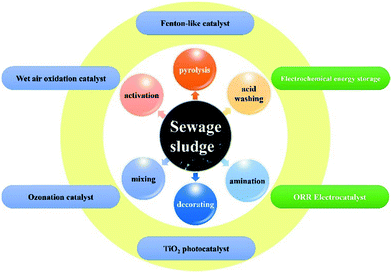
Sewage sludge-based functional nanomaterials: development and applications†
Shi-Jie
Yuan
and
Xiao-Hu
Dai
*
State Key Laboratory of Pollution Control and Resource Reuse, College of Environmental Science and Engineering, Tongji University, Shanghai, 200092, China. E-mail: daixiaohu@tongji.edu.cn; Fax: +86 21 65983602; Tel: +86 21 65986297
First published on 13th October 2016
Abstract
Sewage sludge, the major by-product of wastewater treatment plants, is defined as a pollutant by the US Environmental Protection Agency. The increasing production of sewage sludge worldwide and the imposition of more stringent regulations, together with the restriction of traditional options for sewage sludge disposal, have prompted new approaches for the cost-effective and environmentally benign treatment and reuse of sewage sludge. This paper seeks to review the published research on the development and applications of sewage sludge-based functional nanomaterials. The production methods of nanomaterials are illuminated, and the textural properties of the as-prepared nanomaterials are summarized. The potential applications of the as-prepared nanomaterials in the fields of pollutant degradation and electrochemical energy storage and conversion are reviewed. The synergistically enhanced performances between the as-synthesized nanomaterials and the specific small inorganic molecular or metal oxides in sewage sludge are discussed. Furthermore, the future opportunities and challenges in this emerging research area of designing functional nanomaterials from sewage sludge are addressed.
Environmental significanceDue to the increasing production worldwide and the presence of organic pollutants and heavy metals, the disposal of sewage sludge is currently one of the most important environmental issues. Traditional options for sludge disposal such as ocean dumping, landfilling or combustion are no longer acceptable. This situation has been further aggravated by the imposition of increasingly stringent regulations. Thus, to meet the urgent demand of global requirements, the initiation of more cost-effective, environmentally benign re-use of sewage sludge for synthesizing sewage sludge-based functional nanomaterials is certainly necessary from an environmental standpoint. |
1. Introduction
Sewage sludge (SS), which mainly consists of the solids collected from the primary settling step and the settled microorganisms generated during the biological transformation processes occurring after secondary clarification, is the major residue of wastewater treatment plants (WWTPs).1 It consists of a wide variety of inorganic and organic components including SiO2, CaO, Al2O3, MgO, various transition metals (Fe, Ni, Co, and other species), bacterial cells, biomacromolecules, and toxic organic contaminants and is defined as a pollutant by the U. S. Environmental Protection Agency.2,3 The generation of SS is continually increasing around the world. The amount of SS produced annually now comprises about 30 million tons in China, 74 million tons in Japan, 9.8 million dry tons in the EU, and 5.6–7 million dry tons in the US.4–8Due to the increasing production worldwide and the presence of organic pollutants and heavy metals, the disposal of SS is currently one of the most important environmental issues.4 Traditional options for SS reduction include landfills, fertilization, incineration, and land applications following aerobic and anaerobic digestion.5 However, the accumulation of undesirable substances in SS (e.g., heavy metals, pathogens, and toxic organic contaminants), which can potentially be passed to the food chain, restricts these options (Table S1†). For more information on the conventional disposal practices of SS and their potential effects on the environment, the reader can refer to the excellent review presented by Fytili and coworkers.5 This situation has been further aggravated by the imposition of increasingly stringent regulations, the Title 40 Code of Federal Regulations, Part 503 in the U. S. and the Urban Waste Water Directive 91/271/EEC in Europe, which govern the disposal of SS including lower limit values for heavy metals and more restrictive chemical pollutant and pathogen limits and changes in management practices such as increasing buffer zones between residential areas or drinking water sources and SS-applied land.5,6 Over the past decades, many researchers have focused on the design of innovative SS treatment systems, especially in terms of quality, safety, and cost efficiency.7–10
Recently, sludge pyrolysis, which appears to be less polluting than conventional methods,5,7 has gained noticeable popularity because it can concentrate the heavy metals in a solid carbonaceous residue and convert approximately half of the organic matter into renewable liquid fuels and chemical feedstock. The initiation of value-added, environmentally benign re-use of the pyrolytic residue is certainly necessary from an environmental standpoint.
Stemming from the carbonaceous nature of the SS and the existence of the special inorganic and organic components in it, the treatment and reuse of SS as value-added functional nanomaterials have generated significant scientific interest in the past few years. Functional nanomaterials, especially carbon-based functional nanomaterials, owing to their unique microstructures and physicochemical properties, exhibit great potential in applications such as heterogeneous Fenton reaction catalysts and photocatalysts, electrochemical energy storage and conversion, and pollutant adsorbents, and can be used to resolve many of today's serious global challenges such as environmental pollution, global warming, fossil fuel consumption, and climate change.11,12 However, current state-of-the-art methods for the mass production of carbon-based functional nanomaterials such as chemical vapor deposition and carbonization of synthetic or natural polymers often involve drawbacks, including a tedious synthesis process, expensive fossil fuel-based precursors, complicated apparatus, and low production ability.12,13 Thus, facile, cost-efficient, environmentally friendly, and sustainable synthesis of diverse functional nanomaterials from biomass that are suitable for large-scale production is highly desirable.11,13
Biomass, such as lignocellulose and bacterial cellulose, as a low-cost, easy-to-handle, and nontoxic naturally abundant renewable resource for synthesizing various functional carbon nanomaterials, has been intensively studied and commercially used in recent years.11,14 Compared to other biomasses, SS, as a kind of carbon-rich biomass, has special characteristics owing to its complicated composition and unique physicochemical properties, especially its critical transition metals with various catalytic performances.7,14 Since Kemmer et al. recognized the potential of SS as a feedstock to produce carbon materials and developed the approach to produce adsorbents from SS by its carbonization, numerous studies have investigated the use of sludge as a precursor for synthesizing various carbon nanomaterials as cost-effective adsorbents for pollutant removal.15–17 Details of the current protocols to produce carbon nanomaterials from SS and the effects of various activation conditions on the textural characteristics of the SS-derived carbon nanomaterials, which are beyond the scope of this work, can be found in the excellent reviews by Smith et al. and Hadi et al.7,15
The need to develop more value-added, cost-effective and eco-friendly SS re-use approaches is of particular concern. Together with the discovery and characterization of nanomaterials in SS,18–20 the awareness of the great application potential of SS-based functional nanomaterials (SFNs) has led to a considerable shift in research interest and rapid growth in publications related to both the preparation and applications of these nanomaterials.21–25 The proven thermochemical technique to convert SS into various porous nanomaterials with a range of practical applications has several advantages: (1) it represents a cost-effective, environmentally benign, and value-added approach to the reuse of SS that is facile and suitable for large-scale industrial production; (2) the simultaneous pyrolysis of the complex SS contents improves the quality of the porous structure of the as-synthesized SFNs and the bio-oil generated; (3) synergistically enhanced performances of SS-based nanomaterials are observed.21–25 Thus, the treatment and reuse of SS as value-added functional nanomaterials for pollutant degradation and electrochemical energy storage and conversion have been one of the most popular emerging R&D techniques for sludge re-use.7,26
To date, however, very few comprehensive and critical reviews of the mechanism of creating SFNs, their characteristics, or the functionalization of these nanomaterials for pollutant degradation and electrochemical energy storage and conversion applications are available.13 In particular, a systematic evaluation of the merits and demerits of SFNs, which play a critical role in the application of these nanomaterials and are very different from those of other biomass-based functional materials, is highly desired. Thus, in the present critical review, current progress and innovations in the production of SFNs and their use in various potential applications are summarized, especially in terms of unique SS-enhanced performances. The mechanism of nanomaterial production, the textural properties of the as-prepared SFNs, the tuning of their surface properties and functionality, the potential applications of the as-prepared SFNs, and the future opportunities for expansion and challenges in this emerging research area are discussed. Furthermore, the unique nature and the inevitable drawbacks of SFNs are fully recognized and addressed herein.
2. Development of SFNs
2.1 Characteristic properties of SS
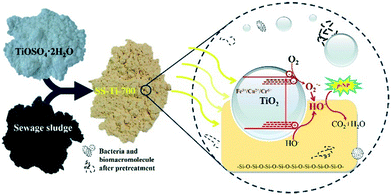
SS, with its content varying from one WWTP to another, is a heterogeneous mixture.2 It consists of various microorganisms, extracellular polymeric substances such as polysaccharides, proteins, and humic substances, biorefractory organics such as fecal material, oils, and plant residues, organic pollutants such as dibenzofurans and polycyclic aromatic hydrocarbons, a wide variety of minerals such as SiO2, microcline, and calcite, mainly from the soil, iron compounds, and heavy metals such as Pb, Cd, Cr, Hg, Cu, Zn, and Ni.2,3,25 Besides the inputs to the WWTP, the composition of SS is also determined by the stabilization and conditioning treatments applied to it. For instance, the different inorganic and organic polymeric coagulants or flocculants widely used in wastewater treatment processes, the decomposition level of the organic materials, the seasonal variation, and the extractive content or the moisture content will all significantly influence the physicochemical properties of SS.26 It is noteworthy that the inputs to the WWTP and the stabilization and conditioning treatments applied to the SS are different from one WWTP to another. Thus, the physical and chemical properties of SS obtained from different WWTPs using the same treatments can vary significantly.26,27Although the composition of SS varies among WWTPs, SS always includes a rich amount of C, relatively medium levels of N, S, P, Si, K, Na, Ca, Mg, and Fe, and a variable content of critical heavy metals.2,3,25 These heavy metals, which are the critical toxic compounds in SS, and minerals all have their own special functions. For instance, Fe, Co, Ni, and Cr can catalyze the partial graphitization of carbon aerogels during the pyrolysis process.28 Doping of TiO2 with these metal ions is one of the most effective and well-known strategies to extend its light absorption into the visible region and maximize its photoactivity.29 An Fe-containing compound has been widely used as a Fenton catalyst,22 CaO and MgO have been demonstrated as candidates for CO2 capture,30 and a Zn and Cu-based catalyst can enhance the methanol synthesis process.31 In addition, SiO2, the special component of SS, is one of the most widely used supports for various applications.32 Thus, given that the characteristic composition of SS differs from those of other biomasses, various special functions are expected for the nanomaterial obtained from SS, either by using it as a support or as an all-in-one precursor with proper resource utilization of all of the various included compounds.33,34
2.2 Design and fabrication of SFNs
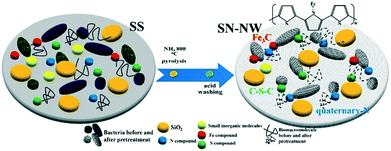
A thermochemical process, mainly pyrolysis of the sludge over a wide temperature range under anoxic conditions, has been widely used to produce functional nanomaterials from SS.7,26,35 The SS pyrolysis process has considerable potential as a promising technology for the disposal of huge amounts of SS.7 It can reduce up to 50% of the SS volume, produce an alternative energy source, and concentrate and stabilize the heavy metals in the pyrolytic residue.15 The pyrolytic residue, a carbonaceous matrix by-product containing various heteroatoms, has gained much popularity as a carbon-based functional nanomaterial for use in many applications such as pollutant control and energy storage and conversion (Table S2†).Compared with lignocellulosic biomass or bacterial cellulose, the physical process and chemical reactions occurring during the thermochemical processes of SS are more complex. Except for the significantly varied nature of SS, the simultaneous pyrolysis of all of these constituents may influence each other and thus have synergetic effects on the products of the obtained functional nanomaterials. For example, the migration and transformation of nitrogen during SS pyrolysis indicate partial reactions between pyrolysis intermediates. The content of the released NH3 can contribute to the etching and doping of carbon frameworks to form N-doped porous carbon material.7 Minerals such as SiO2, microcline or calcite are formed by elements such as K, Ca, Mg, and Fe, which can catalyze certain pyrolysis reactions. Iron compounds can also function as catalysts to increase the degree of graphitization and as the Fe dopant for in situ Fe doping during the SS pyrolysis process.22,33
In addition to the chemical composition of SS, the operational conditions of pyrolysis affect the physicochemical properties and surface functional groups of the obtained functional nanomaterials.7,26,35 These process variables include the pyrolysis temperature, the reaction atmosphere, the acids used for treatment of SS before and after pyrolysis, and the chemicals used for activation. Due to its high moisture content, SS must undergo a drying procedure at relatively low temperature (100–105 °C) or freeze-drying. The obtained dried sludge, as the carbonaceous precursor, is then pyrolyzed at various heating rates and reaction temperatures under anoxic conditions. Additionally, the chemical activation technique, in which the dried sludge is soaked in a dehydrating agent such as KOH, CaO, HNO3, ZnCl2, or K2CO3 and then activated by pyrolysis, offers some additional advantages for the as-synthesized functional nanomaterials including higher carbon yield and larger surface area, but the extra cost of the activating agent, the environmental concerns, and the necessity of extra washing steps are important issues.7,26
Compared to those of lignocellulosic biomass and bacterial cellulose, one of the most distinctive features of SFNs is their high ash content containing various metal oxides and sulfides, SiO2, CaCO3, aluminosilicates, and other materials.7,26,35 During the pyrolysis approach, the inorganic fraction of the SS, including the additional activating agent, can be converted into mineral-like compounds, with some heavy metals being strongly encapsulated into the matrix of the pyrolysis residue. The acid washing step, including the fly-silicon treatment with HF and the HCl/H2SO4/HNO3 washing process, is widely used to lower the inorganic content and enhance the porous structure of the as-prepared SFNs by realizing the partial dissolution of the non-porous inorganic fraction from its frameworks. The enhanced porous structure of the as-prepared SFNs, which can be observed through the dramatically enlarged surface areas, can be attributed to the unblocking of the many pores obscured by the inorganic content and the improvement in the accessibility of the carbon content. The as-obtained SFNs, with their favorable surface area and abundant surface functional groups, can be potentially used in the fields of pollutant degradation and electrochemical energy storage and conversion.22,34,36
In addition to the direct use of SFNs, decorating special metal compounds onto SS can create SS-supported metal compound nanocomposites with multiple functionalities.21,33 The complex SS contents are expected to result in synergistically enhanced performances that SS self-derived nanomaterials or inorganic nanoparticles alone cannot provide (Fig. 1). For example, Yuan et al. have synthesized SS-supported iron oxide and TiO2 with this direct one-step synthetic approach. In the as-synthesized SFNs, nanosized iron oxide and TiO2 were dispersed on the surface of the mesoporous templates derived from the SS.21,33 The decoration of metal compounds onto SS-derived carbon-based SFNs after pyrolysis has also been performed. Zhuang and co-workers reported the ZnCl2-activated SS-derived carbon-supported Fe and Mn oxides catalysts.37 Athalathil and co-workers reported the fabrication of efficient sludge carbon/TiO2 nanocomposites using different methods.38 In general, the one-step synthetic approach would be more favorable due to its facile synthesis method, easy-to-handle process, and suitability for scale-up. However, the additional metal compounds may have special catalytic effects during the SS pyrolysis process and thus can improve the quality of the porous structure of the as-synthesized SFNs and the bio-oil generated.
Specifically, the coexistence of diverse materials in SS has a significant effect on the improved performance of these as-synthesized SFNs.21–25 For the Fenton-like catalytic and TiO2 photocatalytic applications, the co-catalyst effect of SiO2 and Al2O3 was observed, and the photocatalytic activity under visible light attributed to specific small inorganic molecular or metal oxides in the SS was confirmed.21,33 For the energy storage applications, the organic matter in SS can work as a natural dopant and ideal precursor for the synthesis of multi-doped porous carbon-based SFNs, which not only improve the electrical conductivity of the electrodes but also induce additional pseudocapacitance. Then, for the oxygen reduction reaction (ORR) electrocatalyst, the good ORR catalytic activity of the as-prepared SFNs is likely attributable to the synergistic effects of the presence of N, P, and N-coordinated Fe groups originating from the SS.22,23,34 Thus, SS provides a unique but effective precursor for the development of a new generation of functional materials (Table S2†).
3. Application of diverse SFNs
3.1 Pollutant degradation applications
In addition to their use as adsorbents for H2S, NOx, heavy metal ions, and organic pollutants, which has been excellently reviewed by Smith et al. and Hadi et al.,7,15 the application of SFNs as efficient catalysts for pollutant degradation in wastewater treatment has recently been widely reported (Table S2†).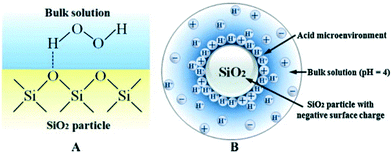 | ||
| Fig. 2 Synergistic co-catalyst effect of SiO2 due to the formation of hydrogen bonds between H2O2 and Si–O–Si (A) and the acidic micro-environment near the SiO2 surface (B) (reprinted from ref. 24). | ||
Yuan et al. synthesized an alternative SFN via a facile one-step calcination method at 350 °C for 3 h and demonstrated its efficiency and stability as a heterogeneous photo-Fenton catalyst under conditions of both ultraviolet and visible irradiation (Fig. 3).21 All of these chemicals usually present in SS were appropriately used for the synthesis of this SFN. The porous structure of the catalyst was attributed to the evaporation of the adsorbed H2O, combustion or carbonization of the small organic molecules and biomacromolecules in the SS during the calcination process. The Si–O–Fe bond between the loaded Fe compound and the SiO2 components of SS ensured the absence of Fe leaching during the heterogeneous photo-Fenton reaction and the stability of the catalysts for reuse. In particular, the synergistic effect between the specific small inorganic molecular or metal oxides in the SS, which were excited by visible light, and the loaded Fe compound via one-electron transfer could initiate the degradation of various organic pollutants under visible light irradiation conditions. The electron spin resonance spectra and rhodamine B and p-nitrophenol degradation reactions under both ultraviolet and visible light irradiation supported the inference and suggested the efficient use of rich and natural solar energy to drive the heterogeneous photo-Fenton reaction due to this synergistic effect.
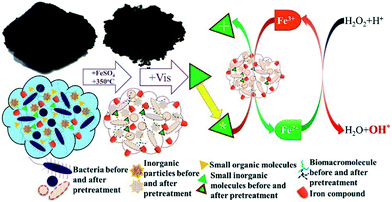 | ||
| Fig. 3 Schematic diagram of the as-prepared SFN catalyst and the mechanism of the photo-Fenton reaction under conditions of visible light irradiation (reprinted from ref. 21). | ||
In addition to the iron(II) salt, the iron sludge can act as the iron source for the synthesis of sludge carbon-supported iron oxide for the heterogeneous electro-Fenton process. Hou et al. reported the preparation of this SFN from iron sludge and SS via a facile approach. Such SFN can serve as a catalytic particle electrode in the 3D electro-Fenton process for the treatment of biologically pretreated coal gasification wastewater.39 Compared to Fe3O4 magnetic nanoparticles, this SFN exhibited a larger absorptivity of 101.1 mg g−1 and higher chemical oxygen demand, total phenols, and total organic carbon (TOC) removal efficiencies of 78.1%, 93.5%, and 65.5%, respectively. Si–O–Fe bonds between the loaded Fe compound from the iron sludge and SiO2 in the SS were detected. The unique Fe species embedded in the carbon matrix structure of this SFN was responsible for the favorable stability and superior catalytic activity by generating more H2O2 and HO*. A co-Fenton-like effect of SiO2 and Al2O3 was also observed when SS carbon prepared by pyrolysis at 800 °C was used as microparticle electrodes in the electrochemical oxidation of acid orange 7.40
Fe species are an inherent component of SS. A large amount of Fe was observed in SS and SS-based activated carbons.21,41 Therefore, SS-derived porous carbon prepared by carbonization and activation without the addition of an extra iron source was also applied to the adsorption and degradation of various organic pollutants. Compared with the SS-supported iron oxide obtained by the facile one-step method, a more complex synthesis process can be adopted to produce SS-derived SFN with different textural properties observed.
A magnetic SFN containing Fe3O4 was synthesized by pretreatment of SS with a physicochemical activation and pyrolysis process, including impregnation into 0.5 M KOH solution and microwave digestion in HNO3 solutions at pH 1, followed by pyrolysis in a N2 atmosphere (Fig. 4).41 The resulting SFN carbonized at 600 °C had a higher Brunauer–Emmett–Teller (BET) surface area (407.7 m2 g−1), higher pore volume (0.504 ml g−1), and a porous structure superior to those of char carbonized at 800 and 1000 °C. It also achieved a 96.6% removal efficiency of Fenton-like naphthalene dye intermediate 1,2,4-acid oxidation, higher than those of the char carbonized at 800 and 1000 °C and commercial Fe3O4 magnetic nanoparticles, which were 67.5%, 38.9%, and 57.5%, respectively. An additional acid washing process with 1 M HCl and 1 M HF was accompanied by the removal of inorganic salts such as silica and alumina, an increase in the BET surface area and carbon content, and a great decrease in the efficiency of naphthalene dye intermediate 1,2,4-acid removal. These results further confirmed the synergistic catalytic effect of Fe, C, SiO2, and Al2O3, which are the special and inherent components of SS and exist in large quantities in SFNs.42 The Al2O3 and iron species provide the catalytic centres for Haber–Weiss reactions, whereas the SiO2 associated with carbon particles contributes to the concentration of H2O2 and organic pollutants on the surface of Fe oxide and the dispersion of the catalytic centres. In addition, the SiO2 along with the carbon matrix can limit the outside effect of strong oxidants like OH*, acting as a stabilizer of the Fe3O4 particles. Furthermore, the large magnetic hysteresis loop (8.7 emu g−1) make it favourable for application in solid–liquid separation.
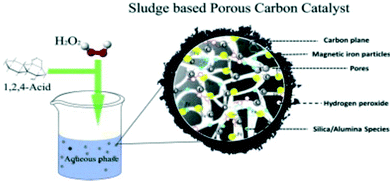 | ||
| Fig. 4 Preparation of SS-derived magnetic carbon-based SFN (reprinted from ref. 41). | ||
By tuning the surface functionality of the SS-derived carbonaceous catalyst through pretreatment with HNO3 and H2SO4, the products exhibited improved catalytic activity in a Fenton-like m-cresol wet peroxide oxidation process.43 After treatment with an oxidizing acid, the SS-derived carbonaceous catalyst presented a larger adsorption capacity and formed a –COOH on the carbon that could react with –OH. Therefore, it was easy for m-cresol to form esters on the carbonaceous surface of this SFN. The co-existence of SiO2 could lead to the absorption of H2O2 by hydrogen bonding with the oxygen in Si–O–Si bridges, and the iron supported on the SFN surface was beneficial in enhancing the Fenton-like wet peroxide oxidation reaction (Fig. 5). Esters were oxidized and m-cresol was degraded after the oxidation reaction. Then, the surface functional groups of the H2SO4-pretreated SS-derived carbonaceous catalyst were transformed into –COOH and Si–O–Si bridges again. The conversion of m-cresol reached 100%, after 3 h of reaction at an initial pH of 7 over a H2SO4-pretreated SS-derived carbonaceous catalyst. Thus, the enhanced catalytic activity of the SS-derived carbonaceous catalyst was attributed to the synergistic co-catalytic effect of the surface-modified groups after oxyacid treatment with the co-catalytic effect of the inherent component of the SS, Fe3+, and SiO2.
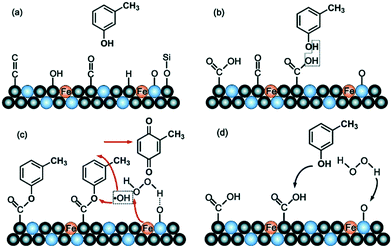 | ||
| Fig. 5 Possible reaction mechanism on the surface of the HNO3-pretreated SFN for the oxidation of m-cresol in the wet peroxide oxidation reaction: the surface of the as-prepared SFN catalyst before HNO3 pretreatment (a), the surface of the as-prepared SFN catalyst (b), esterification and catalytic wet peroxide oxidation degradation of m-cresol on the SFN surface (c), and the catalytic cycle in the wet peroxide oxidation reaction using the SFN catalyst (d). *Black balls, blue balls, and yellow balls represent C, Si, and Fe atoms, respectively (reprinted from ref. 43). | ||
Similar results were also reported by Stüber et al. and Yu et al.45,46 The hardened SS-based carbons produced using a lignosulphonate binder were found to be the most stable catalyst when used in a trickle bed reactor. In particular, the SS-based carbons treated with oxidizing acids such as H2SO4 and HNO3 showed favorable catalytic activity in the wet air oxidation of m-cresol attributed to the synergistic effect of the high iron content and surface functional groups mentioned above. The conversion of m-cresol reached 99.0% with the HNO3-pretreated SS-derived carbonaceous catalyst after 90 min at 160 °C and 0.66 MPa oxygen. Tu et al. reported the preparation of a SS-supported iron oxide-based SFN and its successful use in the catalytic wet air oxidation of 2-chlorophenol. In this system, the iron leaching phenomenon was minimized by using an acetate buffer while keeping its catalytic activity.47 Compared with the well-known, classic, highly efficient noble metal catalyst Ru/ZrO2, the SS-derived carbon-supported iron oxide catalyst displayed higher catalytic activity under identical reaction conditions.
 | ||
| Fig. 6 Schematic diagram of the as-prepared SS-supported TiO2 photocatalyst and the mechanism of the photocatalytic reaction under conditions of visible light irradiation (reprinted from ref. 33). | ||
Athalathil et al. investigated various surface modification processes for improving the photocatalytic efficiency of sludge carbon/TiO2 nanocomposites.38 The SFN obtained by a hydrothermal deposition process exhibited the highest photocatalytic rate compared to those achieved using chemical treatment and the sol–gel process, and a better rate of bisphenol A removal in the catalytic wet air oxidation and catalytic ozonation reaction was also observed with the sludge carbon/TiO2 nanocomposites.
3.2 Electrochemical energy storage and conversion applications
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge/discharge cycles. The unique electrochemical performance of this SFN was attributed to the pseudocapacitive contribution of the particular heteroatom doping compositions of SS and the proper hierarchical porous structure formed during the synthesis process. As mentioned in section 2.2, SiO2, the special component of SS, was used as the template, whereas the organic matter worked as the ideal dopant and natural precursor.
000 charge/discharge cycles. The unique electrochemical performance of this SFN was attributed to the pseudocapacitive contribution of the particular heteroatom doping compositions of SS and the proper hierarchical porous structure formed during the synthesis process. As mentioned in section 2.2, SiO2, the special component of SS, was used as the template, whereas the organic matter worked as the ideal dopant and natural precursor.
Furthermore, by impregnation into 30% HF solution and KOH activation, a 3D honeycomb-like carbon-based SFN can be obtained by using high ash content SS as a precursor (Fig. 7).36 The acid washing and alkali activation process resulted in effective leaching out of the ash content, an enhancement in porosity, an increase in the specific BET surface area (2839 m2 g−1), and the improvement of electrochemical performance. The resulting 3D carbon material exhibited a high specific charge storage capacitance of 379 F g−1 in 1 M Na2SO4 at a current density of 0.5 A g−1. It also exhibited excellent long-term stability of over 90% capacitance retention after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge/discharge cycles at a high current density of 20 A g−1. The superior electrochemical performance of the SFN can be attributed to the hierarchically interconnected honeycomb-like framework, which is suitable for high-power and long-life supercapacitors. The enhanced supercapacitive behavior of the as-assembled symmetric supercapacitor is comparable to those of other biomass-derived carbon materials. Thus, it is suitable for use in high-performance supercapacitor electrode materials.
000 charge/discharge cycles at a high current density of 20 A g−1. The superior electrochemical performance of the SFN can be attributed to the hierarchically interconnected honeycomb-like framework, which is suitable for high-power and long-life supercapacitors. The enhanced supercapacitive behavior of the as-assembled symmetric supercapacitor is comparable to those of other biomass-derived carbon materials. Thus, it is suitable for use in high-performance supercapacitor electrode materials.
 | ||
| Fig. 7 Schematic of the synthesis process of 3D honeycomb-like carbon-based SFN (HSC) and photographs of SS (a), sludge-derived carbon (SC) (b), fly-silicon treatment SC (FSC) (c), and the as-prepared 3D honeycomb-like carbon-based SFN (HSC) (d) (reprinted from ref. 36). | ||
To increase the C content of the SFN, SS was evenly mixed with coconut shells and then converted into a SS-derived SFN by pyrolysis.23 A maximum power density of 969 ± 28 mW m−2 was obtained in a microbial fuel cell with an anode and a cathode made from the as-synthesized carbon nanomaterials, which was around 2.4 times that of a microbial fuel cell with a Pt cathode and a graphite anode. The excellent performance of the as-synthesized SFN electrode could be attributed to the enhanced electrical conductivity of the SFN by modification with the coconut shells. The ORR catalytic activity of the as-prepared SFN was likely attributable to the combined effects of the conductivity and the presence of N, P, and the N-coordinated Fe groups doped into the carbon framework, which greatly enhanced the catalytic activity.
Furthermore, by pyrolysis of SS under NH3 conditions, Yuan et al. constructed an efficient and stable N, Fe, and S multi-doped SS-derived bi-functional electrocatalyst (Fig. 8).22 The as-prepared SFN showed excellent ORR catalytic activities in both acidic and alkaline media that were comparable with those of benchmark commercial Pt/C(20%). The as-prepared SFN also exhibited favorable oxygen evolution reaction performance with good stability. The enhanced electrocatalytic performance of this SFN likely originated from the synergistic effects of the hierarchically porous structure facilitating the reactant and electron transport, and the multi-doped heteroatoms, especially the enhanced doped N content with the NH3 atmosphere and the intrinsic N content in SS as double N dopants.
 | ||
| Fig. 8 Schematic diagram of the synthesis of the SS-derived electrocatalyst produced by green and facile one-step pyrolysis of sewage sludge under NH3 conditions (reprinted from ref. 22). | ||
4. Conclusions and prospects
To address the continuously increasing amounts of SS produced annually and the loss of traditional disposal routes, a series of alternative uses for SS has been developed for the construction of functional nanomaterials through simple, low-cost, and sustainable methods.7–9 This review shows that SS is a highly promising precursor for the production of various functional nanomaterials for applications in the field of pollutant degradation and electrochemical energy storage and conversion (Table S2†). With synergistically enhanced performances between the SS-based carbon and the specific small inorganic molecular or metal oxides in the SS, SFNs represent a dramatic and attractive alternative to existing functional nanomaterials and SS reuse routes.21–25 Furthermore, the use of different functionalization methods, such as chemical activation, acid washing, and decoration of nanoparticles, can improve the capabilities of the synthesized nanomaterials, exhibiting great application potential and making them compete with other biomass-based functional nanomaterials in many fields. Thus, the cost-effective and environmentally benign use of SS, which has the advantage of exceptional structural and compositional traits, can open new avenues for the construction of various highly efficient functional nanomaterials.Despite all of the above successes (Table S2†), it should be noted that research into the design and application of SFNs has occurred over a rather short period of time. A number of challenges and questions still remain before the widespread implementation of these materials and the extension of their practical applications can be realized.
First, the composition of the SS has an important influence on the make-up, surface functionality, and porosity of the as-synthesized SFNs, and sludge from different WWTPs will vary in water, inorganic and organic contents, and composition.26,27 It can be fairly criticized that the effects of these complex contents of SS on the textural properties and the performance of the as-synthesized SFNs should be systematically studied. More effort should be focused on the internal relationship between the contents of SS, the synthesis process, and the textural characterization and performance of the as-synthesized SFNs. For example, the elucidation of the catalytic mechanism of these inherent heavy metals in SS during the pyrolysis process would be very helpful in developing a more effective process for tuning the textural properties of the obtained functional nanomaterials. Based on these results, it is anticipated that functional nanomaterials with diverse textural property designs and repeatable performance can be obtained from SS with corresponding compositions.
Second, SS contains several materials that could be recovered for more eco-friendly, cost-effective, and value-added use. For example, a portion of the volatile organic matter, which was lost to evaporation, combustion, or carbonization during the synthesis process, could be alternatively converted into biogas in advance by anaerobic digestion.9,52 Attempts have been made to recover phosphorus, a scarce and expensive element, from SS.22,53 Thus, it is suggested that combined treatment attempts together with pyrolysis would lead to more thorough use of SS on the premise that the effectiveness of the SFNs should not be affected.
Third, intensified research must be devoted to salient synthesis design, strategies, and theoretical advances to improve the performance of these functional materials and expand their application in new fields by controlling suitable constituents and designing unique structures. For example, heavy metals, the inherent and critical component in SS, also exhibit high catalytic activities in various chemical reactions, such as organic syntheses, CO2 capture, and catalytic reforming.30,31 Therefore, SS with special heavy metals can be converted into corresponding functional nanomaterials for use as alternative catalyst sources.
Finally, the production of other products including liquid (bio-oil) and noncondensable gaseous (pygas) by-products during SS pyrolysis should also be taken into account.16 Factors such as sludge properties and pyrolysis conditions, which affect the textural properties of the functional nanomaterials, will also affect the composition profile of the produced bio-oil and pygas. Therefore, in addition to focusing on the effectiveness of the as-synthesized functional nanomaterials, more attention should be paid to opportunities for the end use of the bio-oil and pygas produced and the efficiency of energy recovery of the pyrolysis process.
Acknowledgements
The authors wish to thank the National Natural Science Foundation of China (51538008; 51308401; 51678426), the National Key Technology Support Program (2013BAC16B03), and the Key Program for International S&T Cooperation Projects of China (2014DFA91650) for their partial support to this study.Notes and references
- S. J. Ren, Environ. Int., 2004, 30, 1151–1164 CrossRef CAS PubMed.
- J. Walker, L. Knight and L. Stein, U.S. Envtl. Protection Agency, 1994 Search PubMed.
- M. Gong, W. Zhu, Z. R. Xu, H. W. Zhang and H. P. Yang, Renewable Energy, 2014, 66, 605–611 CrossRef CAS.
- Y. Suh and P. Rousseaux, Resour., Conserv. Recycl., 2002, 35, 191–200 CrossRef.
- D. Fytili and A. Zabaniotou, Renewable Sustainable Energy Rev., 2008, 12, 116–140 CrossRef CAS.
- E. Viau, K. Bibby, T. Paez-Rubio and J. Peccia, Environ. Sci. Technol., 2011, 45, 5459–5469 CrossRef CAS PubMed.
- K. M. Smith, G. D. Fowler, S. Pullket and N. J. D. Graham, Water Res., 2009, 43, 2569–2594 CrossRef CAS PubMed.
- A. Kelessidis and A. S. Stasinakis, Waste Manage., 2012, 32, 1186–1195 CrossRef CAS PubMed.
- N. N. Duan, B. Dong, B. Wu and X. H. Dai, Bioresour. Technol., 2012, 104, 150–156 CrossRef CAS PubMed.
- R. S. Varma, Green Chem., 2014, 16, 2027–2041 RSC.
- W. J. Liu, H. Jiang and H. Q. Yu, Chem. Rev., 2015, 115, 12251–12285 CrossRef CAS PubMed.
- N. Linares, A. M. Silvestre-Albero, E. Serrano, J. Silvestre-Albero and J. Garcia-Martinez, Chem. Soc. Rev., 2014, 43, 7681–7717 RSC.
- V. K. Sharma, J. Filip, R. Zboril and R. S. Varma, Chem. Soc. Rev., 2015, 44, 8410–8423 RSC.
- C.-H. Zhou, X. Xia, C.-X. Lin, D.-S. Tong and J. Beltramini, Chem. Soc. Rev., 2011, 40, 5588–5617 RSC.
- P. Hadi, M. Xu, C. Ning, C. S. K. Lin and G. McKay, Chem. Eng. J., 2015, 260, 895–906 CrossRef CAS.
- F. N. Kemmer, S. R. Robertson and R. D. Mattix, Sewage Treatment Process Nalco Chemical Company, US Patent Office, Patent No. 3619420, 1971.
- J. M. Beeckmans and P. C. Ng, Environ. Sci. Technol., 1971, 5, 69–71 CrossRef CAS.
- B. Kim, C. S. Park, M. Murayama and M. F. Hochella, Environ. Sci. Technol., 2010, 44, 7509–7514 CrossRef CAS PubMed.
- E. Lombi, E. Donner, E. Tavakkoli, T. W. Turney, R. Naidu, B. W. Miller and K. G. Scheckel, Environ. Sci. Technol., 2012, 46, 9089–9096 CrossRef CAS PubMed.
- C. Meier, A. Voegelin, A. Pradas del Real, G. Sarret, C. R. Mueller and R. Kaegi, Environ. Sci. Technol., 2016, 50, 3503–3510 CrossRef CAS PubMed.
- S. J. Yuan and X. H. Dai, Appl. Catal., B, 2014, 154, 252–258 CrossRef.
- S. J. Yuan and X. H. Dai, Green Chem., 2016, 18, 4004–4011 RSC.
- Y. Yuan, T. Liu, P. Fu, J. Tang and S. Zhou, J. Mater. Chem. A, 2015, 3, 8475–8482 CAS.
- Y. Tu, S. Tian, L. Kong and Y. Xiong, Chem. Eng. J., 2012, 185-186, 44–51 CrossRef CAS.
- G. Ahlberg, O. Gustafsson and P. Wedel, Environ. Pollut., 2006, 144, 545–553 CrossRef CAS PubMed.
- I. Fonts, G. Gea, M. Azuara, J. Ábrego and J. Arauzo, Renewable Sustainable Energy Rev., 2012, 16, 2781–2805 CrossRef CAS.
- I. Fonts, M. Azuara, G. Gea and M. B. Murillo, J. Anal. Appl. Pyrolysis, 2009, 85, 184–191 CrossRef CAS.
- F. J. Maldonado-Hódar, C. Moreno-Castilla, J. Rivera-Utrilla, Y. Hanzawa and Y. Yamada, Langmuir, 2000, 16, 4367–4373 CrossRef.
- E. P. Reddy, B. Sun and P. G. Smirniotis, J. Phys. Chem. B, 2004, 108, 17198–17205 CrossRef CAS.
- B. Feng, H. An and E. Tan, Energy Fuels, 2007, 21, 426–434 CrossRef CAS.
- M. Behrens, S. Zander, P. Kurr, N. Jacobsen, J. Senker, G. Koch, T. Ressler, R. W. Fischer and R. Schlögl, J. Am. Chem. Soc., 2013, 135, 6061–6068 CrossRef CAS PubMed.
- A. L. T. Pham, C. Lee, F. M. Doyle and D. L. Sedlak, Environ. Sci. Technol., 2009, 43, 8930–8935 CrossRef CAS PubMed.
- S. J. Yuan, X. W. Li and X. H. Dai, RSC Adv., 2014, 4, 61036–61044 RSC.
- Y. Yuan, T. Yuan, D. M. Wang, J. H. Tang and S. G. Zhou, Bioresour. Technol., 2013, 144, 115–120 CrossRef CAS PubMed.
- K. Tian, W. J. Liu, T. T. Qian, H. Jiang and H. Q. Yu, Environ. Sci. Technol., 2014, 48, 10888–10896 CrossRef CAS PubMed.
- H. Feng, M. Zheng, H. Dong, Y. Xiao, H. Hu, Z. Sun, C. Long, Y. Cai, X. Zhao, H. Zhang, B. Lei and Y. Liu, J. Mater. Chem. A, 2015, 3, 15225–15234 CAS.
- H. Zhuang, H. Han, B. Hou, S. Jia and Q. Zhao, Bioresour. Technol., 2014, 166, 178–186 CrossRef CAS PubMed.
- S. Athalathil, B. Erjavec, R. Kaplan, F. Stüber, B. Christophe, F. Josep, F. Agusti, P. Albin and F. Azael, J. Hazard. Mater., 2015, 300, 406–414 CrossRef CAS PubMed.
- B. Hou, H. Han, S. Jia, H. Zhuang, P. Xu and K. Li, J. Taiwan Inst. Chem. Eng., 2016, 60, 352–360 CrossRef CAS.
- H. Sun, T. Chen, L. Kong, Q. Cai, Y. Xiong and S. H. Tian, Ind. Eng. Chem. Res., 2015, 54, 5468–5474 CrossRef CAS.
- L. Gu, N. Zhu, H. Guo, S. Huang, Z. Lou and H. Yuan, J. Hazard. Mater., 2013, 246-247, 145–153 CrossRef CAS PubMed.
- L. Gu, N. W. Zhu and P. Zhou, Bioresour. Technol., 2014, 118, 638–642 CrossRef PubMed.
- Y. Yu, H. Z. Wei, L. Yu, T. Zhang, S. Wang, X. N. Li, J. H. Wang and C. L. Sun, RSC Adv., 2015, 5, 41867–41876 RSC.
- R. R. N. Marques, F. Stüber, K. M. Smith, A. Fabregat, C. Bengoa, J. Font, A. Fortuny, S. Pullket, G. D. Fowler and N. J. D. Graham, Appl. Catal., B, 2011, 101, 306–316 CrossRef CAS.
- F. Stüber, K. M. Smith, M. B. Mendoza, R. R. N. Marques, A. Fabregat, C. Bengoa, J. Font, A. Fortuny, S. Pullket, G. D. Fowler and N. J. D. Graham, Appl. Catal., B, 2011, 110, 81–89 CrossRef.
- Y. Yu, H. Wei, L. Yu, B. Gu, X. Li, X. Rong, Y. Zhao, L. Chen and C. Sun, Catal. Sci. Technol., 2016, 6, 1085–1093 CAS.
- Y. Tu, Y. Xiong, S. Tian, L. Kong and C. Descorme, J. Hazard. Mater., 2014, 276, 88–96 CrossRef CAS PubMed.
- G. Wen, Z. H. Pan, J. Ma, Z. Q. Liu, L. Zhao and J. J. Li, J. Hazard. Mater., 2012, 239-240, 381–388 CrossRef CAS PubMed.
- H. Zhuang, H. Han, B. Hou, S. Jia and Q. Zhao, Bioresour. Technol., 2014, 166, 178–186 CrossRef CAS PubMed.
- S. J. Yuan and X. H. Dai, RSC Adv., 2015, 5, 45827–45835 RSC.
- S. J. Yuan and X. H. Dai, Sci. Rep., 2016, 6, 27570 CrossRef CAS PubMed.
- S. J. Yuan, N. H. Liao, B. Dong and X. H. Dai, Cuihua Xuebao, 2016, 37, 735–742 CAS.
- C. Blöcher, C. Niewersch and T. Melin, Water Res., 2012, 46, 2009–2019 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6en00177g |
| This journal is © The Royal Society of Chemistry 2017 |