initial concentration of the adsorbate; t (hour) is the time for the adsorption to reach equilibrium; R is the removal efficiency at equilibrium; Qe (mg g−1) is the adsorbate adsorption capacity at equilibrium; k2 (min−1) is the rate constant of the pseudo-second-order model. | ||||||||
| Graphene oxide (GO)56 | Tetracycline | 3.6 | 181 | 33.33 | 0.5 | — | 126 | 0.065 |
| GO (ref. 57) | Sulfamethoxazole, ciprofloxacin | 5.0 | 100, 10 | 20, 10 | 12, 24 | 47%, 34.5% | 93.8, 345 | 0.133, 0.067 |
| Activated GO (ref. 58 and 59) | Ciprofloxacin | 7.0 | 500 | 150 | 0.036 | 50% | 149.4 | 0.036 |
| Graphene nanoplatelets | Aspirin, acetaminophen, caffeine | 8.0 | 1000 | 20 | 0.17 | 65%, 90%, 100% | 13, 18, 20 | 0.185, 0.188, 0.777 |
| Reduced GO (rGO) (ref. 45) | Ketoprofen, carbamazepine | 6.5 | — | 15, 30 | 170, 96 | — | 45, 75 | — |
| Fe3O4 modified rGO (ref. 48) | Tetracycline | — | 1600 | 12.5, 75 | 24 | 50% | 95, 12 | — |
| Fe3O4-modified graphene (ref. 60) | Oxytetracycline, tetracycline | 7.0 | 3000 | 1 | 1 | 97%, 98% | 2.5, 1.95 | 0.00083, 0.00097 |
| Sodium alginate/GO (ref. 61) | Ciprofloxacin | — | 300 | 50 | 48 | 84.5% | 25.9 | 7.36 |
| SWCNTs45 | Ketoprofen, carbamazepine | 6.5 | — | 15, 30 | 48, 96 | — | 73, 148 | — |
| MWCNTs45 | Ketoprofen, carbamazepine | 6.5 | — | 15, 30 | 24, 48 | — | 20, 54 | — |
| MWCNTs55 | Sulfamethoxazole, thiamphenicol, ibuprofen, diclofenac, carbamazepine | 7.0 | 427–1556 | 0.2 | 12 | — | 5.85, 9.30, 1.45, 20.64, 15.38 | — |
| MWCNTs62 | Oxytetracycline, carbamazepine | 7.0 ± 0.2 | 25 or 50 | 2.5 | 24 | 79–97% | 92.59, 37.59 | 0.317, 0.117 |
| MWCNT-coated biochar63 | Sulfamethazine | 5.0 | 1000 | 10 | 24 | >90% | 9.5 | — |
| Granular CNTs/alumina (ref. 64) | Diclofenac, carbamazepine | 6.0 | 250 | 4.6, 3.7 | 36, 48 | 60%, 73% | 11.4, 10.8 | — |
It is commonly accepted that SWCNTs have a higher adsorption capacity for organic compounds than double and multi-walled CNTs.65 Do the carbonaceous nanomaterials follow the law that a larger surface area gives a higher adsorption capacity? To answer this question, a study measured the maximum adsorption capacities for three PhACs (ketoprofen, carbamazepine and bisphenol A) using five carbonaceous nanomaterials and found that the adsorption followed the sequence SWCNTs > reduced graphene oxides > MWCNTs > graphene > graphite. This order is in line with the order of their surface areas and micro-pore volumes.45 Therefore, efforts have been devoted to increase the adsorption capacity via increasing the specific surface area of CNTs. For instance, a KOH dry etching method was applied to activate CNTs. The surface area of the activated SWCNTs was increased from 410.7 to 652.8 m2 g−1 and that of MWCNTs was increased from 157.3 to 422.6 m2 g−1. Consequently, the adsorption capacity for the tested antibiotics (sulfamethoxazole, tetracycline, and tyrosine) was increased 2–3 and 3–8 times by the activated SWCNTs and MWCNTs, respectively.54
Apart from surface area, some surface properties, such as the amount and form of the functional groups, also greatly affect the adsorption of PhACs by graphene and CNTs. Graphene oxide is the most popular graphene-based adsorbent. It has a high density of functional groups (hydroxyl, carboxyl, carbonyl and epoxy) in the carbon lattice, which makes it extremely hydrophilic and provides it with a high adsorption performance.66 For graphene and CNTs, proper functionalization can prevent aggregation of the nanomaterials, leading to improved selectivity, a higher adsorption capacity or faster adsorption.40 Introducing oxygenated functional groups, such as hydroxyl (–OH), carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and carboxylic groups (–COOH), could enhance the adsorption of graphene and CNTs by affecting the hydrophobic interactions and π–π bonds.67 For example, the reducing of graphene oxide could introduce a large quantity of surface functional groups, such as epoxide, alcohol, carbonyl and carboxylic acid, which serve as efficient adsorption sites for pharmaceutical contaminants.68 Yu et al.58 synthesized an activated graphene by a one-step alkali-activated method, where the activation process introduced oxygen-containing functional groups on the materials and improved the maximum adsorption capacity of ciprofloxacin from 145.9 to 194.6 mg g−1. Another study compared the adsorption performance of norfloxacin by CNTs with different functionalization types, including graphitized MWCNTs (G-MWCNTs), carboxylated MWCNTs (C-MWCNTs) and hydroxylated MWCNTs (H-MWCNTs) with AC; whereby, the functionalized CNTs showed higher selectivity and greater desorption hysteresis. On the basis of unit surface area, the sorption coefficient followed the order G-MWCNTs > H-MWCNTs > C-MWCNTs > AC. Although AC had a much larger BET surface area (664 m2 g−1) than the CNTs (117–228 m2 g−1) in this study, its adsorption performance was poorer.69
O) and carboxylic groups (–COOH), could enhance the adsorption of graphene and CNTs by affecting the hydrophobic interactions and π–π bonds.67 For example, the reducing of graphene oxide could introduce a large quantity of surface functional groups, such as epoxide, alcohol, carbonyl and carboxylic acid, which serve as efficient adsorption sites for pharmaceutical contaminants.68 Yu et al.58 synthesized an activated graphene by a one-step alkali-activated method, where the activation process introduced oxygen-containing functional groups on the materials and improved the maximum adsorption capacity of ciprofloxacin from 145.9 to 194.6 mg g−1. Another study compared the adsorption performance of norfloxacin by CNTs with different functionalization types, including graphitized MWCNTs (G-MWCNTs), carboxylated MWCNTs (C-MWCNTs) and hydroxylated MWCNTs (H-MWCNTs) with AC; whereby, the functionalized CNTs showed higher selectivity and greater desorption hysteresis. On the basis of unit surface area, the sorption coefficient followed the order G-MWCNTs > H-MWCNTs > C-MWCNTs > AC. Although AC had a much larger BET surface area (664 m2 g−1) than the CNTs (117–228 m2 g−1) in this study, its adsorption performance was poorer.69
It should be noted that the adsorption of different PhACs undergoes varied mechanisms. For example, the sorption of ciprofloxacin on graphene oxide is primarily controlled by electrostatic attraction, while sulfamethoxazole sorption is mainly though π–π electron donor–acceptor attraction on the graphene oxide.57 Therefore, the functionalization of carbonaceous materials can exhibit different performances on the adsorption of various PhACs.
The development of computational chemistry offered great opportunities for designing/optimizing nanomaterials. For environmental remediation, it has been widely used to interpret the adsorption mechanism of organic contaminants onto nano-adsorbents.39,70–74 In recent years, density functional theory (DFT) calculations have attracted great research interests and is a powerful tool for elucidating the adsorption process of PhACs on nanomaterials. For example, Song et al.75 studied the competitive adsorption of two pharmaceuticals on rGO, and DFT calculations of the sorption energy revealed that rGO had a higher sorption affinity for tetracycline than that for sulfamethazine. In addition, the high sorption of organics on rGO was ascribed to π–π interactions, hydrogen bonds, and van der Waals interactions. In future, more in-depth insights and details of mechanisms of the adsorption of PhACs should be explored by means of computational calculations.
The application of nanomaterials for environmental remediation is always criticized due to their poor separation property and possible release into the environment due to their small size. Therefore, the immobilization of CNTs and graphene has been extensively studied by fabricating hybrid nanocomposites by coating or by impregnating the nanomaterials onto large solid particles, or by synthesizing CNTs or graphene on membranes,76 for instance, Wu et al.68 loaded GO on calcium alginate fibres via a wet spinning method. The prepared material exhibited good separation performance and also achieved a relatively high ciprofloxacin removal efficiency, i.e. 78.9% of ciprofloxacin was removed at the adsorbent dosage of 2 g L−1 with a GO loading of 6%. The CNTs could form a hybrid with Al2O3 to obtain a separable porous granular hybrid adsorbent-CNTs/alumina, where the composite demonstrated a good adsorption performance for diclofenac sodium and carbamazepine. Moreover, it could be fully thermally regenerated.64
The carbonaceous nanomaterials possess promising potential for use as adsorbents; however, there are some key technical barriers yet to be overcome. First, most studies reported so far have focused on the adsorption of a single solute in deionized water or synthesized wastewater. Real water/wastewater, however, is more complicated as pollutants always coexist with natural organic matter (NOM), inorganic ions or other inorganic materials, which impact the adsorption behaviors. For example, NOM coating on graphene or CNTs can alter their physico-chemical properties, thus affecting the adsorption of PhACs.42 Moreover, carbonaceous nanomaterials are difficult to be separated from water after application. The practical application of the widely used ultra-centrifugation method for separation is hindered by a high energy consumption and high cost;76 hence, immobilization methods should be further developed. The largest disadvantage of carbonaceous nanomaterials-based technology is the cost: bulk purified MWCNTs are sold for less than $100 per kg, which is still 1 to 10 times the price of commercially available carbon fiber, and the cost of graphene is even higher.77 Fortunately, the development of synthesis technologies helps to decrease the cost of the carbonaceous nanomaterials. In addition, materials with relatively low purities could still fulfill the requirement as adsorbents.
2.2 Other nano-adsorbents
Other than graphene, some 2D nanomaterials with a high specific surface area, i.e. molybdenum disulfide (MoS2) nanosheets,78 layered double hydroxides,79 and hexagonal boron nitride nanosheets,80 have also exhibited superior adsorption performance for organic compounds in recent years and possess great application potential for PhACs removal in water treatment processes. In addition, magnetic nano-adsorbents (e.g. nanocomposites combined with Fe3O4) have been developed for facilitating the recovery efficiency.81 Moreover, nano-hydroxyapatite and chitosan also are good adsorbents for PhACs removal.MoS2 nanosheets are graphene-like 2D layered materials that are vertically stacked and held together by van der Waals interactions; Fig. 5a and b show their structure and AFM images. The adsorption of PhACs by MoS2 is primarily due to hydrophobic effects, π–π stacking, and electrostatic interactions.78 The large surface area provides MoS2 nanosheets with a large adsorption capacity and relatively fast kinetics for PhACs removal. It has been reported that a layered MoS2 could remove 83% of doxycycline antibiotic in a test with an adsorption capacity around 310 mg g−1.78 However, MoS2 is more commonly reported as a photocatalyst rather than an adsorbent, and therefore, it is also beneficial to take advantage of its prominent adsorption property to promote the photocatalytic performance.
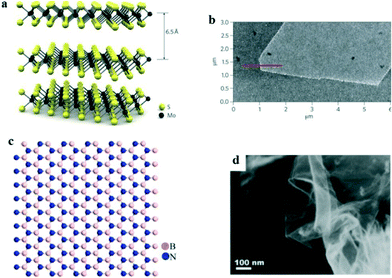 | ||
| Fig. 5 (a) Structure and (b) AFM image of monolayer MoS2;82 (c) structure and (d) SEM images of monolayer boron nitride83 (reproduced with permission from ref. 82 and 83, copyright 2011 nature, and 2014 Elsevier). | ||
Hexagonal boron nitride is isoelectronic with graphene and possesses a similar 2D structure (Fig. 5c and d). Its remarkable properties, i.e. large surface area, high mechanical strength and high stability, render it a good adsorbent.80 Liu et al.80 synthesized highly porous hexagonal boron nitride nanosheets, which exhibited unprecedentedly high adsorption capacities for the tested PhACs (i.e. 1170, 284, 206 and 174 mg g−1 for chlortetracycline, tetracycline, ciprofloxacin and norfloxacin, respectively) in the aqueous phase, and the material showed excellent recyclability after simple regeneration. However, the difficulties involved in the preparation of boron nitride, the high cost and toxicity problems limit its application as an adsorbent for PhACs removal.84 Moreover, information on the adsorption performance, mechanism, effects of the environmental factors and the regeneration of spent materials is still needed, and lots of uncertainties are still present in using boron nitride as an adsorbent.
Nano-hydroxyapatite is an inorganic material widely applied in medical and biological fields, and in recent years it has been claimed to be a promising adsorbent for PhACs removal from wastewater due to its high stability, low solubility and large surface area.85 However, the experimental data showed that only 54.3% and 47.3% of norfloxacin and ciprofloxacin were removed at a high nano-hydroxyapatite dosage (20 g L−1).85 Its relatively low affinity to PhACs makes it hard to compete with other nano-adsorbents or even with conventional AC.
Chitosan is derived from the naturally abundant chitin, and the presence of hydroxyl and amine groups makes it a candidate for use in remove organic pollutants from effluents in pharmaceutical industries. In recent years, chitosan nanoparticles have been reported to exhibit high adsorption kinetics and a capacity for removing diuretic furosemide from water, with a maximum adsorption capacity of furosemide of up to 270 mg g−1 within 10 min.86 Given its high abundance, low cost and renewable and biodegradable properties,87 chitosan possesses great potential as an adsorbent. However, no systematic study concerning the adsorption of various PhACs species by chitosan has yet been published, as well as the environmental parameters on the adsorption process have not been reported, and hence lots of possibilities still remain to be explored.
Magnetic nano-adsorbents (e.g. Fe3O4) are attractive materials because they can be easily retained and separated from treated water.76 To date, several magnetic nano-adsorbents, including magnetic zeolites and magnetic carbon nanocomposite, have been reported for the adsorption of PhACs, and have exhibited high efficiency.81,88 For instance, a study demonstrated that a magnetic nanoparticles (γ-Fe2O3)-coated zeolite achieved >95% removal efficiencies for diclofenac, naproxen, gemfibrozil and ibuprofen within 10 min at an initial concentration of 100 μg L−1 and a sorbent dosage of 1 g L−1.81 Singh et al.88 synthesized a carbon–iron magnetic nanocomposite, which showed an improved ibuprofen removal efficiency compared to its precursor carbon. The synthesis of magnetic nano-adsorbents provides an opportunity for the easy control and fast separation of nano-adsorbents with the ability to retain most of its surface area when compared with other immobilization methods, making the approach of environmental remediation by these nanomaterials more close to the engineering application.
Table 3 compares the merits and shortcomings of the key nano-adsorbents. Undoubtedly, the above-reviewed progress in the adsorptive removal of PhACs by nano-adsorbents demonstrates their promising prospects in wastewater treatment; however, the appropriate cost of nano-adsorbents for practical application has not yet been realized, and some barriers still need to be overcome. Based on our opinion and the pioneering studies, the understanding of the adsorption mechanism should be further improved in future research in order to synthesize functionalized nano-adsorbents with higher selectivity and specificity for PhACs. Moreover, the cost of manufacturing nano-adsorbents needs to be further decreased, and effective regeneration methods should be established. Considering the engineering application, more efforts are required for conducting pilot-scale or even commercial-scale adsorption experiments to fully evaluate the operating costs and efficiency under real water treatment conditions.
| Materials | Advantages | Shortcomings |
|---|---|---|
| CNTs64,66 | High mechanical strength, selectivity, sorption kinetics and capacity, high hydrophobicity and strong affinity to organic pollutants | Hard to recover, potential environmental risks, high cost |
| Graphene40,68 | High mechanical strength, selectivity, quick sorption kinetics and high capacity | Expensive, potential environmental risks |
| MoS2 (ref. 78) | High adsorption capacity and kinetics | High cost, instability, potential environmental risks |
| Boron nitride80,84 | High mechanical strength, high stability, good adsorption capacity | High cost and toxicity |
| Metal oxides81 | High surface area, unique magnetic properties that facilitate their recovery, low toxicity | Low sorption capacity |
3. Photocatalysis
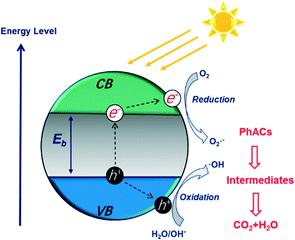
The photocatalytic degradation of PhACs by nanomaterials has attracted significant attention due to the high efficiency of the process and possible complete mineralization of the target organics. Various nanomaterials, such as titanium dioxide (TiO2),89 graphitic carbon nitride (g-C3N4),90 zinc oxide (ZnO),91 iron oxide (Fe2O3)92 and tungsten oxide (WO3),93 have been applied as photocatalysts to degrade PhACs.Fig. 6 presents the photocatalytic process on a semiconductor in detail. The electronic structure of these photocatalysts includes a filled valence band (VB) and an empty conduction band (CB). The electron (e−) in the VB can be excited into the CB when the materials are irradiated with light at a photon energy higher than their band gap energy (Eg). Then, the photo-generated electronic holes (h+) in the VB and electrons will react with the organics directly, or with oxide H2O molecules, to give various reactive oxygen species (ROS) (e.g. ˙OH, ˙O2− and H2O2), which are strong oxidants for PhACs degradation. In addition, the holes can further initiate interfacial electron transfer or other chemical reactions to an adsorbate, or, with the surface-bound ˙OH radicals, or can diffuse into the solvent bulk. The related reactions during photocatalysis are shown as follows:
| Photocatalysts + hv → Photocatalysts(h)+ + e− | (1) |
| Photocatalysts (h)+ + H2O → Photocatalysts + H+ + ˙OH | (2) |
| e− + O2 → ˙O2− | (3) |
| ˙O2− + H+ → HO2˙ | (4) |
| 2HO2˙ → H2O2 + O2 | (5) |
| H2O2 → 2 ˙OH | (6) |
| Photocatalysts (h)+ + Organics → Photocatalysts + CO2 + H2O | (7) |
| ˙OH + Organics → CO2 + H2O | (8) |
Great progress has been made in the photocatalytic degradation of PhACs in the aqueous phase. However, the performance of bulk photocatalysts is still limited by two intrinsic drawbacks: 1) the limited light absorbance spectrum, and 2) the rapid recombination rate of electron–hole pairs. The wide band gap prevents the utilization of visible light, which is a bottleneck for many photocatalysts. Fig. 7 summarizes the typical band gap energies of common photocatalysts with respect to the energy levels of the redox potentials of different species. Until now, metal/non-metal doping and the formation of the heterojunction materials have been commonly applied as band gap adjusting methods to improve the visible light absorbance.94,95 The recombination of photoexcited electron–hole pairs typically takes place on the surface or in the bulk of the catalysts, thus dissipating the energy without reacting with the reactants. Therefore, various strategies have been applied to suppress the recombination process, such as noble metal doping, combining with carbonaceous materials, textural design and copolymerization.95,96
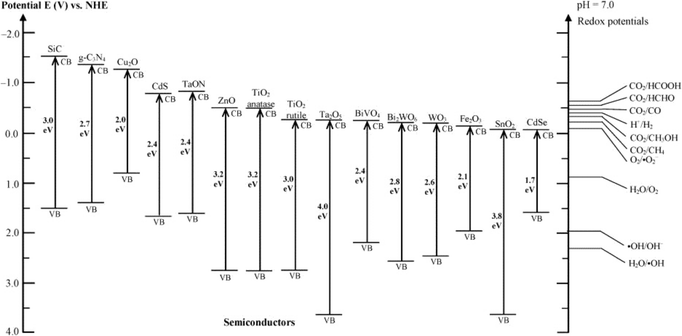 | ||
| Fig. 7 Band gap energies of various photocatalysts and the selected redox potentials of H2O splitting, CO2 reduction and pollutant degradation measured at pH 7 (reproduced with permission from ref. 97, copyright 2016 ACS Publications). | ||
Nano-photocatalysts have exhibit unique properties, such as a large surface area, abundant surface states and the feasibility of nano-surfaces functionalization, which provides them with exciting opportunities to overcome the inherent limitations to achieve supreme photoactivity.
In this part, the most studied nano-photocatalysts and their application towards PhACs removal are summarized and discussed. Since the PhACs removal efficiency of photocatalysis is mainly determined by the activity of the photocatalysts, the modification strategies and examples of the photocatalysts are emphasized.
3.1 Titanium dioxide
Since Fujishima and Honda discovered the photocatalytic splitting of water on TiO2 in 1972,98 interest in its application for contaminant photocatalytic oxidation has significantly increased. TiO2 has been extensively studied as a typical photocatalyst for environmental applications for more than two decades, and it is the most suitable photocatalyst by far due to its high efficiency, low cost and non-toxic and photochemically stable properties. Three TiO2 polymorphs are naturally available; among these, anatase has the highest photocatalytic activity and photoelectron transfer property when compared with rutile, and brookite.99 In recent years, with the development of nanotechnology, a lot of works have focused on the application of nano-TiO2 catalysts, which have shown better photocatalytic degradation ability for organics than bulk TiO2.96,100 The decreased particle size provides shorter carrier-diffusion paths, a lower electron–hole recombination rate, a larger surface area and a better adsorption of contaminants, all of which are beneficial to enhancement of the photocatalytic activity.100The application of TiO2 for environmental remediation has been well documented and has consequently aroused great attention since the 1980s.101 However, it was not until 2004 that researchers started to systematically study the photodegradation of PhACs in the presence of different TiO2 materials, including nano-sized TiO2 (especially P25).102 Thereafter, nano-sized TiO2 with various morphologies have been extensively investigated for PhACs removal, and various modification methods have been applied to TiO2 to promote the photocatalytic activity.
The nano-sized TiO2 with various morphologies, including nanoparticles, nanobelts, nanowires, nanotubes, nanosheets, usually have a large surface area, abundant surface state and many active sites compared with bulk TiO2. It is generally believed that nano-TiO2 presents higher photoactivity in the photocatalysis process. The TiO2 nanoparticles P25 were the first and most extensively studied types of nano-TiO2, and have shown remarkably promoted photocatalytic activity for degrading PhACs compared to bulk TiO2.103 TiO2 nanobelts have also exhibited high degradation kinetics in the photocatalytic degradation of various persistent organics under UV light, where the holes, ˙OH and H2O2 played dominant roles in promoting the degradation rate.104 A highly entangled TiO2 nanowires embedded membrane exhibited higher efficiency for the photocatalytic degradation of pharmaceuticals under UV irradiation compared to TiO2 powder (P25).105 Also, TiO2 nanotubes were found to show high PhACs photocatalytic activity in PhACs degradation, where the activity was closely related to TiO2’s crystallinity and morphology of the photoanodes.106 Moreover, nano-TiO2 possesses an improved charge mobility and fewer localized states near the band edges and in the band gap due to the lower number of unpassivated surface states, which facilitates the photoactivity.
However, the efficacy of TiO2 as a photocatalyst is still limited by the following two inherent limitations: 1) low visible utilization efficiency, and 2) easy recombination of photogenerated electron–hole pairs.96 It is well known that the wide band gap (3.0–3.2 eV) of TiO2 restricts its light-harvesting to UV radiation only, which only accounts for ca. 5% of sunlight energy.96,100 Meanwhile, about 90% of the photoexcited electron–hole pairs are recombined based on time-resolved spectroscopic studies.107 Therefore, it is of great significance to enhance the solar or visible light activity, and to prevent the electron–hole pairs recombination of TiO2. To this end, various methods have been applied; for example, doping or incorporating metal or non-metal atoms to eliminate the charge recombination, coupling with a visible-light-excited semiconductor (e.g. CdS and g-C3N4, carbon quantum dots) to improve the solar utilization efficiency108 and combining with carbonaceous materials (such as CNTs and graphene) to enhance the adsorption property and electron mobility of the catalysts, thus improving their subsequent effectiveness.109 The commonly used methods to enhance the photocatalysis activity of TiO2 are summarized in Table 4.
| Optimization methods | Mechanisms | |
|---|---|---|
| Retardation of electron–hole recombination | Nano-sized catalysts109 | Larger surface area; more reactive sites; lower electron–hole recombination |
| Nanotube formation96,106 | Shorter carrier-diffusion paths; higher reactant mass transfer rate towards the tube surface | |
| Metal (transition and noble) doping96 | Inhibition of electron–hole recombination | |
| Reactive crystallographic facets109,110 | Higher reactant sorption; better electron–hole separation; lower electron–hole recombination | |
| Lattice mismatch111 | Inhibition of electron–hole separation | |
| Combining with carbonaceous nanomaterials26 | Facilitation of electron transfer, acceleration of the electron–hole separation, promotion of the adsorption of reactants on a catalyst surface | |
| Promotion of visible light activity | Metal impurities doping94 | Formation of impurity energy levels, shifting of the adsorption edge to the visible light range |
| Dye sensitizer doping28 | Electron injection | |
| Non-metal atoms (anion doping)28 | Lower band gap; restriction of electron–hole recombination; formation of impurity energy levels | |
| Narrow band gap94 | Electron injection | |
| Oxygen deficient TiO2 (ref. 94) | Rates of recombination; electron transport; charge transfer | |
Doping/deposition with transition metals (e.g. Fe, Co, Ni and Cr) or noble metal ions (e.g. Au, Ag, Pd and Pt) have been the most extensively investigated because they can promote the formation of a hybrid O 2p conduction band with a lower band gap energy, thus extending the visible light absorption. The doping with a metal can also retard electron–hole recombination via facilitating electron transfer to the conduction band, thus enhancing the photoactivity of TiO2.100,112 In recent years, metal-modified TiO2 has showed great potential in the photocatalytic degradation of PhACs. For example, Huo et al.113 modified TiO2-halloysite nanotubes (HNTs) by Fe3+via an impregnation method, and the synthesized photocatalyst with a narrowed band gap showed an enhanced tetracycline degradation rate in simulated wastewater under visible light irradiation. Previous studies also showed that noble metal (i.e. Ag) doping on TiO2 nanoparticles could significantly improve the photocatalytic degradation efficiency of chloramphenicol and tartrazine.89 However, some shortcomings in metal doping also need to be carefully addressed during the research, such as the high cost of the noble metals, electron trapping by the metal centres and thermal instability of transition metal-doped TiO2, as well as the toxicity of metal leaching from the photocatalyst.
Doping TiO2 with non-metal species (e.g. C, N, F and B) can substitute the oxygen vacancy in the TiO2, which will extend the excitation spectrum of TiO2 to the visible range and improve the quantum efficiency of the photocatalysts.30,114 N is the most used, cost-effective and feasible non-metal species. The breakthrough work by Asahi et al.115 in 2001 first reported the enhanced visible light activity of TiO2 by N doping. After that, various non-metals-doped TiO2 have been extensively investigated in the aim to obtain better TiO2 optimization methods, as well as the application of modified TiO2 in the photocatalytic degradation of PhACs. For example, a N-doped TiO2 was applied in the photocatalytic removal of spiramycin under visible light, whereby a high mineralization efficiency (>80%) was achieved within 4 h in real pharmaceutical wastewater. A B-doped TiO2 material was synthesized by a sol–gel method,116 and it was found that the 5% (w/w) B-doped TiO2 degraded metoprolol faster (70% removal) than pure TiO2 (48% removal) in 180 min under simulated solar light irradiation. However, the origin of the visible light photoactivity of modified TiO2 is still under debate. For instance, Asahi et al.115 stated that the mixing of N 2p with O 2p narrowed the band gap, while latter experimental and theoretical studies proposed that the formation of localized midgap states was responsible for the visible light activity.117
Some noncompensated n–p co-doped TiO2 nanoparticles have shown much more narrowed band gaps, which can efficiently photodegrade PhACs under visible irradiation.118 The co-doping of noncompensated materials can provide electrostatic attraction with the n–p dopant pair, which can then increase the thermodynamic, and also create tunable intermediate bands to narrow the band gap. Shi et al.119 synthesized CdS quantum dots-modified N-doped titania plates, where Cd acted as an n-type dopant to replace a host Ti atom, and N as a p-type dopant to replace the neighbouring O atom, which showed a high photocatalytic activity and low electron–hole combination rate under visible light. The mineralization rate of diclofenac by the CdS-N co-doped photocatalyst was enhanced 1.95 times compared to that of N-doped TiO2 under visible light irradiation. Similarly, composites of TiO2 with other materials, such as graphene, SnO2, C3N4 or MoS2, could also help to restrict electron–hole recombination and improve the photocatalytic activity.95
Creating highly reactive crystallographic facets is another approach to enhance the photocatalytic activity of TiO2.95 The average facet surface energy of anatase TiO2 is 0.90 J m−2 for {001} > 0.53 J m−2 for {100}, and >0.44 J m−2 for {101};110 however, the low-reactive {101} facet is dominant in anatase, while the highly reactive {001} facets decreases quickly to minimize its total surface free energy during the crystal growth process. A specific capping agent, such as fluorine ions, doped on the anatase can greatly increase the percentage (up to 89%) of the highly reactive {001} facet in TiO2, and promote the ˙OH production and organic pollutant photodegradation rate.120 The facet modification creates new opportunities for constructing highly efficiency photocatalysts for PhACs removal from wastewater.
The excellent properties of carbonaceous nanomaterials, including their high specific surface area, superior electron mobility, high mechanical strength and high stability, make them ideal high performance candidates for photocatalyst carriers or promoters.121 These promising properties have triggered many investigations into the synthesis of new photocatalysts for PhACs degradation. Pastrana-Martínez et al.122 prepared a rGO–TiO2 composite and tested it in the photocatalytic degradation of diphenhydramine under visible light. They speculated that the rGO served as a visible light sensitizer of TiO2, and promoted the transport of photoexcited electrons, thus eliminating electron–hole recombination. The enrichment of diphenhydramine on the photocatalyst could further promote the photocatalytic performance of the rGO–TiO2 composite.122 Murgolo et al.26 compared the photocatalytic activity of nano-sized TiO2 supported on SWCNTs with TiO2 (P25) for degrading warfarin, acetaminophen, triclosan and carbamazepine. The CNTs efficiently prevented the agglomeration of TiO2 particles, and improved photocatalysis by shifting the UV-vis spectra to longer wavelengths. The results showed that the new material displayed a comparable degradation rate even at a much lower absolute dosage than TiO2 (P25). It should be noted that the carbonaceous material was not incorporated into the TiO2 lattice, and instead it was immobilized on the surface.
One of the great challenges for the application of TiO2 is the separation of TiO2 nanoparticles from the water phase for reuse purposes, and this is also true for most other nanomaterials. The aforementioned magnetic materials could facilitate their separation, while manipulating the TiO2 morphologies, such as nanotubes or nanosheets has been considered as an available approach to promote the settling performance of the nanomaterials.96
3.2 Graphitic carbon nitride
Graphitic carbon nitride (g-C3N4) has recently attracted tremendous attention as a promising metal-free photocatalyst. The heptazine ring structure and high condensation degree provide it with unique properties, such as an appealing electronic structure with a medium band gap of 2.7 eV and excellent physico-chemical stability. Therefore, it is a promising candidate as a visible-light-driven photocatalyst.95 Moreover, g-C3N4 is abundant, non-toxic and facile to synthesize. In 2009, the photocatalytic property of g-C3N4 was observed by Wang et al.123 during water splitting experiments, and since then a g-C3N4-driven “gold rush” has been seen in a growing number of photocatalytic studies. Of course, it is considered as a promising photocatalyst for PhACs removal.90Pristine g-C3N4 suffers from the rapid recombination of photoexcited electron–hole pairs, and unsatisfactory visible-light-utilization efficiency.124 Consequently, g-C3N4-based photocatalysts with a high quantum efficiency are an increasing requirement, and several approaches have been applied to achieve improved visible light photoactivity, e.g. the construction of mesoporous structures, the formation of surface coupling hybridization (using graphene, Bi2WO6, etc.) and elemental doping.124 Among these, the formation of heterostructures is the most promising and extensively studied method (as shown in Fig. 8). As a matter of fact, the heterostructure creates a coupling hybridization with difference in chemical potential between the two coupled semiconductors and generates band bending at the junction interface, where the band bending induces a built-in field to impel the transfer of photoexcited electrons and holes to opposite directions, resulting in a spatially efficient separation of electron–hole pairs at the interface of the heterojunction.124 Lattice mismatch at the interface may occur and produce detects, which can capture the photogenerated electronic carriers and retard the recombination of electron–hole pairs.124 The heterostructures are also reported to shift the optical absorption to higher wavelength regions.
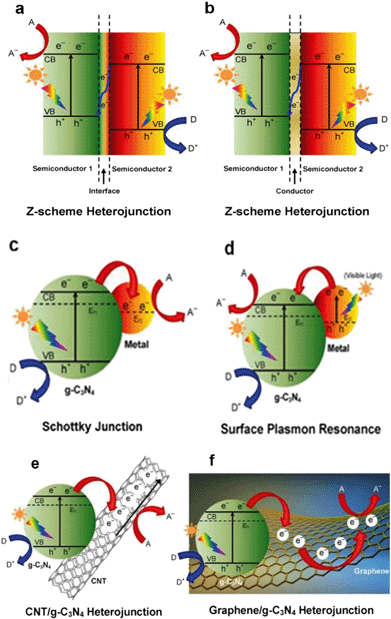 | ||
| Fig. 8 Schematic illustration of the photocatalysis of various types of modification methods for g-C3N4: (a) semiconductor–semiconductor Z-scheme heterojunction, (b) semiconductor-conductor-semiconductor Z-scheme heterojunction, (c) Schottky junction of metal/g-C3N4 nanohybrids, (d) surface plasmon resonance (SPR) effect of noble metal/g-C3N4 nanohybrids, (e) CNT/g-C3N4 heterojunction and (f) graphene/g-C3N4 heterojunction. A, D and Ef denote the electron acceptor, electron donor and Fermi level, respectively (reproduced with permission from ref. 97, copyright 2016 ACS Publications). | ||
Previous studies revealed that the g-C3N4 heterostructure showed higher photocatalytic activity and greatly promoted PhACs degradation efficiency in the aqueous phase. A sunlight-driven g-C3N4/P25 heterostructure was synthesized by Chen et al.125via a facile hydrothermal-calcination approach for the degradation of clofibric acid, and the UV-vis spectra revealed that the light absorption region of new materials was extended to 460 nm. The photocatalytic activity of the new material was 3.36 and 2.29 times higher than that of pristine g-C3N4 and P25 for clofibric acid degradation.125 The deposition of noble metals (e.g. Au and Ag) derives a surface plasmon resonance (SPR) effect from the collective coherent oscillation of surface electrons, which suggests the noble metal nanoparticles are promising candidates for harvesting both UV and visible light. A Au/Pt/g-C3N4 heterostructure was fabricated by Xue et al.126via a facile calcination–photodeposition method, and the obtained new material exhibited 3.4 times higher photocatalytic activity for degrading tetracycline hydrochloride than that of pristine g-C3N4 under visible light irradiation. The enhancement of photocatalytic activity was speculated to be from the surface plasmon resonance effect of Au (Fig. 8d) and the electron-sink function of the Pt nanoparticles.
As we can see to date, although ample studies have put an emphasis on obtaining highly effective g-C3N4-based photocatalysts, but the studies in this field are still in the initial stage and further systematic investigations are required. For example, the mechanism for enhancing the photocatalytic activity of g-C3N4-based photocatalysts is partly still unclear. More work could be devoted to the surface activation of g-C3N4 to derive the specific binding of functional groups, or for dispersing semiconductor nanoparticles to form g-C3N4/semiconductor heterostructures with an improved interfacial contact for photocatalysis, etc. However, based on the aforementioned advantages and reported highly photocatalytic active photocatalyst, it is believed that g-C3N4-based photocatalysts will have promising application prospects for treating PhACs or other organic contaminants in wastewater.
3.3 Zinc oxide
Nano-ZnO is regarded as an efficient and promising candidate for photocatalysis because the large exciton binding energy (60 meV) and direct wide band gap energy (3.37 eV) endow ZnO with a high UV utilization efficiency. In addition, the favourable properties of high electron mobility, long photoexcited electron lifetime, low cost and large surface area are also beneficial to its practical application as a photocatalyst.108,127–129 Consequently, the nano-sized ZnO has received much attention in the photodegradation and mineralization of PhACs in recent years. In the previous studies, Chatzitakis and coworkers130 found that the ZnO nanoparticles behaved with similar photocatalytic activity as TiO2 (P25) for chloramphenicol degradation under identical conditions, and an almost complete mineralization of chloramphenicol was achieved after 90 min. Following this work, El-Kemary et al.,91 Farzadkia et al.131 and many other researchers have investigated the photocatalytic degradation of PhACs with nano-sized ZnO, and high PhACs removal efficiencies were always achieved under various water chemistry conditions.The microstructures of ZnO nanomaterials can be manipulated by the synthesis methods, which are closely related to the photocatalytic activity. For instance, the hierarchical structures, i.e. flower-like, sea-urchin-shaped and dandelion-like, exhibit higher photocatalytic performance than the mono-morphological structure.132 However, quite a few works have studied the effect of ZnO structures on the photodegradation of PhACs.
With a similar band gap to TiO2, ZnO also shows poor visible-light-driven photocatalytic activity. Moreover, fast recombination of the photogenerated electron–hole pairs also limits its application as a photocatalyst. Various methods have been developed for overcoming these intrinsic shortcomings; among these, metal doping and coupling with other semiconductors have proven to be effective approaches for retarding the electron–hole recombination.127 The incorporation of additional elements or impurities into the ZnO framework can distinctly tune the electronic, optical, luminescent and other physical properties, thus modulating the light absorption and enhancing the charge separation efficiency.133,134 Mg-doped ZnO nanocrystallites were prepared via a conventional solid-state reaction for the photocatalytic removal of alprazolam under UV light. The obtained materials showed a hexagonal wurtzite structure with oxygen vacancies. A red-shift in the absorption edge and a narrowed band gap were observed by diffuse reflectance spectra (DRS) analysis. Consequently, a higher alprazolam removal efficiency was achieved when compared with pure ZnO and TiO2 (P25).134 However, another Mg-doped ZnO synthesized via an oxalate coprecipitation method exhibited a blue-shift in the near band edge photoluminescence emission. This new material with a wide band gap and efficient electron–hole separation still showed enhanced solar-driven photocatalytic activity.135 Therefore, more insightful outlooks need to be explored to understand the mechanisms of the metal-doped ZnO, which would be helpful for the rational design of new photocatalysts. Other than Mg, promotion of the photocatalytic degradation of PhACs was also observed by lanthanum (La)- and silver (Ag)-doped ZnO nanoparticles.133,136 In addition, the photocatalytic activity of ZnO could be improved by coupling with small band gap semiconductor quantum dots, such as CdTe and CdS.108
The coupling of ZnO with other semiconductors has been proven to enhance the charge separation via increasing the lifetime of the charge carriers and narrowing the band gap.127,137 A nano-scaled tungsten oxide (WO3) coated on ZnO nanorods with red-shifted light absorption was synthesized by Lam et al.137via combining the hydrothermal and chemical solution processes. The material exhibited enhanced photocatalytic activity for degrading resorcinol and methylparaben, which primarily benefited from the enhanced visible light absorbance and the lower recombination rate of charge carriers. The ZnO/TiO2 heterostructure is one of the most frequently reported photocatalysts due to its high photocatalytic activity and relatively low price. ZnO/TiO2/clay nanoarchitectures with reduced electron–hole recombination and promoted charge carrier migration were synthesized by Tobajas et al.,138 and the material showed enhanced acetaminophen and antipyrine degradation efficiency under simulated solar irradiation.
Their high efficiency and low price make the modified ZnO promising catalysts for the catalytic degradation of PhACs and various organic contaminants. However, the photocorrosion is the major limitation encountered in the wider application of ZnO due to a loss of toxic Zn2+ in the water phase and a subsequent decrease in activity. Meanwhile, a deep understanding of mechanism of the modification approaches is still required for future research.
3.4 Other nano-sized photocatalysts
Many other nanomaterials, such as WO3,93 ZnSnO3 nanospheres139 and NiO/nano-clinoptilolite,140 have also been studied for the photocatalytic removal of PhACs. WO3 is a visible-light-active photocatalyst with a narrow band gap (2.4–2.8 eV), high oxidation power of VB holes (+3.1 to 3.2 VNHE), high stability and non-toxicity. Unfortunately, the low CB edge (+0.3 to 0.5 VNHE) limits its potential to reduce O2 [E0(O2/˙O2−) = −0.33 VNHE and E0(O2/HO2˙) = −0.05 VNHE],141,142 resulting in rapid electron–hole recombination and low photocatalytic activity. The deposition of noble metals (like Pt) can accelerate the multi-electron reduction of dioxygen by WO3, thus promoting photocatalytic activity under visible light.143 ZnSnO3 hollow nanospheres were reported to be highly effective photocatalysts for the degradation of metronidazole under UV light irradiation, and the photocatalytic activity of ZnSnO3–graphene oxide composite yielded a ca. 30.4% higher degradation rate than pure ZnSnO3.139 NiO supported on nano-clinoptilolite also could photodegrade cefuroxime, and the NiO/clinoptilolite composite showed about a double photodegradation rate compared to nano-NiO.140 In addition, Bobu et al.144 synthesized a modified LAPONITE® clay-based Fe nanocomposite and conducted the photo-assisted Fenton mineralization of ciprofloxacin by hydrogen peroxide and UV light. The catalyst exhibited good catalytic activity and photostability, and ciprofloxacin of 0.15 mM was completely degraded within 30 min under the optimized experimental conditions. However, these reported catalysts always suffer from some limitations, such as high cost, low stability, toxicity or comparable low quantum efficiency; therefore, they are still far from ready for engineering applications.Photocatalysis has demonstrated great potential for the removal of PhACs in water and wastewater treatment owing to its low cost, easy operation and environmentally friendly features. The merits and shortcomings of various nano-sized catalysts are summarized in Fig. 9. Of these, TiO2 and g-C3N4 are expected to be particularly promising. However, as aforementioned, some underlying mechanisms of the semiconductor modification methods require further investigation for the sake of the rational design of highly effective photocatalysts. For practical operation, the type of water matrix, the aqueous environmental conditions and the target pollutant type and concentration need to be carefully considered. However, some challenges for the application of photocatalysts still remain, including: (1) developing low cost, high efficiency catalysts with a high quantum yield or visible-light photoactivity; (2) improving the efficiency of photocatalysis under solar light to meet engineering requirements; (3) enhancing the catalyst selectivity to degrade the target contaminants; (4) controlling the turbidity of the wastewater, which limits the photocatalytic process, to <5 nephelometric turbidity units (NTU) for photocatalytic degradation;42 (5) improving the recovery efficiency or immobilization methods of the catalysts; and (6) improving the mineralization degree, since the by-products of the photocatalytic degradation may be more toxic.
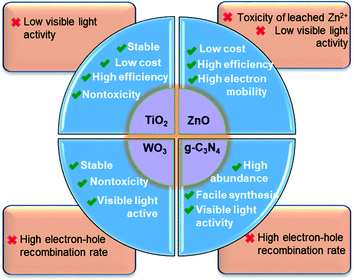 | ||
| Fig. 9 Summary of the merits and shortcomings of the most widely studied nano-sized photocatalysts used for PhACs removal. | ||
4. Heterogeneous catalytic ozonation
Ozonation is a mature organic removal method that has been widely applied in drinking water and wastewater treatment. The oxidation of organic contaminants generally proceeds via direct molecular ozone (O3) reactions and/or indirect O3 degradation by generating ˙OH.145,146 Many PhACs, e.g. quinolones, sulfonamides and tetracyclines, are primarily degraded via oxidation by O3, whereas cephalexin, penicillin G and N4-acetylsulfamethoxazole are transformed predominantly by ˙OH.24 However, the ozonation kinetics of PhACs are relatively slow. Moreover, carboxylic acids and aldehydes are usually formed in the ozonation process, both of which cannot further react with O3.Since O3 has a higher oxidation potential than O2, it can capture electrons excited in the conduction band of the catalyst, which leads to the formation of an ozonide radical ion (˙O3−) and eventually results in producing ˙OH. Consequently, a much higher PhACs removal efficiency can be anticipated for the catalytic ozonation process.147 Catalytic ozonation using nano-sized catalysts, like CNTs148 and metal oxides,149 have shown great potential to effectively degrade/mineralize the refractory PhACs, and thus have received increasing attention in recent years. However, the literature about the catalytic ozonation of PhACs using nanomaterials is still limited.
CNTs have proven to perform with high efficiency as ozonation catalysts for the mineralization of different PhACs.148,150 Goncalves and co-workers148 systematically tested the catalytic ozonation of bezafibrate using MWCNTs. The large surface area, fast internal mass transfer and high abundance of surface sites benefited bezafibrate degradation, and ˙OH attack was observed as the main mechanism for the enhanced mineralization.148 However, it should be noted that the reaction may introduce oxygenated groups on the surface of catalyst, which could lead to deactivation of the material.
It is known that the catalyst activity of catalytic ozonation mainly depends on the surface acid-based property, where the Lewis acid sites on a catalyst induce the chemisorbed water and promote their interaction with O3, leading to more ROS production and enhanced ozonation efficiency.149 A β-FeOOH/mesoporous alumina catalyst with higher surface Lewis acid sites was synthesized. In situ attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR) demonstrated the dissociative chemisorption of D2O on the surface Lewis acid sites of the catalysts, where O3 interacted with surface hydrogen-bonded-O-D to initiate the formation of ROS, which consequently led to enhanced efficiency for degrading and mineralizing ibuprofen and ciprofloxacin.149
Ozonation can be coupled with other physico-chemical processes to enhance the formation of oxidizing species, which can offer enhanced treatment efficiency and lead to the complete mineralization of PhACs. Photocatalytic ozonation is a widely used combination method.147 For example, a TiO2 nanoparticles thin film was supported on a ceramic irradiated by UV light in the proximity of O3 to facilitate the photocatalytic ozonation of phenazopyridine hydrochloride, and a higher amount of ˙OH was produced on the surface of TiO2 through the formation of ˙O3−. Consequently, a notable enhancement in the degradation of phenazopyridine hydrochloride was achieved when compared with photocatalytic degradation or catalytic ozonation alone.147,151
Until now, the understanding of the catalytic ozonation process and its underlying mechanism are still limited and several questions need answering in order to better reveal the mechanism and the role of the nano-catalysts, such as: (1) how does the catalytic ozonation of organic contaminants proceed?; (2) What roles do O3 and organic adsorption play in the catalytic ozonation process?; (3) Which process is responsible for deactivation of the catalysts? The answers to these questions will help the researchers in this area to develop new and high efficiency catalysts, and then to facilitate their engineering applications.
Besides photocatalytic degradation and catalytic ozonation, various other AOPs, such as catalytic sonolysis,152 the Fenton reaction,153 UV/H2O2,154 UV/S2O8 (ref. 2 and 154) and the electro-peroxone process,155 have been investigated for removing PhACs from wastewater. However, these techniques are rarely associated with nanomaterials and thus we do not discuss them in this paper.
5. Filtration
The pressure-driven membrane filtration technologies, including reverse osmosis (RO), nanofiltration (NF) and ultrafiltration (UF), have been widely studied and extensively applied for pollutants removal in water and wastewater treatment, and are believed to be promising technologies due to their high efficiency, easy operation and space saving.156,157 Specifically, RO can effectively remove the dissolved inorganic ions and small organic molecules; while NF, with larger pore size, is capable of filtering the hardness ions (e.g. Ca2+) and small organic matter. Therefore, RO and NF have been considered as promising methods for PhACs removal from the water phase.158,159The membrane fouling and high energy consumption are the most important limitations of the filtration processes.158,160 Nowadays, the development of nanotechnology provides opportunities to incorporate functional nanomaterials into the membrane to promote permeability, stability, fouling resistance or self-cleaning properties for the membrane, thus making filtration more practical for engineering applications.28,30 The most widely used modification techniques include surface coating, chemical treatment and the use of a materials composite (CNTs, nanofibre, zeolites, etc.). Fig. 10 displays the potential performance and commercial viability of nanotechnology for enhancing membrane performance, which is presented here to provide readers with a bird's eye view of the effectiveness and prospects of the nanomaterials in membrane applications. To date, huge numbers of literature reports are available on the modified NF or OS membranes for water purification; however, only a few reports can be found on PhACs removal.160–162
 | ||
| Fig. 10 Comparison of the potential performance and commercial viability of nanomaterial-modified membranes. Performance refers to the permeability, selectivity and robustness; commercial viability refers to the material cost, scalability and compatibility with existing manufacturing infrastructure (modified with permission from ref. 164, copyright 2011 Royal Society of Chemistry). | ||
The modification of RO and NF membranes using nanomaterials (e.g. zeolites and TiO2 nanoparticles) can modify the matrix structure and surface properties of the membrane, thus providing favourable properties for the removal of PhACs. For example, the coating of photocatalytic nanoparticles and antimicrobials on a membrane surface can provide a self-cleaning property, while the incorporation of zeolites provides a membrane with a higher selectivity and permeability and the introduction of aligned CNTs seeks to simultaneously improve the permeability and selectivity.163 The innovative nanomaterials promise unique performance enhancements for membranes and can help to overcome the barriers to its commercially viable.
A zeolite-nanoparticles-embedded thin-film nanocomposite membrane with high surface roughness was prepared by Dong et al.161 The modified membrane material was full of internal pores and microporous defects derived from the zeolite nanoparticles, which exhibited a doubled water permeability and maintained a similar PhACs rejection efficiency when compared with the non-modified membrane.161
The impregnating of nano-TiO2 in a thin-film composite polysulfonate forward osmosis membrane using polydopamine was conducted for removing PhACs. The prepared membrane showed a more negative zeta potential, which increased the electrostatic repulsion between the functional groups on the membrane, resulting in an increased pore size and higher permeation flux. Moreover, the separation behaviours of metoprolol, sulfamethoxazole and triclosan in the modified membrane were improved.165 Another study reported a photoactive microfiltration membrane loaded with non-aggregated TiO2 nanoparticles on the membrane surface. The prepared membrane showed a higher permeation flux, improved anti-fouling properties and higher photocatalytic activity for diclofenac and ibuprofen degradation.160
The incorporation of integrated multi-layered nanostructures and internal domains in porous polymer membranes can decrease the blocking probability of the membrane and improve the chemical stability, hydrophilicity and anti-pollution properties.166,167 A bioinspired propranolol-imprinted multilevel nanocomposite (Br-Ag-pDA@SiO2) membrane with enhanced specific rebinding capacity and permselectivity was prepared through an in situ photoinitiated atom transfer radical polymerization method using propranolol (a PhAC) as the template molecule. A rapid adsorption kinetics of propranolol was achieved as well as an excellent regeneration performance.168 Based on this work, the authors also applied ciprofloxacin as a template molecule and synthesized another similar membrane with a higher specific rebinding capacity and high permselectivity.162
Notably, the greatest obstacles for the application of membrane technologies for PhACs removal are the high energy consumption and membrane fouling. Further effort is still required to improve the membrane selectivity and permeability, and to decrease fouling. Moreover, various other treatment methods, such as AOPs, ultrafiltration and microfiltration, can act as pre-treatment procedures to relieve the fouling of RO and NF. Notwithstanding their higher operational cost, an increasing number of wastewater treatment plants and drinking water facilities have applied membrane filtration methods to meet increasingly stringent water quality standards.169 With the improvement of new membrane synthesis technology, the production and maintenance costs will gradually decrease, leading to a broader application scenario for membrane technologies.
6. Summary and perspectives
Increasing quantities and types of PhACs are consumed every year, and they have been frequently detected in surface water, groundwater and even drinking water. However, the conventional sewage treatment methods can hardly achieve sufficient PhACs removal. The development of nanotechnology offers leapfrogging opportunities for developing innovative technologies for efficient PhACs removal.Nano-sized adsorbents, such as CNTs, graphene, MoS2 and hexagonal boron nitride nanosheets, possess an extremely large surface area and modified surface properties, and can provide a high adsorption capacity and selectivity for PhACs removal. Among these, carbonaceous nanomaterials exhibit superior adsorption performance and excellent reusability; moreover, the development of synthesis methods is continuously lowering the cost of carbonaceous nanomaterials, all of which make them promising materials for large-scale application.
Photocatalysis has demonstrated great potential for the removal of PhACs in water and wastewater treatment owing to its low cost, easy operation and environmentally friendly features. Nano-sized photocatalysts are expected to be particularly promising, e.g. nano-TiO2 and g-C3N4 have good potential for near-term use due to their high efficacy and low cost. TiO2 is in the preponderant position as the archetypical photocatalyst, especially with its modified visible light absorbance and charge carrier mobility. Recently, considerable progress has been made for obtaining high efficiency g-C3N4-based photocatalysts, but the photocatalytic mechanism is still not clear and further systematic investigations are required. Other than photocatalysis, the catalytic ozonation of PhACs using nano-catalysts has gained increasing attention recently. Unfortunately, the mechanisms behind the catalytic processes are still largely unknown, and the cost of ozone production is still high.
Fouling and high energy consumption are the prevalent issues that hinder the application of filtration operations. Efforts towards membrane modification by using nanomaterials have been reported. The introduction of nanomaterials, i.e. CNTs, TiO2 and nano-zeolite, offers the filtration process higher stability, selectivity, permeability and fouling resistance, which help the membrane overcome the barriers to its commercially viability. The nano-zeolite-modified membrane possesses the greatest potential commercial viability, and is likely to achieve engineering application in the near future.
Until now, most of nanotechnologies for environmental remediation are still under laboratory development. From the aspects of economy, feasibility and safety, the following important issues should be addressed before the broader environmental application of nanomaterials: (1) more studies should simulate the real environmental conditions, i.e. the environmental contaminants concentration, ionic strength, pH, DOMs, solar irradiation, to better reveal the reactions in the engineering application; (2) the material and energy costs for manufacturing nanomaterials need to be further reduced, and the reusability and lifespan of nanomaterials should be improved; (3) efficient approaches should be developed for separating spent nanomaterials from the aqueous phase; (4) the potential environmental impacts of nanomaterials, including the fate, transport and toxicity of nanomaterials in the environment, should be carefully evaluated; moreover, the potential risks of nanomaterials when associated with PhACs need to be carefully assessed.
Based on our opinion and the pioneering studies, the suggestions regarding the further research about using nanomaterials to remove PhACs are proposed as follows: (1) the PhACs' adsorption kinetics and isotherms, and the physico-chemical properties of nano-adsorbents have been extensively studied; however, the important thermodynamic parameters, e.g. adsorption free energy and surface energy, have not yet been generally characterized or discussed. Such works, it is suggested, need to be carried out when investigating the mechanism of new nano-adsorbents; (2) the development of visible-light-active photocatalysts has become a hot topic in recent years. However, in our opinion, high solar-light-active photocatalysts should be emphasized rather than concerning only the visible-light activity; (3) it is encouraged to combine band gap engineering with methods to promote the activity of photocatalysts. The surface/interface processes of the photocatalytic degradation reaction should be comprehensively investigated, which will inevitably benefit developing new nano-photocatalysts; (4) the rapid development of numerical modelling methods and computational capabilities offer great opportunities for theoretically calculating the new materials or predicting unknown materials, which can be used to develop or modify nanomaterials, and to reveal the reaction mechanisms.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the financial support from Fudan University (grant number: JIH1829022), the Basic Research Funding of Peking University (No. 7100601190) and the Power China Huadong Engineering Corporation (grant number: KY2016-02-04).References
- M. J. Benotti, R. A. Trenholm, B. J. Vanderford, J. C. Holady, B. D. Stanford and S. A. Snyder, Environ. Sci. Technol., 2009, 43, 597–603 CrossRef CAS PubMed.
- N. Ratola, A. Cincinelli, A. Alves and A. Katsoyiannis, J. Hazard. Mater., 2012, 239, 1–18 CrossRef PubMed.
- T. P. Van Boeckel, S. Gandra, A. Ashok, Q. Caudron, B. T. Grenfell, S. A. Levin and R. Laxminarayan, Lancet Infect. Dis., 2014, 14, 742–750 CrossRef PubMed.
- FDA, Approved drug products with therapeutic equivalence evaluations, Center for Drug Evaluation and Research, Rockville, MD, 2003 Search PubMed.
- Q. Q. Zhang, G. G. Ying, C. G. Pan, Y. S. Liu and J. L. Zhao, Environ. Sci. Technol., 2015, 49, 6772–6782 CrossRef CAS PubMed.
- Y. D. Guan, B. Wang, Y. X. Gao, W. Liu, X. L. Zhao, X. F. Huang and J. H. Yu, Pedosphere, 2017, 27, 42–51 CrossRef.
- Y. Yoon, P. Westerhoff, S. A. Snyder, E. C. Wert and J. Yoon, Desalination, 2007, 202, 16–23 CrossRef CAS.
- S. Yan, B. Yao, L. Lian, X. Lu, S. A. Snyder, R. Li and W. Song, Environ. Sci. Technol., 2017, 51, 2738–2747 CrossRef CAS PubMed.
- S. Castiglioni, R. Bagnati, R. Fanelli, F. Pomati, D. Calamari and E. Zuccato, Environ. Sci. Technol., 2006, 40, 357–363 CrossRef CAS PubMed.
- K. Ikehata, N. J. Naghashkar and M. G. Ei-Din, Ozone: Sci. Eng., 2006, 28, 353–414 CrossRef CAS.
- L. D. Nghiem, A. I. Schafer and M. Elimelech, Environ. Sci. Technol., 2005, 39, 7698–7705 CrossRef CAS PubMed.
- I. Michael, L. Rizzo, C. S. McArdell, C. M. Manaia, C. Merlin, T. Schwartz, C. Dagot and D. Fatta-Kassinos, Water Res., 2013, 47, 957–995 CrossRef CAS PubMed.
- C. Hignite and D. L. Azarnoff, Life Sci., 1977, 20, 337–341 CrossRef CAS PubMed.
- D. W. Kolpin, E. T. Furlong, M. T. Meyer, E. M. Thurman, S. D. Zaugg, L. B. Barber and H. T. Buxton, Environ. Sci. Technol., 2002, 36, 1202–1211 CrossRef CAS PubMed.
- P. E. Stackelberg, E. T. Furlong, M. T. Meyer, S. D. Zaugg, A. K. Henderson and D. B. Reissman, Sci. Total Environ., 2004, 329, 99–113 CrossRef CAS PubMed.
- T. A. der Beek, F. A. Weber, A. Bergmann, S. Hickmann, I. Ebert, A. Hein and A. Kuster, Environ. Toxicol. Chem., 2016, 35, 823–835 CrossRef PubMed.
- A. B. A. Boxall, M. A. Rudd, B. W. Brooks, D. J. Caldwell, K. Choi, S. Hickmann, E. Innes, K. Ostapyk, J. P. Staveley, T. Verslycke, G. T. Ankley, K. F. Beazley, S. E. Belanger, J. P. Berninger, P. Carriquiriborde, A. Coors, P. C. DeLeo, S. D. Dyer, J. F. Ericson, F. Gagne, J. P. Giesy, T. Gouin, L. Hallstrom, M. V. Karlsson, D. G. J. Larsson, J. M. Lazorchak, F. Mastrocco, A. McLaughlin, M. E. McMaster, R. D. Meyerhoff, R. Moore, J. L. Parrott, J. R. Snape, R. Murray-Smith, M. R. Servos, P. K. Sibley, J. O. Straub, N. D. Szabo, E. Topp, G. R. Tetreault, V. L. Trudeau and G. Van Der Kraak, Environ. Health Perspect., 2012, 120, 1221–1229 CrossRef PubMed.
- L. P. Padhye, H. Yao, F. T. Kung'u and C.-H. Huang, Water Res., 2014, 51, 266–276 CrossRef CAS PubMed.
- J. H. Writer, I. Ferrer, L. B. Barber and E. M. Thurman, Sci. Total Environ., 2013, 461, 519–527 CrossRef PubMed.
- S. Chelliapan, T. Wilby and P. J. Sallis, Water Res., 2006, 40, 507–516 CrossRef CAS PubMed.
- S. A. Snyder, P. Westerhoff, Y. Yoon and D. L. Sedlak, Environ. Eng. Sci., 2003, 20, 449–469 CrossRef CAS.
- V. Kårelid, G. Larsson and B. Björlenius, J. Environ. Manage., 2017, 193, 163–171 CrossRef PubMed.
- N. Le-Minh, S. J. Khan, J. E. Drewes and R. M. Stuetz, Water Res., 2010, 44, 4295–4323 CrossRef CAS PubMed.
- M. C. Dodd, H. P. E. Kohler and U. Von Gunten, Environ. Sci. Technol., 2009, 43, 2498–2504 CrossRef CAS PubMed.
- L. Yang, C. Hu, Y. Nie and J. Qu, Appl. Catal., B, 2010, 97, 340–346 CrossRef CAS.
- S. Murgolo, F. Petronella, R. Ciannarella, R. Comparelli, A. Agostiano, M. L. Curri and G. Mascolo, Catal. Today, 2015, 240, 114–124 CrossRef CAS.
- Y. Xu, T. J. Liu, Y. Zhang, F. Ge, R. M. Steel and L. Y. Sun, J. Mater. Chem. A, 2017, 5, 12001–12014 CAS.
- X. L. Qu, P. J. J. Alvarez and Q. L. Li, Water Res., 2013, 47, 3931–3946 CrossRef CAS PubMed.
- T. Masciangioli and W. X. Zhang, Environ. Sci. Technol., 2003, 37, 102a–108a CrossRef CAS PubMed.
- X. L. Qu, J. Brame, Q. L. Li and P. J. J. Alvarez, Acc. Chem. Res., 2013, 46, 834–843 CrossRef CAS PubMed.
- J. Rivera-Utrilla, M. Sánchez-Polo, M. Á. Ferro-García, G. Prados-Joya and R. Ocampo-Pérez, Chemosphere, 2013, 93, 1268–1287 CrossRef CAS PubMed.
- C. Jung, A. Son, N. Her, K.-D. Zoh, J. Cho and Y. Yoon, J. Ind. Eng. Chem., 2015, 27, 1–11 CrossRef CAS.
- A. Cincinelli, T. Martellini, E. Coppini, D. Fibbi and A. Katsoyiannis, J. Nanosci. Nanotechnol., 2015, 15, 3333–3347 CrossRef CAS PubMed.
- N. C. Mueller, B. van der Bruggen, V. Keuter, P. Luis, T. Melin, W. Pronk, R. Reisewitz, D. Rickerby, G. M. Rios, W. Wennekes and B. Nowack, J. Hazard. Mater., 2012, 211–212, 275–280 CrossRef CAS PubMed.
- T. X. Bui and H. Choi, J. Hazard. Mater., 2009, 168, 602–608 CrossRef CAS PubMed.
- W. Liu, X. Zhao, Z. Cai, B. Han and D. Zhao, RSC Adv., 2016, 6, 67260–67270 RSC.
- P. G. Collins, K. Bradley, M. Ishigami and A. Zettl, Science, 2000, 287, 1801–1804 CrossRef CAS PubMed.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed.
- G. X. Zhao, L. Jiang, Y. D. He, J. X. Li, H. L. Dong, X. K. Wang and W. P. Hu, Adv. Mater., 2011, 23, 3959–3963 CrossRef CAS PubMed.
- M. Khajeh, S. Laurent and K. Dastafkan, Chem. Rev., 2013, 113, 7728–7768 CrossRef CAS PubMed.
- W. B. Yang, Y. P. Lu, F. F. Zheng, X. X. Xue, N. Li and D. M. Liu, Chem. Eng. J., 2012, 179, 112–118 CrossRef CAS.
- P. Lens, J. Virkutyte, V. Jegatheesan, S. H. Kim and S. Al-Abed, Nanotechnology for water and wastewater treatment, IWA Publishing, 2013 Search PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- S. Iijima, Nature, 1991, 354, 56–58 CrossRef CAS.
- F. F. Liu, J. Zhao, S. G. Wang, P. Du and B. S. Xing, Environ. Sci. Technol., 2014, 48, 13197–13206 CrossRef CAS PubMed.
- A. McWilliams, BCC Research, AVM075B, 2011 Search PubMed.
- X. M. Yan, B. Y. Shi, J. J. Lu, C. H. Feng, D. S. Wang and H. X. Tang, J. Colloid Interface Sci., 2008, 321, 30–38 CrossRef CAS PubMed.
- Y. Zhang, B. Chen, L. Zhang, J. Huang, F. Chen, Z. Yang, J. Yao and Z. Zhang, Nanoscale, 2011, 3, 1446–1450 RSC.
- Y. L. Zhao and J. F. Stoddart, Acc. Chem. Res., 2009, 42, 1161–1171 CrossRef CAS PubMed.
- M. I. Katsnelson and A. Fasolino, Acc. Chem. Res., 2013, 46, 97–105 CrossRef CAS PubMed.
- T. Hayashi and M. Endo, Composites, Part B, 2011, 42, 2151–2157 CrossRef.
- H. Ba, S. Podila, Y. F. Liu, X. K. Mu, J. M. Nhut, V. Papaefthimiou, S. Zafeiratos, P. Granger and P. H. Cuong, Catal. Today, 2015, 249, 167–175 CrossRef CAS.
- S. J. Zhang, T. Shao, H. S. Kose and T. Karanfil, Environ. Sci. Technol., 2010, 44, 6377–6383 CrossRef CAS PubMed.
- L. L. Ji, Y. Shao, Z. Y. Xu, S. R. Zheng and D. Q. Zhu, Environ. Sci. Technol., 2010, 44, 6429–6436 CrossRef CAS PubMed.
- H. Zhao, X. Liu, Z. Cao, Y. Zhan, X. D. Shi, Y. Yang, J. L. Zhou and J. Xu, J. Hazard. Mater., 2016, 310, 235–245 CrossRef CAS PubMed.
- Y. Gao, Y. Li, L. Zhang, H. Huang, J. Hu, S. M. Shah and X. Su, J. Colloid Interface Sci., 2012, 368, 540–546 CrossRef CAS PubMed.
- H. Chen, B. Gao and H. Li, J. Hazard. Mater., 2015, 282, 201–207 CrossRef CAS PubMed.
- F. Yu, J. Ma and D. S. Bi, Environ. Sci. Pollut. Res., 2015, 22, 4715–4724 CrossRef CAS PubMed.
- L. A. Al-Khateeb, S. Almotiry and M. A. Salam, Chem. Eng. J., 2014, 248, 191–199 CrossRef CAS.
- Y. Zhang, Z. Jiao, Y. Y. Hu, S. H. Lv, H. B. Fan, Y. Y. Zeng, J. Hu and M. M. Wang, Environ. Sci. Pollut. R., 2017, 24, 2987–2995 CrossRef CAS PubMed.
- Y. Fei, Y. Li, S. Han and J. Ma, J. Colloid Interface Sci., 2016, 484, 196–204 CrossRef CAS PubMed.
- P. Oleszczuk, B. Pan and B. S. Xing, Environ. Sci. Technol., 2009, 43, 9167–9173 CrossRef CAS PubMed.
- C. Zhang, C. Lai, G. Zeng, D. Huang, C. Yang, Y. Wang, Y. Zhou and M. Cheng, Water Res., 2016, 95, 103–112 CrossRef CAS PubMed.
- H. R. Wei, S. B. Deng, Q. Huang, Y. Nie, B. Wang, J. Huang and G. Yu, Water Res., 2013, 47, 4139–4147 CrossRef CAS PubMed.
- H. Kim, Y. S. Hwang and V. K. Sharma, Chem. Eng. J., 2014, 255, 23–27 CrossRef CAS.
- F. Perreault, A. Fonseca de Faria and M. Elimelech, Chem. Soc. Rev., 2015, 44, 5861–5896 RSC.
- Y. Z. Tian, W. H. Li, G. L. Shi, Y. C. Feng and Y. Q. Wang, J. Hazard. Mater., 2013, 248, 89–96 CrossRef PubMed.
- M. J. Zhi, C. C. Xiang, J. T. Li, M. Li and N. Q. Wu, Nanoscale, 2013, 5, 72–88 RSC.
- Z. Y. Wang, X. D. Yu, B. Pan and B. S. Xing, Environ. Sci. Technol., 2010, 44, 978–984 CrossRef CAS PubMed.
- J. Comer, R. Chen, H. Poblete, A. Vergara-Jaque and J. E. Riviere, ACS Nano, 2015, 9, 11761–11774 CrossRef CAS PubMed.
- Y. D. Zou, X. X. Wang, Y. J. Ai, Y. H. Liu, Y. F. Ji, H. Q. Wang, T. Hayat, A. Alsaedi, W. P. Hu and X. K. Wang, J. Mater. Chem. A, 2016, 4, 14170–14179 CAS.
- S. J. Yu, X. X. Wang, Y. J. Ai, X. L. Tan, T. Hayat, W. P. Hu and X. K. Wang, J. Mater. Chem. A, 2016, 4, 5654–5662 CAS.
- S. J. Yu, X. X. Wang, W. Yao, J. Wang, Y. F. Ji, Y. J. Ai, A. Alsaedi, T. Hayat and X. K. Wang, Environ. Sci. Technol., 2017, 51, 3278–3286 CrossRef CAS PubMed.
- Z. X. Jin, X. X. Wang, Y. B. Sun, Y. J. Ai and X. K. Wang, Environ. Sci. Technol., 2015, 49, 9168–9175 CrossRef CAS PubMed.
- W. C. Song, T. T. Yang, X. X. Wang, Y. B. Sun, Y. J. Ai, G. D. Sheng, T. Hayat and X. K. Wang, Environ. Sci.: Nano, 2016, 3, 1318–1326 RSC.
- M. M. Khin, A. S. Nair, V. J. Babu, R. Murugan and S. Ramakrishna, Energy Environ. Sci., 2012, 5, 8075–8109 CAS.
- M. F. L. De Volder, S. H. Tawfick, R. H. Baughman and A. J. Hart, Science, 2013, 339, 535–539 CrossRef CAS PubMed.
- Y. Chao, W. Zhu, X. Wu, F. Hou, S. Xun, P. Wu, H. Ji, H. Xu and H. Li, Chem. Eng. J., 2014, 243, 60–67 CrossRef CAS.
- S. J. Yu, X. X. Wang, Z. S. Chen, J. Wang, S. H. Wang, T. Hayat and X. K. Wang, J. Hazard. Mater., 2017, 321, 111–120 CrossRef CAS PubMed.
- D. Liu, W. Lei, S. Qin, K. D. Klika and Y. Chen, Phys. Chem. Chem. Phys., 2016, 18, 84–88 RSC.
- T. M. S. Attia, X. L. Hu and Y. D. Qiang, Chemosphere, 2013, 93, 2076–2085 CrossRef PubMed.
- B. Radisavljevic, A. Radenovic, J. Brivio, V. Giacometti and A. Kis, Nat. Nanotechnol., 2011, 6, 147–150 CrossRef CAS PubMed.
- W. Lei, H. Zhang, Y. Wu, B. Zhang, D. Liu, S. Qin, Z. Liu, L. Liu, Y. Ma and Y. Chen, Nano Energy, 2014, 6, 219–224 CrossRef CAS.
- D. Golberg, Y. Bando, Y. Huang, T. Terao, M. Mitome, C. Tang and C. Zhi, ACS Nano, 2010, 4, 2979–2993 CrossRef CAS PubMed.
- Y. J. Chen, T. Lan, L. C. Duan, F. H. Wang, B. Zhao, S. T. Zhang and W. Wei, PLoS One, 2015, 10, e0145025 Search PubMed.
- J. Zhi, Y. Wang and G. Luo, React. Funct. Polym., 2005, 65, 249–257 CrossRef CAS.
- N. Zhuo, Y. Lan, W. Yang, Z. Yang, X. Li, X. Zhou, Y. Liu, J. Shen and X. Zhang, Sep. Purif. Technol., 2017, 177, 272–280 CrossRef CAS.
- K. P. Singh, A. K. Singh, U. V. Singh and P. Verma, Environ. Sci. Pollut. Res., 2012, 19, 724–738 CrossRef CAS PubMed.
- A. Jodat and A. Jodat, Desalin. Water Treat., 2014, 52, 2668–2677 CrossRef CAS.
- D. B. Hernández-Uresti, A. Vázquez, D. Sanchez-Martinez and S. Obregón, J. Photochem. Photobiol., A, 2016, 324, 47–52 CrossRef.
- M. El-Kemary, H. El-Shamy and I. El-Mehasseb, J. Lumin., 2010, 130, 2327–2331 CrossRef CAS.
- A. Nezamzadeh-Ejhieh and A. Shirzadi, Chemosphere, 2014, 107, 136–144 CrossRef CAS PubMed.
- Y. F. Rao, W. Chu and Y. R. Wang, Appl. Catal., A, 2013, 468, 240–249 CrossRef CAS.
- A. Fujishima, X. T. Zhang and D. A. Tryk, Surf. Sci. Rep., 2008, 63, 515–582 CrossRef CAS.
- Y. J. Yuan, Z. J. Ye, H. W. Lu, B. Hu, Y. H. Li, D. Q. Chen, J. S. Zhong, Z. T. Yu and Z. G. Zou, ACS Catal., 2016, 6, 532–541 CrossRef CAS.
- Z. Cai, X. Zhao, T. Wang, W. Liu and D. Zhao, ACS Sustainable Chem. Eng., 2017, 5, 547–555 CrossRef CAS.
- W. J. Ong, L. L. Tan, Y. H. Ng, S. T. Yong and S. P. Chai, Chem. Rev., 2016, 116, 7159–7329 CrossRef CAS PubMed.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS PubMed.
- Z. Li, S. Cong and Y. Xu, ACS Catal., 2014, 4, 3273–3280 CrossRef CAS.
- X. Zhao, Z. Q. Cai, T. Wang, S. E. O'Reilly, W. Liu and D. Y. Zhao, Appl. Catal., B, 2016, 187, 134–143 CrossRef CAS.
- H. Kawaguchi, Environ. Technol. Lett., 1984, 5, 471–474 CrossRef CAS.
- T. E. Doll and F. H. Frimmel, Water Res., 2004, 38, 955–964 CrossRef CAS PubMed.
- X. Zhang, F. Wu, X. W. Wu, P. Y. Chen and N. S. Deng, J. Hazard. Mater., 2008, 157, 300–307 CrossRef CAS PubMed.
- R. Liang, A. M. Hu, W. J. Li and Y. N. Zhou, J. Nanopart. Res., 2013, 15, 1990 CrossRef.
- A. M. Hu, X. Zhang, K. D. Oakes, P. Peng, Y. N. Zhou and M. R. Servos, J. Hazard. Mater., 2011, 189, 278–285 CrossRef CAS PubMed.
- X. Nie, J. Y. Chen, G. Y. Li, H. X. Shi, H. J. Zhao, P. K. Wong and T. C. An, J. Chem. Technol. Biotechnol., 2013, 88, 1488–1497 CrossRef CAS.
- Q. Guo, C. Zhou, Z. Ma, Z. Ren, H. Fan and X. Yang, Chem. Soc. Rev., 2016, 45, 3701–3730 RSC.
- Y. J. Wang, Q. S. Wang, X. Y. Zhan, F. M. Wang, M. Safdar and J. He, Nanoscale, 2013, 5, 8326–8339 RSC.
- S. N. Xiao, W. Zhu, P. J. Liu, F. F. Liu, W. R. Dai, D. Q. Zhang, W. Chen and H. X. Li, Nanoscale, 2016, 8, 2899–2907 RSC.
- X. G. Han, Q. Kuang, M. S. Jin, Z. X. Xie and L. S. Zheng, J. Am. Chem. Soc., 2009, 131, 3152–3153 CrossRef CAS PubMed.
- H. McDaniel, M. Pelton, N. Oh and M. Shim, J. Phys. Chem. Lett., 2012, 3, 1094–1098 CrossRef CAS PubMed.
- S. N. R. Inturi, T. Boningari, M. Suidan and P. G. Smirniotis, Appl. Catal., B, 2014, 144, 333–342 CrossRef CAS.
- P. W. Huo, X. Gao, Z. Y. Lu, X. L. Liu, Y. Y. Luo, W. N. Xing, J. Q. Li and Y. S. Yan, Desalin. Water Treat., 2014, 52, 6985–6995 CrossRef CAS.
- C. J. Lin and W. T. Yang, Chem. Eng. J., 2014, 237, 131–137 CrossRef CAS.
- R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, Science, 2001, 293, 269–271 CrossRef CAS PubMed.
- R. P. Cavalcante, R. F. Dantas, B. Bayarri, O. Gonzalez, J. Gimenez, S. Esplugas and A. Machulek, Catal. Today, 2015, 252, 27–34 CrossRef CAS.
- C. Di Valentin, G. Pacchioni, A. Selloni, S. Livraghi and E. Giamello, J. Phys. Chem. B, 2005, 109, 11414–11419 CrossRef CAS PubMed.
- W. G. Zhu, X. F. Qiu, V. Iancu, X. Q. Chen, H. Pan, W. Wang, N. M. Dimitrijevic, T. Rajh, H. M. Meyer, M. P. Paranthaman, G. M. Stocks, H. H. Weitering, B. H. Gu, G. Eres and Z. Y. Zhang, Phys. Rev. Lett., 2009, 103, 1–4 Search PubMed.
- J. W. Shi, Z. Y. Wang, C. He, H. K. Wang, J. W. Chen, M. L. Fu, G. D. Li and C. M. Niu, J. Mol. Catal. A: Chem., 2015, 399, 79–85 CrossRef CAS.
- S. W. Liu, J. G. Yu and M. Jaroniec, Chem. Mater., 2011, 23, 4085–4093 CrossRef CAS.
- N. Zhang, Y. H. Zhang and Y. J. Xu, Nanoscale, 2012, 4, 5792–5813 RSC.
- L. M. Pastrana-Martínez, S. Morales-Torres, V. Likodimos, J. L. Figueiredo, J. L. Faria, P. Falaras and A. M. T. Silva, Appl. Catal., B, 2012, 123, 241–256 CrossRef.
- X. C. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- Z. Zhao, Y. Sun and F. Dong, Nanoscale, 2015, 7, 15–37 RSC.
- P. Chen, F. Wang, Q. Zhang, Y. Su, L. Shen, K. Yao, Z.-F. Chen, Y. Liu, Z. Cai, W. Lv and G. Liu, Chemosphere, 2017, 172, 193–200 CrossRef CAS PubMed.
- J. J. Xue, S. S. Ma, Y. M. Zhou, Z. W. Zhang and M. He, ACS Appl. Mater. Interfaces, 2015, 7, 9630–9637 CAS.
- K. M. Lee, C. W. Lai, K. S. Ngai and J. C. Juan, Water Res., 2016, 88, 428–448 CrossRef CAS PubMed.
- C. Han, Z. Chen, N. Zhang, J. C. Colmenares and Y. J. Xu, Adv. Funct. Mater., 2015, 25, 221–229 CrossRef CAS.
- Z. Q. Cai, Y. M. Sun, W. Liu, F. Pan, P. Z. Sun and J. Fu, Environ. Sci. Pollut. Res., 2017, 24, 15882–15904 CrossRef CAS PubMed.
- A. Chatzitakis, C. Berberidou, I. Paspaltsis, G. Kyriakou, T. Sklaviadis and I. Poulios, Water Res., 2008, 42, 386–394 CrossRef CAS PubMed.
- M. Farzadkia, K. Rahmani, M. Gholami, A. Esrafili, A. Rahmani and H. Rahmani, Korean J. Chem. Eng., 2014, 31, 2014–2019 CrossRef CAS.
- L. Jing, W. Zhou, G. Tian and H. Fu, Chem. Soc. Rev., 2013, 42, 9509–9549 RSC.
- M. Shakir, M. Faraz, M. A. Sherwani and S. I. Al-Resayes, J. Lumin., 2016, 176, 159–167 CrossRef CAS.
- T. B. Ivetić, M. R. Dimitrievska, N. L. Finčur, L. R. Đačanin, I. O. Gúth, B. F. Abramović and S. R. Lukić-Petrović, Ceram. Int., 2014, 40, 1545–1552 CrossRef.
- V. Etacheri, R. Roshan and V. Kumar, ACS Appl. Mater. Interfaces, 2012, 4, 2717–2725 CAS.
- Y. Li, Y. M. Wang, L. H. Liu, D. W. Wang and W. L. Zhang, Environ. Sci. Pollut. Res., 2014, 21, 5177–5186 CrossRef CAS PubMed.
- S. M. Lam, J. C. Sin, A. Z. Abdullah and A. R. Mohamed, Ceram. Int., 2013, 39, 2343–2352 CrossRef CAS.
- M. Tobajas, C. Belver and J. J. Rodriguez, Chem. Eng. J., 2017, 309, 596–606 CrossRef CAS.
- S. Y. Dong, J. Y. Sun, Y. K. Li, C. F. Yu, Y. H. Li and J. H. Sun, Appl. Catal., B, 2014, 144, 386–393 CrossRef CAS.
- A. Pourtaheri and A. Nezamzadeh-Ejhieh, Spectrochim. Acta, Part A, 2015, 137, 338–344 CrossRef CAS PubMed.
- J. Kim, C. W. Lee and W. Choi, Environ. Sci. Technol., 2010, 44, 6849–6854 CrossRef CAS PubMed.
- M. Miyauchi, Phys. Chem. Chem. Phys., 2008, 10, 6258–6265 RSC.
- R. Abe, H. Takami, N. Murakami and B. Ohtani, J. Am. Chem. Soc., 2008, 130, 7780–7781 CrossRef CAS PubMed.
- M. Bobu, A. Yediler, I. Siminiceanu and S. Schulte-Hostede, Appl. Catal., B, 2008, 83, 15–23 CrossRef CAS.
- B. Kasprzyk-Hordern, M. Ziolek and J. Nawrocki, Appl. Catal., B, 2003, 46, 639–669 CrossRef CAS.
- R. Oulton, J. P. Haase, S. Kaalberg, C. T. Redmond, M. J. Nalbandian and D. M. Cwiertny, Environ. Sci. Technol., 2015, 49, 3687–3697 CrossRef CAS PubMed.
- M. Fathinia and A. Khataee, Appl. Catal., A, 2015, 491, 136–154 CrossRef CAS.
- A. Goncalves, J. J. M. Orfao and M. F. R. Pereira, Appl. Catal., B, 2013, 140, 82–91 CrossRef.
- L. Yang, C. Hu, Y. L. Nie and J. H. Qu, Appl. Catal., B, 2010, 97, 340–346 CrossRef CAS.
- A. G. Gonçalves, J. J. M. Órfão and M. F. R. Pereira, Catal. Commun., 2013, 35, 82–87 CrossRef.
- M. Fathinia, A. Khataee, S. Aber and A. Naseri, Appl. Catal., B, 2016, 184, 270–284 CrossRef CAS.
- Y. A. J. Al-Hamadani, C. Jung, J.-K. Im, L. K. Boateng, J. R. V. Flora, M. Jang, J. Heo, C. M. Park and Y. Yoon, Chem. Eng. Sci., 2017, 162, 300–308 CrossRef CAS.
- I. Epold, M. Trapido and N. Dulova, Chem. Eng. J., 2015, 279, 452–462 CrossRef CAS.
- M. Kwon, S. Kim, Y. Yoon, Y. Jung, T. M. Hwang, J. Lee and J. W. Kang, Chem. Eng. J., 2015, 269, 379–390 CrossRef CAS.
- W. K. Yao, X. F. Wang, H. W. Yang, G. Yu, S. B. Deng, J. Huang, B. Wang and Y. J. Wang, Water Res., 2016, 88, 826–835 CrossRef CAS PubMed.
- F. L. Fu and Q. Wang, J. Environ. Manage., 2011, 92, 407–418 CrossRef CAS PubMed.
- M. Taheran, S. K. Brar, M. Verma, R. Y. Surampalli, T. C. Zhang and J. R. Valero, Sci. Total Environ., 2016, 547, 60–77 CrossRef CAS PubMed.
- A. Azaïs, J. Mendret, E. Petit and S. Brosillon, Water Res., 2016, 104, 156–167 CrossRef PubMed.
- Y. L. Lin, J. H. Chiou and C. H. Lee, J. Hazard. Mater., 2014, 277, 102–109 CrossRef CAS PubMed.
- K. Fischer, M. Grimm, J. Meyers, C. Dietrich, R. Gläser and A. Schulze, J. Membr. Sci., 2015, 478, 49–57 CrossRef CAS.
- L. Dong, X. Huang, Z. Wang, Z. Yang, X. Wang and C. Tang, Sep. Purif. Technol., 2016, 166, 230–239 CrossRef CAS.
- Y. Wu, J. Lu, M. Meng, J. Dai, X. Lin, J. Gao, C. Li and Y. Yan, Chem. Eng. J., 2017, 309, 263–271 CrossRef CAS.
- S. Ben Abdelmelek, J. Greaves, K. P. Ishida, W. J. Cooper and W. H. Song, Environ. Sci. Technol., 2011, 45, 3665–3671 CrossRef CAS PubMed.
- M. M. Pendergast and E. M. V. Hoek, Energy Environ. Sci., 2011, 4, 1946–1971 CAS.
- M. Huang, Y. Chen, C.-H. Huang, P. Sun and J. Crittenden, Chem. Eng. J., 2015, 279, 904–911 CrossRef CAS.
- F. Liu, N. A. Hashim, Y. T. Liu, M. R. M. Abed and K. Li, J. Membr. Sci., 2011, 375, 1–27 CrossRef CAS.
- Z. Zhang, X. Y. Kong, K. Xiao, Q. Liu, G. H. Xie, P. Li, J. Ma, Y. Tian, L. P. Wen and L. Jiang, J. Am. Chem. Soc., 2015, 137, 14765–14772 CrossRef CAS PubMed.
- Y. Wu, J. Zhao, C. Wang, J. Lu, M. Meng, X. Dai, Y. Yan and C. Li, Chem. Eng. J., 2016, 306, 492–503 CrossRef CAS.
- K. Kümmerer, Pharmaceuticals in the environment: sources, fate, effects and risks, Springer, 2008 Search PubMed.
| This journal is © The Royal Society of Chemistry 2018 |