Visible-light-driven TiO2/Ag3PO4 heterostructures with enhanced antifungal activity against agricultural pathogenic fungi Fusarium graminearum and mechanism insight†
Bingkun
Liu
*a,
Yongfei
Xue
a,
Jingtao
Zhang
b,
Bing
Han
a,
Jie
Zhang
a,
Xinying
Suo
b,
Lilong
Mu
a and
Hengzhen
Shi
*a
aSchool of Material and Chemical Engineering, Zhengzhou University of Light Industry, Zhengzhou 450002, People's Republic of China. E-mail: liubk2015@zzuli.edu.cn; shihz@zzuli.edu.cn
bSchool of Food and Bioengineering, Zhengzhou University of Light Industry, Zhengzhou 450002, People's Republic of China
First published on 21st November 2016
Abstract
A series of TiO2/Ag3PO4 heterostructures were fabricated via a facile in situ precipitation process. The as-synthesized composites were found to exhibit markedly enhanced photocatalytic inactivation of E. coli under visible light irradiation compared with bare TiO2 and Ag3PO4 owing to the improved light absorption and the efficient separation of photo-induced electron–hole pairs. Moreover, the antifungal activity of the composite materials was studied against the phytopathogen Fusarium graminearum (one of the main pathogens of wheat). With 100 min of visible light illumination, the TiO2/Ag3PO4 composite reduced the survival ratio of the F. graminearum macroconidia to about 2%. The photocatalytic disinfection mechanism of the macroconidia was attributed to the damage to their cell wall/membrane caused by the attack of reactive oxygen species as demonstrated by fluorescence staining. In addition, ESR measurement revealed that hydroxyl radicals were the predominant active species in the photocatalytic disinfection system. Our results suggest that this hybrid photocatalyst has great potential as an environmentally-friendly alternative method to disinfect plant pathogens for agricultural areas.
Environmental significanceFusarium head blight (FHB) is one of the devastating diseases in small grain cereal crops, which could cause significant loss of the grain quality and yield. FHB could also produce various mycotoxins which are detrimental to livestock and pose potential safety concerns to human food. Fusarium graminearum is the pathogen which causes FHB in wheat. Conventionally, the measurement strategy for plant fungal disease prevention and control mainly depends on the use of chemical agents. However, chemical pesticides are usually hazardous to animals and the environment owing to their residual toxicity. Furthermore, fungi have developed resistance to some traditional fungicides, which makes it more difficult to control fungal growth. In this case, it is essential to develop alternative antifungal approaches, which could overcome the fungicide resistance and also pose no harmful effect on the environment. To our best knowledge, a variety of nanomaterials including TiO2 and Ag3PO4 have been used as antibacterial agents. However, little is known about whether the TiO2–Ag3PO4 composite has antifungal activity toward fungal pathogens. This work first demonstrated that the TiO2–Ag3PO4 composite exhibits good antifungal activity against the typical F. graminearum that causes FHB disease in wheat plants, which is a promising material for prevention of pathogenic fungal or bacterial infections in crop protection. |
1. Introduction
Titanium dioxide has been widely used in various areas such as photocatalytic systems, antimicrobial materials, self-cleaning coatings, and solar cells due to its excellent chemical and physical properties.1–6 In 1985, Matsunaga et al. firstly reported the photocatalytic disinfection of microorganisms by platinum-doped TiO2.7 Since then, extensive research has been conducted on TiO2-based photocatalysts for disinfection of various microorganisms, including viruses,8 bacteria,9,10 fungi,11 algae,12 and protozoa.13 It is generally believed that photocatalytic microorganism disinfection relies on the interaction between microorganisms and reactive oxygen species (ROSs) generated from electron–hole pairs under light illumination, such as ˙OH and ˙O2−, which not only destroys the microorganisms but also destroys a large variety of chemical contaminants.14–16 However, the conventional TiO2 photocatalyst has certain disadvantages in practical applications such as its wide band gap (e.g., 3.2 eV for anatase) and rapid recombination of photo-generated electron–hole pairs.17–19Silver and silver-based compounds such as AgX (X = Cl, Br, I), Ag3PO4, Ag2CO3, and Ag2Mo2O7 have been used for photocatalytic and antibacterial applications.20–23 Among them, silver orthophosphate (Ag3PO4) has already attracted attention from many researchers because of its stronger oxidation power and higher O2 evolution under visible light irradiation.24,25 Nonetheless, Ag3PO4 suffers from high cost and stability issues, which hinders its broad use in practical applications. To solve this problem, many efforts have been made to overcome the disadvantages and a variety of Ag3PO4-based composite photocatalysts were suggested.26–28 Yao and coworkers reported that the heterostructured TiO2/Ag3PO4 photocatalysts showed significantly improved photocatalytic degradation of organic dyes compared to those of pure Ag3PO4 and TiO2.26 Yang et al. have demonstrated that the TiO2/Ag3PO4-based composites exhibit excellent antibacterial activity against common bacteria.29,30 Meanwhile, the photocatalytic stability and recyclability were also enhanced for the TiO2/Ag3PO4 composite materials.
Antimicrobial activity towards phytopathogens has been an important issue. As a common plant disease, Fusarium head blight (FHB) is one of the devastating diseases in small grain cereal crops, which could cause significant loss of the grain quality and yield.31,32 FHB could also produce various mycotoxins which are detrimental to livestock and pose potential safety concerns to human food. Fusarium graminearum is the pathogen which causes FHB in wheat.33 To save crops from the disease, pesticides and fungicides are frequently used in agriculture. However, chemical pesticides are usually hazardous to animals and the environment owing to their residual toxicity. Furthermore, fungi have developed resistance to some traditional fungicides, which makes it more difficult to control fungal growth.34 Therefore, it is necessary to develop alternative antifungal approaches, which could overcome the fungicide resistance and also pose no harmful effect on the environment.
To the best of our knowledge, it has been found that no reported studies are available on the application of TiO2/Ag3PO4 heterostructures on fungal phytopathogens in the presence of light. Herein, we have reported a facile process to synthesize Ag3PO4 nanoparticles on the surface of TiO2 hierarchical microspheres (TiO2-HMS). The photocatalytic inactivation of E. coli (Gram-negative bacteria) using the TiO2/Ag3PO4 heterostructure has been studied in the absence and presence of visible light. Combined with E. coli activity tests, the selected composite materials were used for plant-pathogenic fungus (F. graminearum) experiments. The mechanisms of killing or inhibiting F. graminearum were investigated by the change in the cell viability of the pathogenic fungi attacked by radical species using fluorescence staining under visible light irradiation.
2. Experimental section
2.1. Synthesis of TiO2/Ag3PO4 heterostructure
TiO2-HMS was prepared via a very simple acid thermal process. Briefly, 2 mL of titanium n-butoxide (TBT) was added dropwise to 60 mL of acetic acid (AcOH, 99.8%), reacting in a 100 mL Teflon-lined stainless steel autoclave and using ultrasonic dispersion for 15 min. And then the autoclave was sealed and kept at 160 °C for 5 h. After cooling down to room temperature, the products were washed with ethanol three times and dried overnight at 70 °C in air.Ag3PO4 nanoparticles were deposited onto the as-prepared crystallized TiO2-HMS surfaces via an in situ precipitation process. In a typical process, 50 mg of TiO2-HMS was first dispersed in 10 mL of deionized water, and then sonicated for 1 h. Subsequently, 10 mL of AgNO3 solution with a certain concentration was added to the TiO2-HMS suspension (pH = 7) with magnetic stirring for 12 h. The previously dissolved 1 mol L−1 Na2HPO4 was added dropwise to the suspension, and the mole ratio of Na2HPO4 to AgNO3 was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The resulting suspension was magnetically stirred for 5 h in the dark. The powder was then centrifuged, washed with water five times and dried at room temperature. The composite samples were marked as 10-TiO2/Ag3PO4, 20-TiO2/Ag3PO4, 40-TiO2/Ag3PO4 and 80-TiO2/Ag3PO4, according to the final concentration of AgNO3 (10, 20, 40 and 80 mmol L−1, respectively) in the reaction solution. For comparison, Ag3PO4 particles were also prepared under the same conditions without TiO2-HMS.
3. The resulting suspension was magnetically stirred for 5 h in the dark. The powder was then centrifuged, washed with water five times and dried at room temperature. The composite samples were marked as 10-TiO2/Ag3PO4, 20-TiO2/Ag3PO4, 40-TiO2/Ag3PO4 and 80-TiO2/Ag3PO4, according to the final concentration of AgNO3 (10, 20, 40 and 80 mmol L−1, respectively) in the reaction solution. For comparison, Ag3PO4 particles were also prepared under the same conditions without TiO2-HMS.
2.2. Characterization
The morphology and lattice structure of the samples were examined by field emission scanning electron microscopy (JSM-7001F, JEOL, Japan) and transmission electron microscopy (JEM-2100, JEOL, Japan). A SEM equipped with an energy dispersive X-ray spectrometer (EDX) was used to analyze the composition of the nanostructures. The crystal structure of the samples was characterized by X-ray diffraction (XRD) using a D8 Advance X-ray diffractometer (Bruker Corporation) with Cu Kα radiation (λ = 1.5405 Å) at a scanning rate of 5° min−1. X-ray photoelectron spectroscopy (XPS) spectra were collected with a monochromatic Al Kα X-ray source on an ESCALAB250 multitechnique X-ray photoelectron spectrometer. Optical absorbance spectra were recorded by UV-vis-NIR diffuse reflectance spectroscopy (Hitachi U-3900H, Japan). The concentration of Ag+ was measured using an inductively coupled plasma mass spectrometer (ICP-MS, ELAN 9000, PerkinElmer, USA). Steady state photoluminescence (PL) was recorded using an F-7000 fluorescence spectrometer (Hitachi 7000, Japan). Time-resolved photoluminescence decay (TRPL) measurements were carried out using a time-correlated single-photon counting (TCSPC) spectrometer (FLS 980, Edinburgh Instruments, UK). A diode laser (250 nm) was used as the excitation source, and a photomultiplier (R928P PMT) was used as the detector. Time-resolved photoluminescence decay profiles were analyzed and fitted using a biexponential kinetics model that generates two lifetime values, τ1 and τ2, and the corresponding amplitudes, A1 and A2. The intensity-average lifetime <τ> was determined using the following expression: <τ> = (A1τ12 + A2τ22)/(A1τ1 + A2τ2).2.3. Culture of bacteria and fungi
E. coli (ATCC 15597) cells were cultivated in Luria–Bertani nutrient solution, followed by incubation in a BS-1E rotary shaker at 150 rpm and 37 °C for 12 h. After that, the bacterial cells were harvested by centrifugation at 6000 rpm for 5 min at room temperature, then washed twice with phosphate buffer solution (PBS, pH 7.0), and finally suspended with the same volume of the LB media in PBS. The final cell density was adjusted to about 1 × 109 colony forming units (CFU) mL−1. All solid/liquid materials had been autoclaved for 30 min at 121 °C before use.The Fusarium strain was originally derived from wild fungi in soil cultures in Henan province, P. R. China. Fusarium colonies were inoculated into solid PDA medium, which was composed of 200 g of potato, 20 g of agar, and 1000 mL of H2O mixed together and then treated with an autoclave. The strain was incubated in a constant temperature incubator at 26 °C for 4 days. Four pieces of Fusarium fungi mycelia (1 cm in diameter) were directly transplanted into the sporulation mung bean soup (MBS) medium. The fungi were cultivated on a rotary shaker (KYC-100C, Shanghai CIMO Medical Instrument Manufacturing Co., Ltd.) at 120 rpm and 26 °C for another 2 days. Then, the mycelia were filtered using a four-layer sterile gauze, and spores were harvested from the filterable MBS medium by centrifugation at 6000 rpm for 10 min at room temperature and then washing twice with distilled water. The macroconidia concentration was determined with the chamber for counting blood cells under a phase contrast microscope. The macroconidia suspension was diluted in distilled water to a concentration of ∼1 × 106 CFU mL−1 before its use in photocatalytic inactivation experiments. All solid or liquid materials had been autoclaved for 30 min at 121 °C before use.
2.4. Photocatalytic disinfection performance
A fixed concentration of 1 mg photocatalyst per mL microorganism suspension was used in the experiments. 10 mg of photocatalyst with 9.9 mL of buffer solution was first injected into a sterile 60 mm × 15 mm Petri dish and was dispersed ultrasonically for 10 min. Then, 0.1 mL of microorganism suspensions were added into the Petri dish, so that the initial microorganism concentrations used in the photocatalytic disinfection experiments were ca. 107 CFU mL−1 for E. coli and 105 CFU mL−1 for Fusarium. A 300 W xenon lamp (HSX-F300, Beijing NBET Technology Co. Ltd., Beijing, China) was used for the photocatalytic inactivation experiments, and the light with wavelengths below 400 nm and above 700 nm was blocked by glass filters. The intensity of light striking the cell or spore suspensions was ca. 10 mW cm−2, as measured by an FZ-A optical radiometer (Photoelectric Instrument Factory of Beijing Normal University, Beijing, China). At regular time intervals, 0.1 mL of the cell or spore suspensions were withdrawn in sequence. After appropriate dilution with PBS buffer solution (pH ∼7), aliquots of 0.1 mL were spread onto an agar medium plate, and incubated at 37 °C for 24 h. Then, the number of viable cells in terms of colony-forming units was counted. The survival ratio of microorganisms was determined by the ratio Nt/N0, where N0 and Nt are the numbers of CFU at the initial time and each following time interval, respectively. All analyses were conducted in triplicate.2.5. Determination test of radicals
The electron spin resonance (ESR) signals of radicals spin-trapped by 5,5-dimethyl-1-pyrroline N-oxide (DMPO) were recorded using a JEOL-JES-FA200 electron spin resonance spectrometer. The samples were prepared by mixing 0.05 g of the as-prepared photocatalyst in a 100 mM DMPO solution with a 50 mL aqueous dispersion for DMPO–˙OH or a 50 mL methanol dispersion for DMPO–˙O2− under irradiation with visible light (λ > 400 nm) for 5 min.The determination of ˙OH was carried out with a terephthalic acid (TA) fluorescence probe.35,36 The measurements for the amount of ˙OH were performed as follows: 0.06 g of the TiO2/Ag3PO4 composite was added into a beaker with 60 mL of aqueous solution containing 0.01 M NaOH, 0.1 M KCl, 0.3 mM H2O2, and 3 mM TA and then irradiated under visible light (λ > 400 nm) with stirring. At different time intervals, 6 mL suspensions were extracted and centrifuged at a speed of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The fluorescence spectra of these supernatant liquids were obtained on a fluorescence spectrometer (FLS 980, Edinburgh Instruments, UK)
000 rpm. The fluorescence spectra of these supernatant liquids were obtained on a fluorescence spectrometer (FLS 980, Edinburgh Instruments, UK)
2.6. Fluorescence-based live/dead cell test
The FungaLight CFDA, AM/Propidium Iodide Yeast Vitality Kit (Molecular Probes, USA) was applied to reveal the vitality of macroconidia with or without photocatalytic treatment. The FungaLight CFDA, AM/Propidium Iodide Yeast Vitality Kit combines a cell permeable esterase substrate with a membrane integrity indicator to evaluate the vitality of macroconidia cells by fluorescence microscopy. 5-Carboxyfluorescein diacetate (CFDA), acetoxymethyl ester (AM), and propidium iodide were dissolved in DMSO, respectively, and then kept at −20 °C with protection from light. Then, 1 μL of each was added to 1 mL of cell sample. The samples were then incubated in the dark at 37 °C for 30 min. After incubation, the samples were washed 3 times and resuspended in a 50 μL volume of PBS. A 20 μL suspension was dropped onto a glass slide and then covered with a clear glass coverslip. A Nikon NIE upright research-grade microscope was configured to perform the fluorescence and phase contrast microscopy observation. A mercury lamp (U-LH100HG, 100 W) was used as the light source, and an FITC/TRITC filter was used. Images were recorded through a color cooled digital camera head (DSFi1c) with 10× and 40× objectives.3. Results and discussion
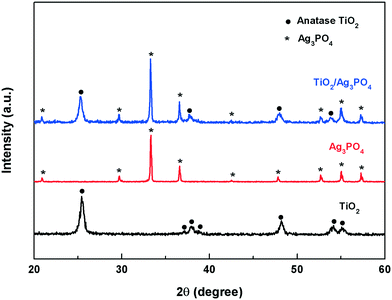
Fig. 1 shows the XRD patterns of the as-prepared bare TiO2, Ag3PO4 and TiO2/Ag3PO4 heterostructure photocatalysts. In the XRD pattern of TiO2, the characteristic peaks at 2θ values of 25.3°, 37.7°, 48.0° and 53.8° can be readily indexed to the anatase phase (JCPDS 21-1272). Besides the anatase crystal peaks, the pattern of the TiO2/Ag3PO4 sample contains diffraction peaks at 2θ values of 20.8°, 29.7°, 33.3°, 36.6°, 52.6°, 55.0° and 57.2°, which are ascribed to the (110), (200), (210), (211), (222), (320) and (321) crystal planes of Ag3PO4 (JCPDS 06-0505), respectively. Meanwhile, the intensity of the Ag3PO4 peaks is high, suggesting a high degree of crystallinity.The morphologies of the as-synthesized samples are observed by using FESEM, TEM and HRTEM. Fig. 2a shows that TiO2-HMS is obtained with a size of 2–4 μm and the microspheres are composed of one-dimensional nanoribbons. In the TiO2/Ag3PO4 sample (Fig. 2b), the surface of TiO2-HMS becomes rough and many nanoparticles are loaded on the microspheres. Fig. 2c shows the morphology of pure Ag3PO4 particles obtained by the precipitation method. It can be seen that pure Ag3PO4 particles are irregular sphere-like structures with the particle size in the range of 200–500 nm. From the TEM and HRTEM images of the TiO2/Ag3PO4 heterostructure (Fig. 2d and e), it can be seen that the Ag3PO4 nanoparticles with size ranging from 2 to 5 nm were uniformly coated on the surface of the TiO2 microspheres. In comparison with the pure Ag3PO4, the smaller particle size of Ag3PO4 supported on TiO2 is attributed to the pre-adsorption of silver cations onto the TiO2 surface, which limits the growth of Ag3PO4 particles. Further, two lattice fringes with d-spacings of 0.27 nm and 0.35 nm corresponding to the (210) plane of Ag3PO4 and the (101) plane of anatase TiO2 as well as the EDX spectrum also support the formation of the TiO2/Ag3PO4 heterostructure.
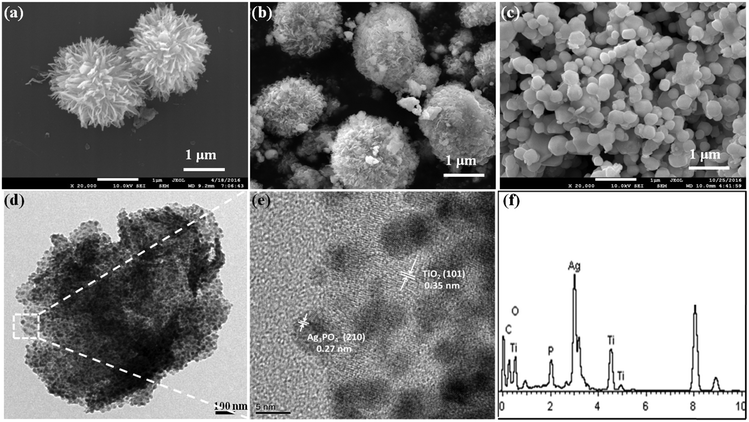 | ||
| Fig. 2 FESEM images of (a) pure TiO2-HMS, (b) the TiO2/Ag3PO4 composite and (c) Ag3PO4 particles. (d) TEM image, (e) HRTEM image and (f) EDX spectrum of the TiO2/Ag3PO4 composite. | ||
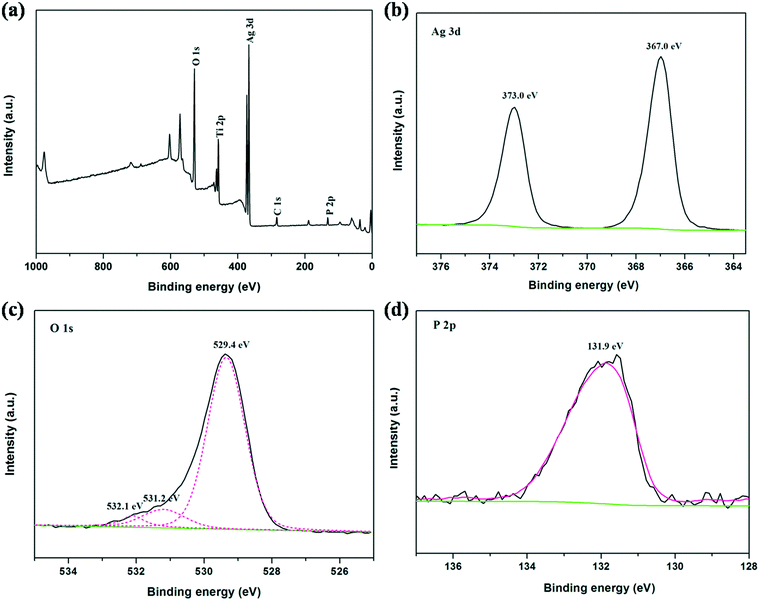
To investigate the elemental composition and chemical states of the TiO2/Ag3PO4 samples, we also carried out XPS measurements, and the results are shown in Fig. 3. The survey XPS spectrum (Fig. 3a) reveals that the obtained hierarchical structures are primarily composed of Ti, Ag, O and P. Fig. 3b exhibits the narrow scan spectrum of Ag 3d at the binding energies of 367.0 eV (Ag 3d5/2) and 373.0 eV (Ag 3d3/2), which can be attributed to the Ag+ ions of Ag3PO4. The oxygen on the sample surface exists in different forms with binding energies at 532.1 eV, 531.2 eV and 529.4 eV (Fig. 3c). The main peaks at 529.4 eV and 531.2 eV could be ascribed to the oxygen lattices in TiO2 and Ag3PO4, respectively. The peak at 532.1 eV is caused by the external –OH group or the water molecule adsorbed on the surface of the TiO2/Ag3PO4 heterostructure. Furthermore, a weak and broad band centered at ca. 131.9 eV can be observed in Fig. 3d, which corresponds to the characteristic P 2p from Ag3PO4.
The UV-vis diffuse reflectance spectra of the as-prepared bare TiO2, Ag3PO4, and TiO2/Ag3PO4 photocatalysts are shown in Fig. 4. It is observed that bare TiO2 exhibits a 390 nm absorption band-edge in the UV region, corresponding to a band gap energy of 3.28 eV. Upon growth of Ag3PO4 on the surface of TiO2-HMS, the composite sample shows a red shift and strong absorption in the wavelength region of 400–540 nm. The calculated band gap of the TiO2/Ag3PO4 composite is about 2.87 eV according to the band gap relation.37,38 Meanwhile, we find that the visible light absorption of the TiO2/Ag3PO4 composite is further increased after illumination in the photocatalytic process (Fig. S1†), which is attributed to the characteristic surface plasmon absorption of metallic silver on the Ag3PO4 surface.
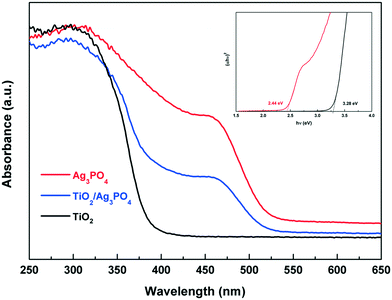 | ||
| Fig. 4 UV-vis absorption spectra of TiO2, Ag3PO4 and the TiO2/Ag3PO4 composite. The inset is the plot of (αhv)2versus energy (hv) characteristics for bare TiO2 and Ag3PO4. | ||
The transfer, migration and recombination processes of the photo-generated charge carriers could be explored from the photoluminescence (PL) spectra of the samples. Fig. 5a shows the steady state PL spectra of pure TiO2 and the TiO2/Ag3PO4 composite with an excitation wavelength of 250 nm. It is found that introducing Ag3PO4 greatly influences the PL intensity. Compared with bare TiO2-HMS and Ag3PO4, the PL intensity of the TiO2/Ag3PO4 composite reveals a significant decrease, which is mainly ascribed to the efficient charge carrier transfer between TiO2 and Ag3PO4. To gain more understanding of this phenomenon, the time-resolved PL spectra are investigated. Fig. 5b represents the PL decay curves of TiO2, Ag3PO4 and the TiO2/Ag3PO4 composite. The spectra are fitted with a biexponential function which yields a slow (τ1) and a fast (τ2) decay component relatively assigned to the radiative recombination and nonradiative relaxation pathways.39,40 The fitting results are summarized in Table 1. For pristine TiO2 and Ag3PO4, the mean lifetimes are 2.52 ns and 2.87 ns, respectively. As for the TiO2/Ag3PO4 composite, a shortened emission lifetime of 1.81 ns is observed, indicating the more effective charge-carrier formation and faster interfacial charge transfer occurring in the TiO2/Ag3PO4 photocatalyst.41 According to the energy band structures of TiO2 and Ag3PO4,26,30 the band matching facilitates the quick transfer of photo-induced holes in Ag3PO4 to TiO2 under light irradiation while photo-induced electrons migrate to the surface of Ag3PO4 from TiO2. Consequently, the TiO2/Ag3PO4 heterostructure significantly accelerates the charge carrier transfer between these two semiconductors, enabling the efficient separation of photo-induced electron–hole pairs.
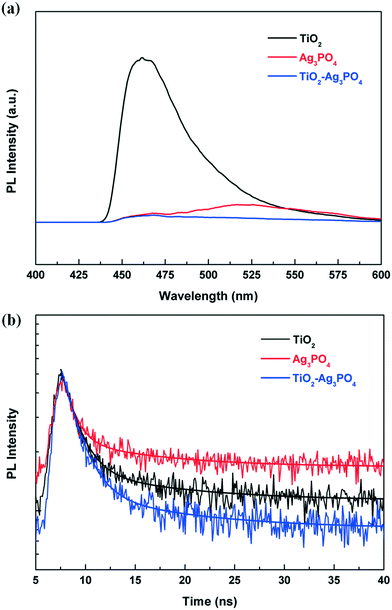 | ||
| Fig. 5 (a) Steady-state PL spectra and (b) time-resolved PL spectra of TiO2, Ag3PO4 and the TiO2/Ag3PO4 composite. In (b), the fitting results (solid curves) were also included for comparison. | ||
| A 1 | τ 1 (ns) | A 2 | τ 2 (ns) | <τ> (ns) | |
|---|---|---|---|---|---|
| TiO2 | 378 | 11.30 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 853 853 |
1.71 | 2.52 |
| Ag3PO4 | 245 | 13.18 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 465 465 |
2.23 | 2.87 |
| TiO2/Ag3PO4 | 98 | 10.8 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 674 674 |
1.66 | 1.81 |
The photocatalysis effect of the TiO2/Ag3PO4 composite is evaluated by the disinfection of E. coli under visible light irradiation (λ = 400–700 nm). Fig. 6 shows the survival ratio of E. coli treated with different photocatalysts. Control experiments such as pure TiO2 under visible light illumination and TiO2/Ag3PO4 in the dark are also carried out. From Fig. 6, it can be seen that the pure TiO2-HMS shows very low antibacterial ability, where only 20% of E. coli are killed after 50 min under visible light irradiation. After Ag3PO4 modification, the disinfection efficiencies of the TiO2/Ag3PO4 composite show a significant enhancement in terms of photocatalytic antibacterial activities compared to pure TiO2. Among them, 80-TiO2/Ag3PO4 exhibits the best photocatalytic performance, and the antibacterial rate reaches 99.53% after 50 min of reaction, far exceeding those of pure TiO2, pure Ag3PO4 and other TiO2/Ag3PO4 composites. Under dark conditions, the survival ratio of E. coli remains at ∼90% after 50 min of treatment with the 80-TiO2/Ag3PO4 composite, which is due to the released silver ions (1.25 μg mL−1) from the TiO2/Ag3PO4 composite. Meanwhile, it is found that the concentration of Ag+ ions released under light irradiation is higher than that in the dark, and there is about 40% cell loss after 50 min of light irradiation (Fig. S2†). Thus, the released Ag+ ions can only cause limited bacterial inactivation, and the primary bactericidal effect of visible light irradiated TiO2/Ag3PO4 should result from the sustainable generated reactive oxygen species.
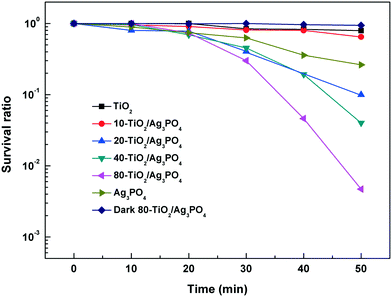 | ||
| Fig. 6 Survival ratio of E. coli (107 CFU mL−1) versus various treatments using the as-fabricated photocatalysts. | ||
The stability of a photocatalyst is one of the important parameters for its usefulness. To study the stability of the TiO2/Ag3PO4 composite photocatalyst, circulating runs in the photocatalytic disinfection experiments are examined under visible light irradiation. As shown in Fig. 7a, although some antibacterial activity losses are observed for the TiO2/Ag3PO4 composite, the antibacterial rate of pure Ag3PO4 exhibits more significant decreases under the same conditions. The improved stability of the TiO2/Ag3PO4 photocatalyst is ascribed to the chemical adsorption between the O2− anions in TiO2 and Ag+ cations in Ag3PO4.26 Further analysis of the XRD patterns of Ag3PO4 and TiO2/Ag3PO4 after repeated experiments is presented in Fig. 7b. It is found that a new diffraction peak located at 2θ = 38.1° corresponding to the (111) plane of silver (JCPDS no. 65-2871) is observed for both samples. But the intensity of used TiO2/Ag3PO4 is much lower than that of Ag3PO4, confirming that bare Ag3PO4 is more easily decomposed into Ag than the TiO2/Ag3PO4 composite. Therefore, the incorporation of TiO2 into the Ag3PO4 photocatalyst can not only enhance the visible light photocatalytic performance, but also inhibit photo-corrosion and promote the stable durability of its photocatalytic activity.
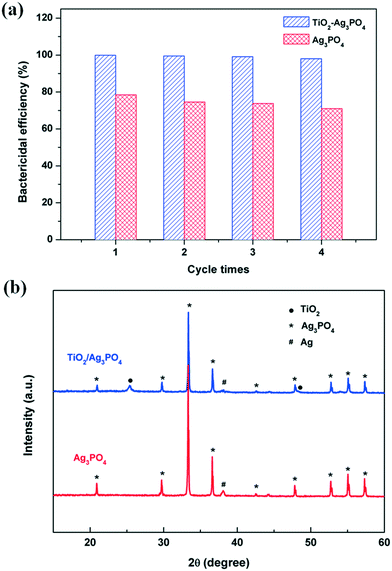 | ||
| Fig. 7 (a) Photocatalytic antibacterial recycling experiments of E. coli. (b) XRD patterns of Ag3PO4 and the 80-TiO2/Ag3PO4 composite after recycling experiments under visible light irradiation. | ||
Then, we use the selected materials for plant-pathogenic fungus experiments. Fig. 8 presents the survival ratio of the wild type F. graminearum macroconidia treated with bare TiO2 and TiO2/Ag3PO4 composites under visible light illumination. We find no obvious loss in the survival ratio of the wild type F. graminearum macroconidia for TiO2, suggesting its low photocatalytic disinfection capability against F. graminearum macroconidia under visible light illumination. When Ag3PO4 is present, the TiO2/Ag3PO4 composites show obviously improved photocatalytic disinfection activities against the wild type F. graminearum macroconidia compared to pure TiO2 under visible light illumination. As for the 80-TiO2/Ag3PO4 composite, the survival ratio decreases slowly to ∼70% and ∼65.5% at 20 and 40 min after the beginning of the photocatalytic treatment, respectively, and then drops sharply. With 100 min of visible light irradiation, the survival ratio of F. graminearum treated with the 80-TiO2/Ag3PO4 composite decreases to ∼2%, which is 50 times lower than that for bare TiO2-HMS.
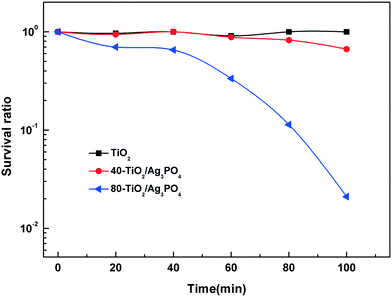 | ||
| Fig. 8 Survival ratio of F. graminearum macroconidia under visible light irradiation using bare TiO2 and TiO2/Ag3PO4 composites. | ||
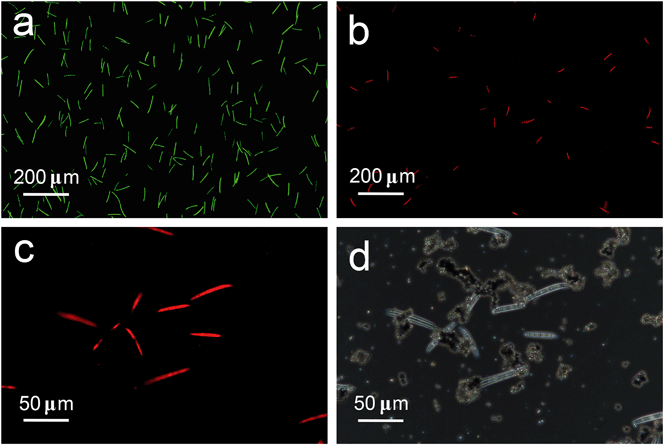
The photocatalytic disinfection of F. graminearum macroconidia is further examined by fluorescence observation of these macroconidia after being stained with the FungaLight CFDA, AM/Propidium Iodide Yeast Vitality Kit to reveal their vitality. The acetoxymethyl ester (AM) of the esterase substrate 5-carboxyfluorescein diacetate (CFDA) allows this reagent to permeate cell membranes, while propidium iodide (PI) could only penetrate cell with damaged membranes. With an appropriate mixture of the CFDA, AM, and PI stains, esterase-active cells with intact cell membranes are stained fluorescent green, whereas cells with damaged membranes are stained fluorescent red. As shown in Fig. 9a, most of the macroconidia in the sample are stained green when treated with the TiO2/Ag3PO4 composite in the dark. In contrast, after 100 min of visible light illumination, almost all of the F. graminearum macroconidia are stained red by the probe of PI (Fig. 9b), indicating that the F. graminearum macroconidia are damaged by the photocatalytic treatment, which allows PI to penetrate into the cells and also leads to the leakage of the cell's contents through the damaged membranes. Fig. 9c and d are the enlarged fluorescence observation image and corresponding phase contrast image of F. graminearum macroconidia with photocatalytic treatment. It is found that the macroconidia still keep their structure even after they lost their viability, suggesting that the damage to the cell membranes of the dead macroconidia is not severe enough to break their structures. This is further confirmed by the SEM images of F. graminearum macroconidia with various inactivation treatments (Fig. S3†). The results above demonstrate that the photocatalytic disinfection mechanism of TiO2/Ag3PO4 on these macroconidia could be attributed to the damage to their cell wall/membrane caused by the attack of photo-induced oxidative active species, while the breakage of their cell structure is not necessary for their loss of viability.
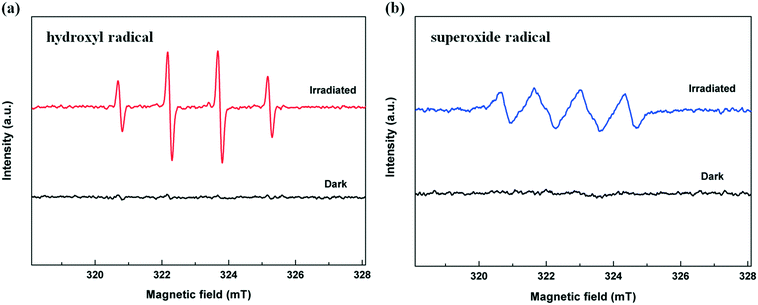
To further study the radical generation during the photocatalytic disinfection process under visible-light irradiation, ESR spin-trapping with the DMPO technique is carried out on the TiO2/Ag3PO4 composite. As shown in Fig. 10, no signals in both ESR spectra could be detected in dark environments. Under visible light irradiation, a 4-fold characteristic peak with an intensity ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is observed (Fig. 10a), indicating that ˙OH radicals were really generated on the TiO2/Ag3PO4 under visible light irradiation. In Fig. 10b, DMPO–˙O2− adduct signals are also detected in the system under irradiation, but are weaker than DMPO–˙OH signals. The results demonstrate that both O2˙− and ˙OH are produced on the surface of the visible light-irradiated TiO2/Ag3PO4 composites in the disinfection process, with ˙OH radicals as the predominant species.
1 is observed (Fig. 10a), indicating that ˙OH radicals were really generated on the TiO2/Ag3PO4 under visible light irradiation. In Fig. 10b, DMPO–˙O2− adduct signals are also detected in the system under irradiation, but are weaker than DMPO–˙OH signals. The results demonstrate that both O2˙− and ˙OH are produced on the surface of the visible light-irradiated TiO2/Ag3PO4 composites in the disinfection process, with ˙OH radicals as the predominant species.
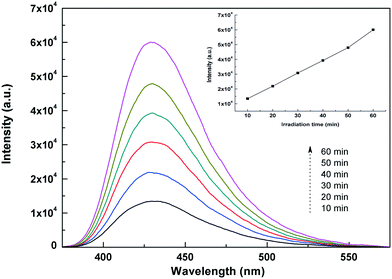
We further detect the formation of the major active species ˙OH over the TiO2/Ag3PO4 composites by the photoluminescence technique, using terephthalic acid as the probe molecule. Fig. 11 shows the changes in the PL spectra of terephthalic acid solution under visible light irradiation with the irradiation time. A gradual increase of PL intensity occurs at about 426 nm, indicating that the amount of ˙OH originating from the TiO2/Ag3PO4 composites increases with the visible light irradiation time. The result is consistent with the disinfection efficiency, which further reveals that ˙OH radicals play an important role in the photocatalytic disinfection process. Furthermore, the nearly linear relationship between the fluorescence intensity and irradiation time confirms the stability of the TiO2/Ag3PO4 photocatalyst.42
On the basis of all of the results above, an overall mechanism leading to the visible-light-driven photocatalytic disinfection of F. graminearum macroconidia is proposed in Fig. 12. Under visible light illumination, the electron–hole pairs are created within Ag3PO4, and then the photo-generated holes in Ag3PO4 are transferred to the valence band (VB) of TiO2 and further react with the adsorbed H2O molecules to form hydroxyl radicals (˙OH). Meanwhile, the photo-generated electrons in the conduction band (CB) migrate to the surface of Ag3PO4 and further react with absorbed oxygen to form superoxide radicals (˙O2−). The improved separation of electron–hole pairs and reduced recombination rate result in more efficient production of highly active radicals such as ˙OH and ˙O2−. All of these photo-induced reactive species could decompose the microbial cell wall and cell membrane, and finally enhance the photocatalytic disinfection efficiency for F. graminearum macroconidia.
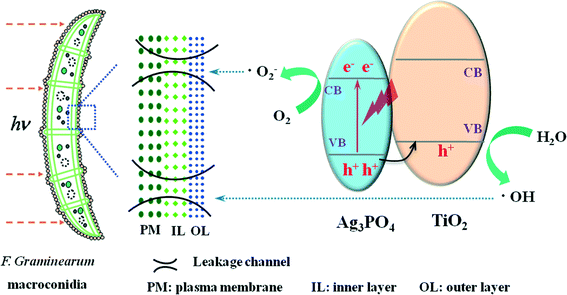 | ||
| Fig. 12 Schematic illustration of photocatalytic inactivation toward F. graminearum macroconidia with the TiO2/Ag3PO4 heterostructure under visible-light irradiation. | ||
4. Conclusions
In summary, efficient TiO2/Ag3PO4 heterostructures were fabricated by a facile in situ precipitation process. The coupled photocatalyst exhibited an enhanced photocatalytic disinfection effect on E. coli and F. graminearum macroconidia under visible light illumination. The enhancement was the result of a synergistic effect between Ag3PO4 and TiO2, which improved light absorption and the effective separation of photo-induced electron–hole pairs. Our results demonstrated that the photocatalytic disinfection mechanism of the TiO2/Ag3PO4 composite attached on the macroconidia was mainly attributed to the damage to their cell wall/membrane caused by the attack of active ˙OH and ˙O2− species. Our results suggest that this hybrid photocatalyst has great potential as an environmentally-friendly alternative method to disinfect plant pathogens for agricultural areas.Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (No. 21571160 and 21501153), the National Natural Science Foundation of China-Henan Talents Fostering Joint Funds (No. U1504311), the Key Research Projects of the Science and Technology Department of Henan Province (No. 152102210147), the Doctoral Scientific Research Foundation of Zhengzhou University of Light Industry (No. 2015BSJJ044) and the Graduate's Scientific Research Foundation of Zhengzhou University of Light Industry.Notes and references
- X. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891–2959 CrossRef CAS PubMed.
- M. Ni, M. K. H. Leung, D. Y. C. Leung and K. Sumathy, Renewable Sustainable Energy Rev., 2007, 11, 401–425 CrossRef CAS.
- H. A. Foster, I. B. Ditta, S. Varghese and A. Steele, Appl. Microbiol. Biotechnol., 2011, 90, 1847–1868 CrossRef CAS PubMed.
- C. Kapridaki, L. Pinho, M. J. Mosquera and P. Maravelaki-Kalaitzaki, Appl. Catal., B, 2014, 156–157, 416–427 CrossRef CAS.
- G. K. Mor, K. Shankar, M. Paulose, O. K. Varghese and C. A. Grimes, Nano Lett., 2006, 6, 215–218 CrossRef CAS PubMed.
- B. Liu, Y. Sun, X. Wang, L. Zhang, D. Wang, Z. Fu, Y. Lin and T. Xie, J. Mater. Chem. A, 2015, 3, 4445–4452 CAS.
- T. Matsunaga, R. Tomoda, T. Nakajima and H. Wake, FEMS Microbiol. Lett., 1985, 29, 211–214 CrossRef CAS.
- M. Cho, H. Chung, W. Choi and J. Yoon, Appl. Environ. Microbiol., 2005, 71, 270–275 CrossRef CAS PubMed.
- A.-G. Rincón and C. Pulgarin, Appl. Catal., B, 2004, 51, 283–302 CrossRef.
- H. M. Coleman, C. P. Marquis, J. A. Scott, S. S. Chin and R. Amal, Chem. Eng. J., 2005, 113, 55–63 CrossRef CAS.
- C. Maneerat and Y. Hayata, Int. J. Food Microbiol., 2006, 107, 99–103 CrossRef CAS PubMed.
- J. R. Peller, R. L. Whitman, S. Griffith, P. Harris, C. Peller and J. Scalzitti, J. Photochem. Photobiol., A, 2007, 186, 212–217 CrossRef CAS.
- V. Aruoja, S. Pokhrel, M. Sihtmae, M. Mortimer, L. Madler and A. Kahru, Environ. Sci.: Nano, 2015, 2, 630–644 RSC.
- Y. Li, W. Zhang, J. Niu and Y. Chen, ACS Nano, 2012, 6, 5164–5173 CrossRef CAS PubMed.
- V. Etacheri, G. Michlits, M. K. Seery, S. J. Hinder and S. C. Pillai, ACS Appl. Mater. Interfaces, 2013, 5, 1663–1672 CAS.
- T. Tong, A. Shereef, J. Wu, C. T. T. Binh, J. J. Kelly, J.-F. Gaillard and K. A. Gray, Environ. Sci. Technol., 2013, 47, 12486–12495 CrossRef CAS PubMed.
- A. L. Linsebigler, G. Lu and J. T. Yates, Chem. Rev., 1995, 95, 735–758 CrossRef CAS.
- J. Du, X. Lai, N. Yang, J. Zhai, D. Kisailus, F. Su, D. Wang and L. Jiang, ACS Nano, 2011, 5, 590–596 CrossRef CAS PubMed.
- D. C. Hurum, K. A. Gray, T. Rajh and M. C. Thurnauer, J. Phys. Chem. B, 2005, 109, 977–980 CrossRef CAS PubMed.
- H. Shi, G. Li, H. Sun, T. An, H. Zhao and P.-K. Wong, Appl. Catal., B, 2014, 158–159, 301–307 CrossRef CAS.
- W.-S. Wang, H. Du, R.-X. Wang, T. Wen and A.-W. Xu, Nanoscale, 2013, 5, 3315–3321 RSC.
- G. Dai, J. Yu and G. Liu, J. Phys. Chem. C, 2012, 116, 15519–15524 CAS.
- C.-C. Shen, Q. Zhu, Z.-W. Zhao, T. Wen, X. Wang and A.-W. Xu, J. Mater. Chem. A, 2015, 3, 14661–14668 CAS.
- Y. Bi, S. Ouyang, N. Umezawa, J. Cao and J. Ye, J. Am. Chem. Soc., 2011, 133, 6490–6492 CrossRef CAS PubMed.
- Y. Bi, H. Hu, S. Ouyang, G. Lu, J. Cao and J. Ye, Chem. Commun., 2012, 48, 3748–3750 RSC.
- W. Yao, B. Zhang, C. Huang, C. Ma, X. Song and Q. Xu, J. Mater. Chem., 2012, 22, 4050–4055 RSC.
- J. Xie, Y. Yang, H. He, D. Cheng, M. Mao, Q. Jiang, L. Song and J. Xiong, Appl. Surf. Sci., 2015, 355, 921–929 CrossRef CAS.
- L. Liu, J. Liu and D. D. Sun, Catal. Sci. Technol., 2012, 2, 2525–2532 CAS.
- X. Yang, J. Qin, Y. Jiang, K. Chen, X. Yan, D. Zhang, R. Li and H. Tang, Appl. Catal., B, 2015, 166–167, 231–240 CrossRef CAS.
- X. Yang, J. Qin, Y. Jiang, R. Li, Y. Li and H. Tang, RSC Adv., 2014, 4, 18627–18636 RSC.
- D. W. Parry, P. Jenkinson and L. McLeod, Plant Pathol., 1995, 44, 207–238 CrossRef.
- R. S. Goswami and H. C. Kistler, Mol. Plant Pathol., 2004, 5, 515–525 CrossRef CAS PubMed.
- S. D. Harris, Mycologia, 2005, 97, 880–887 CrossRef PubMed.
- Y. Elad, H. Yunis and T. Katan, Plant Pathol., 1992, 41, 41–46 CrossRef CAS.
- G. Liu, L. Wang, C. Sun, X. Yan, X. Wang, Z. Chen, S. C. Smith, H.-M. Cheng and G. Q. Lu, Chem. Mater., 2009, 21, 1266–1274 CrossRef CAS.
- L. Sun, Y. Qin, Q. Cao, B. Hu, Z. Huang, L. Ye and X. Tang, Chem. Commun., 2011, 47, 12628–12630 RSC.
- Y. Peng, P.-P. Yu, Q.-G. Chen, H.-Y. Zhou and A.-W. Xu, J. Phys. Chem. C, 2015, 119, 13032–13040 CAS.
- H. Li, Q. Zhang, X. Duan, X. Wu, X. Fan, X. Zhu, X. Zhuang, W. Hu, H. Zhou, A. Pan and X. Duan, J. Am. Chem. Soc., 2015, 137, 5284–5287 CrossRef CAS PubMed.
- S. Sadhu, P. Saha Chowdhury and A. Patra, J. Lumin., 2008, 128, 1235–1240 CrossRef CAS.
- Y.-F. Lin and Y.-J. Hsu, Appl. Catal., B, 2013, 130–131, 93–98 CrossRef CAS.
- Z. Zhang, Y. Huang, K. Liu, L. Guo, Q. Yuan and B. Dong, Adv. Mater., 2015, 27, 5906–5914 CrossRef CAS PubMed.
- G. Liu, H. G. Yang, X. Wang, L. Cheng, J. Pan, G. Q. Lu and H.-M. Cheng, J. Am. Chem. Soc., 2009, 131, 12868–12869 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6en00415f |
| This journal is © The Royal Society of Chemistry 2017 |