Open Access Article
Open Access ArticleIndigenous 14C-phenanthrene biodegradation in “pristine” woodland and grassland soils from Norway and the United Kingdom
Uchechukwu V.
Okere
a,
Jasmin K.
Schuster
b,
Uchenna O.
Ogbonnaya
c,
Kevin C.
Jones
d and
Kirk T.
Semple
 *d
*d
aUniversity of Derby, UK
bEnvironment Canada, Canada
cFederal University Oye-Ekiti, Nigeria
dLancaster University, UK. E-mail: k.semple@lancaster.ac.uk
First published on 4th October 2017
Abstract
In this study, the indigenous microbial mineralisation of 14C-phenanthrene in seven background soils (four from Norwegian woodland and three from the UK (two grasslands and one woodland)) was investigated. ∑PAHs ranged from 16.39 to 285.54 ng g−1 dw soil. Lag phases (time before 14C-phenanthrene mineralisation reached 5%) were longer in all of the Norwegian soils and correlated positively with TOC, but negatively with ∑PAHs and phenanthrene degraders for all soils. 14C-phenanthrene mineralisation in the soils varied due to physicochemical properties. The results show that indigenous microorganisms can adapt to 14C-phenanthrene mineralisation following diffuse PAH contamination. Considering the potential of soil as a secondary PAH source, these findings highlight the important role of indigenous microflora in the processing of PAHs in the environment.
Environmental significanceHistorical and on-going industrial activities have resulted in the release of polycyclic aromatic hydrocarbons (PAHs) into the environment. As a result of the physicochemical properties of PAHs, soils serve as a major sink for these organic contaminants, even in soils which are remote from point sources. It is well known that microorganisms can evolve to degrade organic contaminants in polluted soils; however, what is less well known is whether microorganisms can degrade PAHs at much lower concentrations in ‘remote’ soils. PAH biodegradation patterns were investigated in soils along a transect from the UK to northern Norway. Although biodegradation was measured in all of the soils, the PAH concentration and organic matter content were found to be linked to the rates and extents of biodegradation. |
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are an important group of organic contaminants in the environment. Concerns over their chronic toxicity (mutagenicity and carcinogenicity), environmental persistence and ubiquitous presence in the environment have driven research interest into their sources and processing in the environment.1,2 Most spatial distributions of PAHs in soils vary in scale and occur through diffuse pollution,3–6 owing to the degree of remote economic development, population density, landscape, vegetation and land use.7,8 This results in the contamination of large areas with hotspots having high PAH concentrations and is characterised by the absence of identifiable point sources.9 Understanding soil–PAH interactions is important because soils can store up to 90% of environmental PAHs and act as a secondary PAH source to the environment.10,11 Interactions between PAHs and soil matrices are governed by several factors, including soil type (mineral and organic matter content), PAH properties (aqueous solubility, polarity, hydrophobicity, lipophilicity and molecular structure), aging and environmental factors (e.g. temperature and precipitation).12,13 This can result in sequestration within the soil fractions (mineral or organic), transfer to soil organisms or loss due to leaching to groundwater, volatilisation to air, or microbial degradation.14–16Microbial degradation is widely accepted as the most important means of loss of PAHs from soil.17–19 The size of the indigenous microflora that possesses the necessary genetic capacity for PAH degradation is a principal factor in the microbial degradation of PAHs in soil.12,16 Though the processes on which the evolution of PAH catabolic activity in soil depends are not fully understood, the presence of naturally occurring organic compounds with structures similar to PAHs and the presence of PAHs and PAH concentration levels in soil are believed to be important.20–23 Since PAHs are ubiquitous soil contaminants, microorganisms capable of degrading PAHs have been found in both contaminated and “pristine” soils from different climates.22,24,25
It is currently believed that the principal control on PAH levels in the environment is transiting from primary to secondary sources. The need for further research into the role of microorganisms and biodegradation as major PAH processors has therefore been expressed.26–28 Studying PAH biodegradation in background soils from areas remote from PAH point sources holds the potential for shedding more light on the degradative capacity of indigenous microbial population not previously exposed directly to high levels of contamination.29 Therefore, this study aimed to investigate the indigenous biodegradation of 14C-phenanthrene (radio-labelled) in remote woodland and grassland soils from Norway and the United Kingdom (UK) not directly impacted by PAHs. Phenanthrene was used as it uniquely exhibits both recalcitrant and biodegradable properties of PAHs as well as being commonly found in the soils studied. Soils from Norway and the UK provide interesting samples for study because of their differences in human population (and therefore activity) and histories of industrialisation. While the UK became industrialised in the 19th century due to coal burning which continued until the 20th century, Norway is a comparatively less populated and pristine country.30–32
Materials and methods
Materials
Phenanthrene (>99.6%) and [9-14C] phenanthrene (specific activity = 50 mCi mmol−1, radiochemical purity >95%) standards were obtained from Sigma Aldrich, UK. Chemicals for the minimal basal salt (MBS) solution were obtained from BDH Laboratory Supplies and Fisher Chemicals. The liquid scintillation cocktail (Ultima Gold) and 7 ml glass scintillation vials were obtained from Canberra Packard, UK. Sodium hydroxide was obtained from Sigma Aldrich, UK. Sodium sulphate was supplied by VWR International. Dichloromethane (DCM) was supplied by Rathburn Chemicals, UK. Acetonitrile was supplied by Fisher Scientific. Agar-agar and plate count agar were obtained from Oxoid Ltd., UK.Soil sampling and characterisation
Soil samples were chosen from soils previously studied to investigate the latitudinal distribution, fractionation, cold condensation, and “hopping” of different classes of persistent organic pollutants (POPs) including polychlorinated biphenyls (PCBs), polychlorinated dibenzo-p-dioxins (PCDDs) and PAHs.32,33Fig. 1 shows the sampling spots on a map; soils 1–4 are Norwegian and 5–7 are UK soils. Sampling methods and sample information have been previously published.32–35 In summary, the soil samples were taken from rural/remote sites at 0–10 cm depth. Individual samples were collected with the aid of a hand-held corer that was cleaned before and after collecting each sample.33 The samples were wrapped in two layers of aluminium foil, sealed in two plastic bags, and stored in a cool box for transportation to Lancaster University. Upon arrival at Lancaster University, the samples were immediately transferred to a freezer where they were stored at −20 °C.All the Norwegian soils (1–4) used in this study are woodland soils. Two UK soils (5 and 7) are grassland soils and one (soil 6) is woodland soil. Total carbon was determined by analysing 4 mg of oven dried (105 °C) and sieved (2 mm) soil samples on a Carlo Erba CHNS-OEA 1108 CN-Elemental analyser. For total organic carbon (TOC) analysis, soils were heated to 430 °C to remove all organic carbon; the ash containing inorganic carbon alone was measured on the analyser and the TOC determined by mass balance.36
Analysis of PAH concentrations in the soils
The soil water content was measured by oven drying after the soil samples were thawed. Soil (10–40 g) (depending on the water content and density of the individual samples) was mixed with anhydrous sodium sulphate and then extracted with dichloromethane (DCM) for 16 h by Soxhlet extraction. Clean-up was performed on a silica and alumina gravity fed chromatography column. After clean-up, the solvent was evaporated and exchanged to acetonitrile. Six PAH standards at concentrations from 0.01 to 0.4 ng μl−1 were prepared. Separation was achieved on a ChromSpher5 150 × 4.6 mm HPLC column with 5 μm particle size (Varian, UK). Quantification was performed by an external calibration method.37Quality control
For every twelve samples, a laboratory blank and a duplicate sample were incorporated into the analytical procedure. Method detection limits were derived from the blanks and quantified as three times the standard deviation of the mean concentration of the blanks. The method detection limit ranged from 0.1 to 17 ng ml−1 for the 12 PAHs. The recovery efficiency was determined by analyses of blanks, spiked with 80 ng of each PAH before extraction which was 95%.Catabolism of 14C-phenanthrene in soil
The mineralisation of 14C-phenanthrene was determined in 250 ml screw-cap Erlenmeyer flasks (respirometers) using methods described by Reid et al. (2001), Doick and Semple (2003), and Semple et al. (2006).38–40 The respirometers contained 10 g of soil (spiked with 50 mg kg−1 unlabelled and 14C-phenanthrene [80 Bq 14C-phenanthrene g−1 soil]) using toluene as a carrier solvent in 30 ml mineral basal salt (MBS) medium to make a slurry.40 The respirometers were fastened on a SANYO® Gallenkamp orbital incubator which was set at 100 rpm to agitate and ensure adequate mixing over the 32-day incubation period (Norwegian soils) stored in the dark at 22 °C (ref. 36 and 40), and the contents were analysed using a Packard Canberra Tri-Carb 2250CA liquid scintillation counter.36 Lag phases were measured as the time before 14C-phenanthrene mineralisation reached 5%. The fastest rates of mineralisation (%) were measured as the highest rate of mineralisation per day divided by 24 hours to obtain per hour, whilst the extents of mineralisation were measured as the total amount of 14C-phenanthrene mineralised after 14 days (the UK) and 32 days (Norway) when the 14CO2 curve plateaued. Analytical blanks containing no 14C-phenanthrene were used for the determination of the levels of background radioactivity.In order to attain a standard rate constant and half-life for the mineralisation of 14C-phenanthrene in the soils, the residual PAH percentages were calculated by subtracting the mineralised fraction (%) from 100%. The mineralisation data collected were fitted into the first-order kinetic model, S = S0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp (−kt), t1/2 = 0.693/k, where S0 is the substrate fraction at the beginning of the experiment prior to mineralisation and S corresponds to the amount of 14C-phenanthrene remaining in the soil after mineralisation at time t (d) and k the degradation rate constant.
exp (−kt), t1/2 = 0.693/k, where S0 is the substrate fraction at the beginning of the experiment prior to mineralisation and S corresponds to the amount of 14C-phenanthrene remaining in the soil after mineralisation at time t (d) and k the degradation rate constant.
Enumeration of soil bacteria
Colony forming units (CFUs) of heterotrophic bacteria and 14C-phenanthrene degrading bacteria were enumerated on plate count agar using a viable plate count technique.4112C-phenanthrene was used as a sole carbon source on plate count agar for the measurement of 14C-phenanthrene degrading bacteria following standard microbiological techniques.Statistical analysis
The levels of 14C-phenanthrene detected by the liquid scintillation counter were corrected for background radioactivity. All samples were analysed in triplicate and error bars presented in graphs are standard error mean for n = 3. The SIGMA STAT version 3.5 software package was used for statistical analysis of data. The significance of 14C-phenanthrene degradation between soils was assessed by implementing ANOVA and Tukey's tests, while the Pearson product moment correlation was used for analysis of correlations between data.Results
Soil characteristics
Soil total carbon (% C), TOC and PAH concentrations (ng g−1 dw soil) are presented in Table 1. The Norwegian woodland soils (1–4) generally showed higher % TOC (up to 47.56% in soil 2) than the woodland and grassland UK soils (5–7). The highest TOC level in a UK soil (13.99%) was in soil 6. The highest ∑PAHs (285.54 ng g−1 dw soil) was in soil 7 (the UK) and the lowest (16.39 ng g−1 dw soil) in a Norwegian soil (2). A weak positive correlation (r = 0.32) was found between % TOC and ∑PAHs in Norwegian soils alone. No direct correlation was found between ∑PAHs in all soils and the numbers of 14C-phenanthrene degraders (r = 0.00).| PAH (ng g−1 dry wt soil) | Soil 1 | Soil 2 | Soil 3 | Soil 4 | Soil 5 | Soil 6 | Soil 7 |
|---|---|---|---|---|---|---|---|
| Naphthalene | 0.60 | 2.39 | 4.00 | 4.35 | 1.67 | 0.62 | 2.16 |
| Fluorene | 0.21 | 0.61 | 1.06 | 1.34 | 0.54 | 0.12 | 0.77 |
| Phenanthrene | 1.35 | 4.73 | 15.01 | 16.16 | 7.14 | 2.32 | 0.04 |
| Anthracene | 0.07 | 0.37 | 0.61 | 0.61 | 0.28 | 0.10 | 1.73 |
| Pyrene | 0.23 | 0.08 | 0.13 | 1.43 | 0.06 | 2.53 | 0.36 |
| Benzo(a)anthracene | 0.14 | 2.67 | 22.40 | 36.6 | 8.15 | 4.24 | 2.16 |
| Chrysene | 0.39 | 3.61 | 14.54 | 24.17 | 1.46 | 4.22 | 2.24 |
| Benzo(b)fluoranthene | 0.58 | 5.50 | 33.15 | 52.9 | 39.04 | 2.13 | 2.27 |
| Benzo(k)fluoranthene | 11.3 | 0.93 | 0.30 | 10.57 | 3.73 | 0.41 | 92.44 |
| Benzo(a)pyrene | 0.34 | 1.57 | 10.01 | 11.31 | 10.80 | 0.80 | 178.72 |
| Benzo(ghi)perylene | 0.68 | 2.63 | 16.25 | 22.96 | 24.88 | 1.93 | 0.29 |
| Coronene | 0.5 | 1.58 | 15.04 | 15.04 | 0.16 | 0.63 | 2.36 |
| ∑PAHs | 16.39 | 26.67 | 132.5 | 197.44 | 97.91 | 20.05 | 285.54 |
| % C | 4.30 | 48.54 | 15.64 | 46.12 | 12.43 | 14.31 | 5.87 |
| % TOC | 4.12 | 47.56 | 15.47 | 44.94 | 12.38 | 13.99 | 5.72 |
Enumeration of bacteria in soils
The numbers of colony forming units (CFUs) of total heterotrophic bacteria and 14C-phenanthrene degrading bacteria present in the soils before and after 14C-phenanthrene mineralisation to 14CO2 are presented in Table 2. Total heterotrophic and phenanthrene degrading bacteria present ranged between 104–106 and 103–106 CFU g−1 soil, respectively. The highest numbers of total heterotrophs before mineralisation were found in soil 2 (5.16 × 106 ± 0.03 × 106 CFU g−1) and the lowest in soil 5 (1.34 × 104 ± 0.36 × 104 CFU g−1). Phenanthrene degraders were highest in soil 3 (3.80 × 105 ± 0.05 × 105 CFU g−1) and lowest in soil 4 (4.56 × 103 ± 1.45 × 103 CFU g−1). No significant difference (P > 0.05) was found between the numbers of both total heterotrophic bacteria and phenanthrene degraders in UK and Norwegian soils. The CFUs of total heterotrophs and phenanthrene degraders increased in all soils after mineralisation except in soils 1 and 2 where they decreased.| Soil | Before mineralisation assay | After mineralisation assay | ||
|---|---|---|---|---|
| Heterotrophs (CFU g−1) | 14C-phe. degraders (CFU g−1) | Heterotrophs (CFU g−1) | 14C-phe. degraders (CFU g−1) | |
| 1 | 4.11 × 105 ± 0.14 × 105 | 1.68 × 105 ± 0.08 × 105 | 9.00 × 104 ± 1.29 × 104 | 1.41 × 105 ± 0.07 × 105 |
| 2 | 5.16 × 106 ± 0.03 × 106 | 6.70 × 104 ± 0.60 × 104 | 2.54 × 105 ± 0.04 × 105 | 5.77 × 104 ± 0.58 × 104 |
| 3 | 9.18 × 105 ± 0.01 × 105 | 3.80 × 105 ± 0.05 × 105 | 1.52 × 106 ± 0.07 × 106 | 5.10 × 106 ± 0.52 × 106 |
| 4 | 2.25 × 104 ± 0.01 × 104 | 4.56 × 103 ± 1.45 × 103 | 2.76 × 106 ± 0.23 × 106 | 1.53 × 104 ± 0.08 × 104 |
| 5 | 1.34 × 104 ± 0.36 × 104 | 2.73 × 104 ± 0.03 × 104 | 9.91 × 104 ± 0.11 × 104 | 5.73 × 105 ± 0.48 × 105 |
| 6 | 4.38 × 104 ± 0.02 × 104 | 1.17 × 105 ± 0.29 × 105 | 4.94 × 106 ± 0.17 × 106 | 5.82 × 106 ± 0.48 × 106 |
| 7 | 9.13 × 104 ± 1.83 × 104 | 1.17 × 104 ± 0.24 × 104 | 0.73 × 104 ± 0.04 × 104 | 7.31 × 105 ± 1.22 × 105 |
Microbial degradation of 14C-phenanthrene
Fig. 2 shows the catabolism of 14C-phenanthrene in background UK and Norwegian soils as determined by the mineralisation of 14C-phenanthrene to 14CO2 by indigenous microflora after a 14-day (UK soils) and 30-day (Norwegian soils) assay. The lag phases in the Norwegian soils were noticeably longer than those in UK soils. The shortest lag phase observed in a Norwegian soil was approximately 18 days (soil 3) and the longest lag phase in a UK soil was 6.39 days (soil 7). Soils 5 and 6 specifically had the shortest lag phases (P < 0.05) of approximately 3 days when compared to other soils (1, 2, 3, 4 and 7). The observed fastest rates of 14C-phenanthrene mineralisation were generally higher in the UK soils than those in the Norwegian soils (Table 3). Norwegian soils 2 and 3 showed significantly lower levels (P < 0.05) of rate of mineralisation when compared to soils 1 and 4. Similarly, soils 5 and 6 from the UK had a significantly higher (P < 0.05) fastest rate of 14C-phenanthrene mineralisation when compared to soil 7 from UK and other Norwegian soils. Regarding the extents of 14C-phenanthrene mineralisation in both Norwegian and UK soils, soils exhibiting shorter lag phases and higher rates of mineralisation also showed higher extents of mineralisation. The highest extents of 14C-phenanthrene mineralisation were observed in soil 6 (86.16%) and soil 5 (74.79%), which were significantly higher (P < 0.01) than those in soils from Norway and soil 7 from the UK (Table 3). There was however no statistically significant difference between the extents of 14C-phenanthrene mineralised in Norwegian soils (P > 0.05), where they were below 42%, except soil 1. In contrast, soil 7 (the UK) exhibited the shortest (P < 0.05) extent of mineralisation (23.49%) amongst all soils.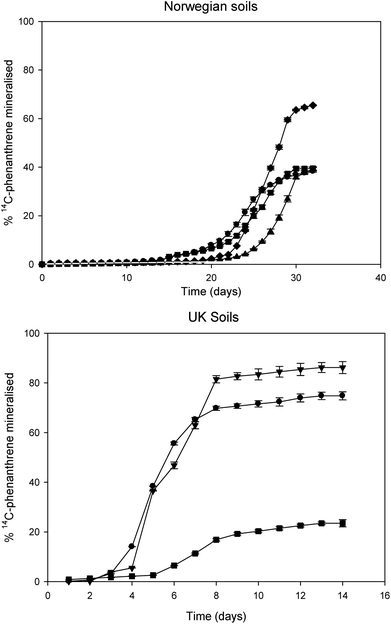 | ||
| Fig. 2 14C-phenanthrene mineralisation in four (4) Norwegian soils [soil 1 (♦); soil 2 (■); soil 3 (●); soil 4 (▲)] and three (3) UK soils [soil 5 (●); soil 6 (▼); soil 7 (■)]. | ||
| Soil | Lag phase (time before mineralisation > 5%) (d) | Fastest rate of phenanthrene mineralisation (% hour−1) | Extent of phenanthrene mineralisation (%) | K value (% day−1) | Half-life – t½ (days) |
|---|---|---|---|---|---|
| 1 | 22.30 ± 0.01 | 0.47 ± 0.00 | 65.47 ± 0.21 | 0.110 | 6.30 |
| 2 | 18.22 ± 0.21 | 0.25 ± 0.01 | 41.44 ± 0.43 | 0.011 | 60.85 |
| 3 | 18.00 ± 0.33 | 0.22 ± 0.01 | 38.26 ± 0.35 | 0.012 | 57.74 |
| 4 | 24.50 ± 0.10 | 0.37 ± 0.02 | 39.13 ± 0.74 | 0.010 | 69.30 |
| 5 | 3.19 ± 0.00 | 1.01 ± 0.02 | 74.79 ± 1.61 | 0.261 | 2.66 |
| 6 | 3.89 ± 0.01 | 1.29 ± 0.04 | 86.16 ± 2.44 | 0.407 | 1.70 |
| 7 | 6.39 ± 0.01 | 0.28 ± 0.01 | 23.49 ± 1.47 | 0.040 | 36.47 |
When fitted into a first-order kinetic model, the biodegradation rate constants for phenanthrene in the soil samples 1–7 were estimated at 0.110, 0.011, 0.012, 0.010, 0.261, 0.407 and 0.040% day−1, and half-lives at 6.30, 60.85, 57.75, 69.30, 2.66, 1.70 and 17.33 days, respectively (Table 3). As the biodegradation rate constants increased, the extents of mineralisation also increased with shorter half-lives and lag phases. In general, UK soils exhibited higher rate constants and shorter half-lives compared to Norwegian soils, except for soil 1 having 4.12% TOC, irrespective of the variation in CFUs. A positive linear correlation (r2 = 0.80) was observed between TOC (%) and the lag phase for UK soils, indicating that 80% of data points fall on the regression line. Also, a strong correlation relationship (r2 = 0.97 and r2 = 0.95) was observed between TOC (%) and the half-life and TOC (%) and rate constants of phenanthrene biodegradation in UK soils, respectively. In Norwegian soils, there were weak but obvious relationships between TOC (%) and lag phase (r2 = 0.65); half-lives (r2 = 0.65) and rate constants (r2 = 0.56), respectively. There were no correlations (r = 0.00) found between 14C-phenanthrene degraders and extent of 14C-phenanthrene mineralisation in all the Norwegian and UK soils.
Discussion
PAH concentrations in background soils are important to indigenous PAH biodegradation because their presence has been linked to the development and retention of PAH degrading genes in the soil microflora.42 The highest ∑PAH concentration in all of the seven soils studied in this experiment was in soil 7, a grassland soil from the UK. Comparing the three UK soils interrogated in this study showed that the highest TOC was in the woodland soil 6, but unlike in Norwegian soils, ∑PAHs was not found to correlate with TOC. This suggests that TOC may not be the only primary controlling factor in the biodegradation of PAHs in the UK soils. The TOC is composed of amorphous and carbonaceous organic matter fractions, with the potential to alter the desorption kinetics of hydrophobic organic compounds, such as phenanthrene, which was used in this study.43–47 Previous studies have also shown that PAH levels were higher in UK soils than those in Norwegian soils.32,48 This was attributed to the UK's longer history of industrialisation and coal burning, larger human population and closer proximity to PAH point sources.32,34,49 PAH concentrations in Norwegian soils are thought to be predominantly influenced by long range atmospheric transport and not deposition from local sources. As a result, for PAH partitioning within soil, TOC plays a dominant role in the retention of PAHs in such soils, particularly in those with higher TOC.37 TOC concentrations in 2–4 of the Norwegian soils investigated in this experiment were higher than those in all of the UK soils (Table 1), as they were all woodland soils.32 The weak positive correlation found between TOC and ∑PAHs in Norwegian soils further demonstrates the importance of TOC in the retention of PAHs in Norwegian woodland soils.32The numbers of 14C-phenanthrene-degrading bacteria found in all of the soils were at similar levels to those previously found in uncontaminated background soils.50 PAH-degrading bacteria are present in both contaminated and uncontaminated soils because of the ubiquitous presence of PAHs in the environment resulting from atmospheric deposition.9,22,51 In addition to atmospheric deposition, there is also evidence for the biogenic synthesis of PAHs in soils.3,52–55 As an example, Wilcke et al. (2003)56 and Krauss et al. (2005)57 compared the levels of PAHs in the atmosphere to those in the soils of a tropical rainforest and found that the levels of phenanthrene, naphthalene and perylene in plants, termite nests, and soils were higher than the levels in the atmosphere, supporting the claim for biological sources of PAHs. Although the numbers of soil microflora have been shown to increase to reflect the introduction or the presence of carbon sources (e.g. PAHs, organic matter) in their environment,50,58–60 this study showed no direct correlation between ∑PAHs in soils and the numbers of 14C-PAH degraders. Reasons for this may be the weakness of the method employed for microbial enumeration or that not all 14C-phenanthrene-degrading bacteria may be culturable. For example, Bodour et al. (2003)61 studied the temporal changes in the culturable phenanthrene degraders in response to exposure to phenanthrene in a soil. The authors found that a diverse microbial community participated in phenanthrene degradation in soil, some of which were temporally unculturable before, during or after the experiment. Many 14C-phenanthrene degraders can also utilise other more readily degradable carbon sources for growth in soil. Therefore, a direct correlation between the total PAH content or even the bioaccessible PAH content in soils and the number of PAH degraders should not always be expected.50,62–64
Lag phases can be affected by the number of degrading cells and soil TOC.65 Here, the observed lag phases in the biodegradation of 14C-phenanthrene were markedly longer in all the Norwegian woodland soils than those in the UK grassland and woodland soils. Since the numbers of PAH-degrading bacteria were found to be similar in all the soils, it was not thought of as a reason for the different lag phases. In soils with high TOC, PAHs tend to be sequestered over time thereby reducing their bioavailability/bioaccessibility to microbial degradation.15,66,67 However, this depends on the composition, structure and pore distribution of organic matter,44,68 which can delay the onset of mineralisation, hence longer lag phases. While this is true in the natural environment, the higher TOC content of the Norwegian soils is believed to be the factor responsible for the longer lag phases before the on-set of 14C-phenanthrene.32 More specifically, organic matter composition of soils has been shown in the literature to be composed of both rubbery and glassy domains which govern the sorption mechanisms (partitioning, adsorption) of non-polar organic compounds such as phenanthrene.69,70 Although 14C-phenanthrene was freshly added to the soil slurry, diffusion of 14C-phenanthrene into accessible high energetic sites (aliphatic or aromatic moieties) still occurred in all soils, thus reducing the rapidly desorbable fractions of phenanthrene for degradation. Catabolism of 14C-phenanthrene in UK soils by indigenous microorganisms has been shown by Oyelami et al. (2015),47 where the increased porosity of activated carbon (AC) delayed lag phases from 4.5 days (control) to 6.7–8.0 days (0.1% AC) as 14C-phenanthrene was rapidly sorbed. Although not measured, ACs represent a class of modified carbonaceous geosorbents within a group of similar materials collectively named black carbons (BCs). BCs are higher in Norwegian soils compared to UK soils owing to long range atmospheric transport and deposition32 and represent a chemically recalcitrant component of the glassy (condensed) fraction of organic matter in soils that significantly affects the fate of PAHs in soils.44,69,70 Investigating the nature of organic matter in the soils can reveal more about the integral sorption capacities of the soils.
In contrast, soil 7 from the UK contained the lowest TOC level, except for soil 4, and had a longer lag phase compared to the other UK soils. Apparently, the benzo[a]pyrene (B[a]P) level in this soil was responsible for this anomaly. The B[a]P concentration in soil 7 (178.7 ng g−1) exceeded the B[a]P concentrations in all other soils by over 90% and also exceeded the ∑PAHs of the majority of the studied soils except soil 4 (197.4 ng g−1). Although the co-metabolism of B[a]P was not determined, it is suspected that owing to its high concentration there would have either been inhibition of 14C-phenanthrene with or without co-metabolism of B[a]P or toxicity of B[a]P derived metabolites leading to the delayed onset of degradation.17,71 The implication is that even in remote soils, not directly impacted by high levels of PAH contamination, microbial activity can, in addition to the effect of natural PAH analogues,71 still be stimulated by the low levels of PAHs present due to diffuse pollution. In one of the few available studies on indigenous PAH biodegradation in background soils, Johnsen and Karlson (2005)50 found a clear correlation between the amount of soil PAHs and the potential for biodegradation of phenanthrene and pyrene; however, they did not find any lag phase in phenanthrene mineralisation. This current study shows that the onset of 14C-phenanthrene degradation in soil can vary depending on the concentration of PAHs present in the soil.
Faster rates of 14C-phenanthrene mineralisation were observed in the UK soils (Table 3). Rates of PAH degradation are determined by the availability of the contaminants to the degrading microorganisms, the numbers of degrading microorganisms present in the soil and the activity of degrading microorganisms.72,73 Therefore, as with the lag phases, the slurry system would have enhanced the availability of 14C-phenanthrene to the degrading microorganisms, thereby describing the maximum amount of 14C-phenanthrene that may be mineralised in the soils (bioaccessibility).16 Mass transfer of 14C-phenanthrene to a designated diffusion boundary layer is enhanced during shaking, thus encouraging dissolution for catabolism.17 However, in the natural environment, the higher TOC of the Norwegian woodland soils may have decreased the rate of transfer of the 14C-phenanthrene to the degrading microorganisms, thereby reducing the rate of mineralisation.44,47 This study further showed that the TOC and inherent B[a]P composition can strongly influence the degradation rates of 14C-phenanthrene in soils, irrespective of the source. When 55 mg kg−1 phenanthrene was spiked into a sediment slurry by Chen et al. (2008),74 the extent of biodegradation was between 85% and 96% with varying rate constants higher than that of this present study owing to the absence of other PAHs, salinity of the system and microbial inoculum size. More specifically, degradation of phenanthrene in a long-term PAH contaminated industrial site in Germany showed similar rate constants found in the Norwegian soils, having higher TOC values; reduced desorption and diffusion were responsible and not microbial activity.75 Additionally, the rate constants determined in this study (soils 1, 5 and 6) were higher than phenanthrene degradation rates observed by Antizar-Ladislao et al. (2006)76 and Crampon et al. (2014),77 where reduced bioavailability and microbial population, respectively, were critical factors. This current study supports that TOC-reduced bioavailability and rates of degradation, together with the presence of higher concentrations of five ring PAHs (e.g. B[a]P), can also affect rates and in-turn affect the half-life of target contaminants in the soil.
The extents to which 14C-phenanthrene were mineralised in the soils (up to 86% in soil 6) in this study can be attributed to the use of soil slurry assays because soil slurries and agitation are a measure of the maximum catabolic potential of the soil.39,40 This means that even in pristine soils that are not directly impacted by PAHs, the genetic potential for PAH biodegradation can be determined and maintained.78,79 Biochemical pathways for degradation of phenanthrene in soil have been well documented in the literature,18,80,81 and most indigenous bacteria possess the multicomponent dioxygenase enzyme system responsible for degradation.18 Although the microbial diversity was not determined, Uroz et al. (2016)82 and Martin et al. (2012)83 have shown that the diversity and structure of indigenous microbial populations can be altered through biomass type (trees, plants) and PAH concentrations with time. Indeed, most bacterial phenanthrene degraders include Pseudomonas, Burkholderia, Mycobacterium, Nocardia, Alcaligenes, Acidovorax, Polaromonas, and Rhodoferax genus,18,82,83 and fungal degraders frequently identified include Candida, Irpex, Pleurotus, Fusarium, Cunninghamella, and Phanerochaete.18,84 However, microbial composition and distribution in this study varied due to the difference in the distribution of inherent PAHs and TOC composition in the soils. Since PAHs were present in all soils, it is suggested that variation in microbial diversity will still vary due to the soil type, TOC composition and content. Additionally, TOC and the presence of the high B[a]P concentration (soil 7) also affected half-lives and extents of 14C-phenanthrene mineralisation in the soils. Despite indigenous microbial consortium supporting each other to improve bioavailability and metabolism, dead-end products from co-metabolism or B[a]P may have inhibited 14C-phenanthrene mineralisation in soil 7.85,86
Conclusion
The results from this study show that PAH concentrations found in soils vary and long range transport and deposition in pristine soils can encourage microbial catabolic potential in such soils. Surprisingly, TOC of soils did not correlate with the sum of PAHs found within the soils sampled but indicated that the composition of the organic matter domain in soils needs to be studied. Bacterial enumeration of PAH-degrading bacteria in CFUs was done but their numbers did not determine the greatest extent of PAH mineralisation. UK soils showed highest extent of phenanthrene mineralisation (>80%) with shortest lag phases, shorter half-lives, highest rate constants and highest fastest rates of mineralisation when compared to soils from Norway. Our findings show that the nature of the degrading microorganisms and composition of organic matter are accountable for differing catabolic activities by indigenous microorganisms.Conflicts of interest
There are no conflicts of interest to declare.References
- A. K. Haritash and C. P. Kaushik, J. Hazard. Mater., 2009, 169, 1–15 CrossRef CAS PubMed.
- Q. Yang, H. Chen and B. Li, PLoS One, 2015a, 10, e0118141 Search PubMed.
- W. Wilcke, J. Plant Nutr. Soil Sci., 2000, 163, 229–248 CrossRef CAS.
- M. Tsapakis, E. G. Stephanou and I. Karakassis, Mar. Chem., 2003, 80, 283–298 CrossRef CAS.
- E. J. Villanneau, N. P. Saby, T. G. Orton and C. C. Jolivet, et al. , Environ. Chem. Lett., 2013, 11, 99–104 CrossRef CAS.
- C. Peng, M. Wang and W. Chen, Sci. Rep., 2016a, 6, 37185, DOI:10.1038/srep37185.
- W. Wang, S. L. M. Simonich, M. Xue and J. Zhao, et al. , Environ. Pollut., 2010, 158, 1245–1251 CrossRef CAS PubMed.
- C. Peng, M. Wang, Y. Zhao and W. Chen, Environ. Monit. Assess., 2016b, 188, 1–12 Search PubMed.
- A. Johnsen and U. Karlson, Appl. Microbiol. Biotechnol., 2007, 76, 533–543 CrossRef CAS PubMed.
- S. R. Wild and K. C. Jones, Environ. Pollut., 1995, 88, 91–108 CrossRef CAS PubMed.
- T. Agarwal, P. S. Khillare, V. Shridhar and S. Ray, J. Hazard. Mater., 2009, 163, 1033–1039 CrossRef CAS PubMed.
- K. T. Semple, A. W. J. Morriss and G. I. Paton, Eur. J. Soil Sci., 2003, 54, 809–818 CrossRef CAS.
- G. Wu, X. Li, C. Kechavarzi, R. Sakrabani, H. Sui and F. Coulon, Chemosphere, 2014, 107, 43–50 CrossRef CAS PubMed.
- K. C. Jones, R. E. Alcock, D. L. Johnson, G. L. Northcock, K. T. Semple and P. J. Woolgar, Land Contam. Reclamat., 1996, 3, 189–197 Search PubMed.
- B. J. Reid, K. C. Jones and K. T. Semple, Environ. Pollut., 2000, 108, 103–112 CrossRef CAS PubMed.
- M. J. Riding, K. J. Doick, F. L. Martin, K. C. Jones and K. T. Semple, J. Hazard. Mater., 2013, 261, 687–700 CrossRef CAS PubMed.
- A. R. Johnsen, L. Y. Wick and H. Harms, Environ. Pollut., 2005, 133, 71–84 CrossRef CAS PubMed.
- R. H. Peng, A. S. Xiong, Y. Xue, X. Y. Fu, F. Gao, W. Zhao, Y. S. Tian and Q. H. Yao, FEMS Microbiol. Rev., 2008, 32, 927–955 CrossRef CAS PubMed.
- P. Pandey, H. Pathak and S. Dave, Res. J. Environ. Toxicol., 2016, 10, 1–15 CrossRef.
- G. J. Leahy and R. R. Colwell, Microbiol. Rev., 1990, 54, 305–315 Search PubMed.
- T. N. P. Bosma, P. J. M. Middeldorp, G. Schraa and A. J. G. Zehnder, Environ. Sci. Technol., 1996, 31, 248–252 CrossRef.
- U. V. Okere, A. Cabrerizo, J. Dachs, K. C. Jones and K. T. Semple, FEMS Microbiol. Lett., 2012, 329, 69–77 CrossRef CAS PubMed.
- A. E. Ite and K. T. Semple, Int. J. Environ. Biorem. Biodegrad., 2015, 3, 66–78 CAS.
- L. A. M. Ruberto, S. C. Vazquez, A. Curtosi and M. C. Mestre, et al. , Biorem. J., 2006, 10, 191–201 CrossRef CAS.
- C. Muangchinda, S. Chavanich, V. Viyakarn and K. Watanabe, et al. , Environ. Sci. Pollut. Res. Int., 2015, 22, 4725–4735 CrossRef CAS PubMed.
- L. Nizzetto, M. Macleod, K. Borgå, A. Cabrerizo and J. Dachs, et al. , Environ. Sci. Technol., 2010, 44, 6526–6531 CrossRef CAS PubMed.
- F. M. Nakamura, M. G. Germano and S. M. Tsai, Diversity, 2014, 6, 339–353 CrossRef CAS.
- D. Ghosal, S. Ghosh, T. K. Dutta and Y. Ahn, Front. Microbiol., 2016, 7, 1369, DOI:10.3389/fmicb.2016.01369.
- U. V. Okere and K. T. Semple, J. Biorem. Biodegrad., 2012, S1, 006, DOI:10.4172/2155-6199.S1-006.
- G. Lunde and A. L. F. Bjorseth, Nature, 1977, 268, 518–519 CrossRef CAS PubMed.
- E. Aamot, E. Steinnes and R. Schmid, Environ. Pollut., 1996, 92, 275–280 CrossRef CAS PubMed.
- J. J. Nam, G. O. Thomas, F. M. Jaward and E. Steinnes, et al. , Chemosphere, 2008, 70, 1596–1602 CrossRef CAS PubMed.
- S. N. Meijer, E. Steinnes, W. A. Ockenden and K. C. Jones, Environ. Sci. Technol., 2002, 36, 2146–2153 CrossRef CAS PubMed.
- S. N. Meijer, W. A. Ockenden, A. Sweetman and K. Breivik, et al. , Environ. Sci. Technol., 2003, 37, 667–672 CrossRef CAS PubMed.
- A. Hassanin, R. G. M. Lee, E. Steinnes and K. C. Jones, Environ. Sci. Technol., 2005, 39, 4784–4792 CrossRef CAS PubMed.
- A. H. Rhodes, S. M. Owen and K. T. Semple, FEMS Microbiol. Lett., 2007, 269, 323–330 CrossRef CAS PubMed.
- J. J. Nam, A. J. Sweetman and K. C. Jones, J. Environ. Monit., 2009, 11, 45–48 RSC.
- B. J. Reid, C. J. A. MacLeod, P. H. Lee and A. W. J. Morriss, et al. , FEMS Microbiol. Lett., 2001, 196, 141–146 CrossRef CAS PubMed.
- K. J. Doick and K. T. Semple, FEMS Microbiol. Lett., 2003, 220, 29–33 CrossRef CAS PubMed.
- K. T. Semple, N. M. Dew, K. J. Doick and A. H. Rhodes, Environ. Pollut., 2006, 140, 164–172 CrossRef CAS PubMed.
- H. J. Lorch, G. Benckieser and J. C. G. Ottow, Methods in Applied Soil Microbiology and Biochemistry, ed. K. Alef and P. Nannipieri, Academic Press, New York, 1995, vol. 4, pp. 146–161 Search PubMed.
- Y. Yang, J. Wang, J. Liao, S. Xie and Y. Huang, Appl. Microbiol. Biotechnol., 2015b, 99, 1935–1946 Search PubMed.
- A. Kan, W. Chen and M. Tomson, Environ. Pollut., 2000, 108, 81–89 CrossRef CAS PubMed.
- G. Cornelissen, Ö. Gustafsson, T. D. Bucheli and M. T. Jonker, et al. , Environ. Sci. Technol., 2005, 39, 6881–6895 CrossRef CAS PubMed.
- A. H. Rhodes, L. E. McAllister and K. T. Semple, Environ. Pollut., 2010, 158, 1348–1353 CrossRef CAS PubMed.
- A. H. Rhodes, M. J. Riding, L. E. McAllister, K. Lee and K. T. Semple, Environ. Sci. Technol., 2012, 46, 12445–12451 CrossRef CAS PubMed.
- A. O. Oyelami, U. Ogbonnaya, C. Muotoh and K. T. Semple, Environ. Sci.: Processes Impacts, 2015, 17, 1173–1181 CAS.
- R. Gioia, E. Steinnes and G. O. Thomas, et al. , J. Environ. Monit., 2006, 8, 700–710 RSC.
- S. L. Simonich and R. A. Hites, Environ. Sci. Technol., 1994, 28, 939–943 CrossRef CAS PubMed.
- A. R. Johnsen and U. Karlson, Microb. Ecol., 2005, 50, 488–495 CrossRef CAS PubMed.
- W. Wilcke, Geoderma, 2007, 141, 157–166 CrossRef CAS.
- H. Azuma, M. Toyota, Y. Asakawa and S. Kawano, Phytochemistry, 1996, 42, 999–1004 CrossRef.
- J. Aislabie, M. Balks and N. Astori, et al. , Chemosphere, 1999, 39, 2201–2207 CrossRef CAS PubMed.
- W. Wilcke, M. Krauss and W. Amelung, Environ. Sci. Technol., 2002, 36, 3530–3535 CrossRef CAS PubMed.
- A. Cabrerizo, C. Galbán-Malagón, S. Del Vento and J. Dachs, Global Biogeochem. Cycles, 2014, 28, 1424–1436 CrossRef CAS.
- W. Wilcke, W. Amelung, M. Krauss and C. Martius, et al. , Org. Geochem., 2003, 34, 1405–1417 CrossRef CAS.
- M. Krauss, W. Wilcke, C. Martius and A. G. Bandeira, et al. , Environ. Pollut., 2005, 135, 143–154 CrossRef CAS PubMed.
- J. C. Spain and J. C. Van Veld, Appl. Environ. Microbiol., 1983, 45, 428–435 CAS.
- J. W. Davis and S. Madsen, Chemosphere, 1996, 33, 107–130 CrossRef CAS PubMed.
- M. Vinas, J. Sabate, M. J. Espuny and A. M. Solanas, Appl. Environ. Microbiol., 2005, 71, 7008–7018 CrossRef CAS PubMed.
- A. A. Bodour, J. M. Wang, M. L. Brusseau and R. M. Maier, Environ. Microbiol., 2003, 5, 888–895 CrossRef CAS PubMed.
- R. J. Grosser, D. Warshawsky and J. R. Vestal, Appl. Environ. Microbiol., 1991, 57, 3462–3469 CAS.
- R. J. Grosser, J. R. Vestal and D. Warshawsky, Environ. Toxicol. Chem., 1995, 14, 375–382 CrossRef CAS.
- L. M. Carmichael and F. K. Pfaender, Environ. Toxicol. Chem., 1997, 16, 666–675 CrossRef CAS.
- C. J. A. Macleod and K. T. Semple, Environ. Pollut., 2002, 119, 357–364 CrossRef CAS PubMed.
- J. C. White, J. W. Kelsey, P. B. Hatzinger and M. Alexander, Environ. Toxicol. Chem., 1997, 16, 2040–2045 CrossRef CAS.
- K. T. Semple, M. J. Riding and L. E. McAllister, et al. , J. Hazard. Mater., 2013, 261, 808–816 CrossRef CAS PubMed.
- U. Ogbonnaya, J. Thomas, S. A. Fasina and K. T. Semple, Environ. Technol. Innovation, 2016, 6, 80–93 CrossRef.
- A. S. Gunasekara and B. Xing, J. Environ. Qual., 2003, 32, 240–246 CrossRef CAS PubMed.
- R. Lohmann, J. K. Macfalane and P. M. Gschwend, Environ. Sci. Technol., 2005, 39, 141–148 CrossRef CAS PubMed.
- A. L. Juhasz, M. L. Britz and G. A. Stanley, J. Appl. Microbiol., 1997, 83, 189–198 CAS.
- J. L. Stroud, G. I. Paton and K. T. Semple, FEMS Microbiol. Lett., 2007, 272, 120–126 CrossRef CAS PubMed.
- C. J. A. Macleod, A. W. J. Morriss and K. T. Semple, Adv. Appl. Microbiol., 2001, 48, 171–212 CAS.
- J. Chen, M. H. Wong, Y. S. Wong and N. F. Y. Tam, Mar. Pollut. Bull., 2008, 57, 695–702 CrossRef CAS PubMed.
- S. Thiele-Bruhn and G. W. Brummer, Plant Soil, 2005, 275, 31–42 CrossRef CAS.
- B. Antizar-Ladislao, J. Lopez-Real and J. A. Beck, Environ. Pollut., 2006, 141, 459–468 CrossRef CAS PubMed.
- M. Crampon, F. Bureau, M. Akpa-Vinceslas, J. Bodilis, N. Machour, F. Le Derf and F. Portet-Koltalo, Environ. Sci. Pollut. Res., 2014, 21, 8133–8145 CrossRef CAS PubMed.
- R. Margesin, D. Labbe, F. Schinner, C. W. Greer and L. G. Whyte, Appl. Environ. Microbiol., 2003, 69, 3085–3092 CrossRef CAS PubMed.
- A. Cébron, M. P. Norini, T. Beguiristain and C. Leyval, J. Microbiol. Methods, 2008, 73, 148–159 CrossRef PubMed.
- O. Pinyakong, H. Habe and T. Omori, J. Gen. Appl. Microbiol., 2003, 49, 1–19 CrossRef CAS PubMed.
- S. M. Bamforth and I. Singleton, J. Chem. Technol. Biotechnol., 2005, 80, 723–736 CrossRef CAS.
- S. Uroz, P. Oger, E. Tisserand, A. Cébron, M.-P. Turpault, M. Buée, W. De Boer, J. H. J. Leveau and P. Frey-Klett, Sci. Rep., 2016, 6, 27756, DOI:10.1038/srep27756.
- F. Martin, S. Torelli, D. Le Paslier, A. Barbance, F. Martin-Laurent, D. Bru, R. Geremia, G. Blake and Y. Jouanneau, Environ. Pollut., 2012, 162, 345–353 CrossRef CAS PubMed.
- A. Ding, Y. Sun, J. Dou, L. Cheng, L. Jiang, D. Zhang and X. Zhao, Characterizing Microbial Activity and Diversity of Hydrocarbon-Contaminated Sites, In Tech, 2013, DOI:10.5772/50480.
- W. T. Stringfellow and M. D. Aitken, Appl. Environ. Microbiol., 1995, 61, 357–362 CAS.
- S. Boonchan, M. L. Britz and G. A. Stanley, Appl. Environ. Microbiol., 2000, 66, 1007–1019 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |