Formation and alteration of airborne particles in the subway environment
T.
Moreno
 *a,
X.
Querol
a,
V.
Martins
a,
M. C.
Minguillón
a,
C.
Reche
a,
L. H.
Ku
b,
H. R.
Eun
b,
K. H.
Ahn
b,
M.
Capdevila
c and
E.
de Miguel
c
*a,
X.
Querol
a,
V.
Martins
a,
M. C.
Minguillón
a,
C.
Reche
a,
L. H.
Ku
b,
H. R.
Eun
b,
K. H.
Ahn
b,
M.
Capdevila
c and
E.
de Miguel
c
aInstitute of Environmental Assessment and Water Research (IDÆA-CSIC), C/Jordi Girona 18-24, 08034 Barcelona, Spain. E-mail: teresa.moreno@idaea.csic.es; Tel: +34 934006123
bDepartment of Mechanical Engineering, Hanyang University, Ansan 425-791, Republic of Korea
cTransports Metropolitans de Barcelona (TMB), Santa Eulalia, Av. del Metro s/n L'Hospitalet de Llobregat, 08902, Spain
First published on 28th November 2016
Abstract
Most particles in the rail subway environment are sub-micron sized ferruginous flakes and splinters generated mechanically by frictional wear of brake pads, wheels and rails. To better understand the mechanisms of formation and the alteration processes affecting inhalable particles in subways, PM samples (1–2.5 μm and 2.5–10 μm) were collected in the Barcelona Metro and then studied under a scanning electron microscope. Most particles in these samples are hematitic (up to 88%), with relatively minor amounts of mineral matter (up to 9%) and sulphates (up to 5%). Detailed microscopy (using back scattered and TEM-DRX imaging) reveals how many of the metallic particles comprise the metallic Fe nucleus surrounded by hematite (Fe2O3) and a coating of sulphate and chloride salts mixed with mineral matter (including Ca-carbonates, clay minerals and quartz). These observations record the emission of fine to ultrafine FePM by frictional wear at elevated temperatures that promote rapid partial (or complete) oxidation of the native metal. Water condensing on the PM surface during cooling leads to the adsorption of inorganic mineral particles that coat the iron oxide. The distinctively layered polymineralic structure that results from these processes is peculiar to particles generated in the subway environment and very different from PM typically inhaled outdoors.
Environmental impactThe processes involved in the generation of airborne particles in the subway air and their subsequent alteration are very different from those happening in other environments. The immense majority of the particles are produced by the movement of trains, with the mechanical friction between wheels, rails and brakes, with metals such as Zn, Ba, Cr, Mn, Fe and Cu being detected by EDX coupled to electron microscopy. The morphologies of these particles are generally planar with few spherical forms, conforming to a mechanical origin rather than condensation of Fe vapour. However during the friction that caused the emission of abrasion products high temperatures were reached and water could condense on the surface forming a water coating in which soluble and insoluble phases were adsorbed. |
Introduction
Air quality in the rail subway environment is an issue of concern due to high particle concentrations that can be registered, especially on platforms. The variables influencing these concentrations are numerous and include the type and intensity of ventilation, station design, number and location of accesses to platforms and number of trains and passengers.1 However, a major factor in common is that the airborne particles being inhaled in this environment are very similar in all subway systems worldwide due to their common emission sources. The friction between rail tracks, wheels and brakes results in the generation of particle flakes that are chemically distinct, mostly ferruginous. Carbon particles derived from brake materials are also common and typically associated with various distinctive trace metals such as Ba, Sb, Sn (from brakes), Cu (from brakes and catenary), and Cr and Mn (from wheels and rails).2Given that subway PM is distinctively metallic and very different from PM typically inhaled outdoors, it is particularly amenable to study using high resolution transmission electron microscopy (TEM) and scanning electron microscopy (SEM). In recent years, several scientific papers have published electron microscope images of PM in the subway system, and its general appearance and composition are correspondingly well documented.3–10 These studies have demonstrated that most particles in the subway environment are nanometric-scale oxidised Fe-rich flakes and splinters generated mechanically by frictional wear.5,10–13 This paper aims to take the subject further by using electron microscopy to make detailed observations that clarify the likely mechanisms of formation and progressive alteration affecting inhalable particles in the subway system. The PM samples examined for this study were collected in the Barcelona Metro during ongoing intensive campaigns carried out under the work programme of the IMPROVE LIFE project aiming to provide a benchmark study that will lead to real improvement in subway air quality (http://improve-life.eu/). During the project, measures that can reduce PM concentrations on platforms and inside trains are being tested, taking into account variations in all the key factors such as the station depth, date of construction, station design, type of ventilation, types of brakes used on the trains, train frequency and the presence or absence of platform screen door systems.
Materials and methods
Barcelona's Metro includes 8 subway lines built from 1929 to 2016, and the latest (L9–L11) has platform screen door systems and advanced ventilation systems. All trains are operated electrically (80% of them being motor carriages) and run from 5 a.m. until midnight every day, with additional services on Friday nights (finishing at 2 a.m. of Saturday) and Saturday nights (running all night long), and with a frequency between 2 and 15 minutes, depending on the day (weekend or weekday) and time of day. The braking system is electric when approaching the platform, changing to non-asbestos pneumatic braking when slowing down below a 5 km h−1 velocity for all lines independently of the platform design, using either frontal or lateral brake pads.For PM sample collection a rotating impactor with a flow rate of 1 L min−1 was employed. The impactor collects samples in two size stages (1–2.5 μm and 2.5–10 μm), onto Quantifoil® gold (Au) grids with 1 μm diameter holes – 4 μm separation of 200-mesh for microscopic analysis. Gold grids were mounted covering the whole impacting area for each of the size-fractionated particle stages.
The equipment was transported in a rucksack, with sampling being performed for 30 minutes in the central part of 8 platforms from different lines (L1, 2, 3, 4, and 10). Each station was selected based on different architectural designs, chosen to obtain a wider range of PM sample characteristics, including stations with open double rail tracks (Santa Coloma and Rocafort on L1), double tracks separated by a wall in the station (Joanic on L4, Palau Reial and Poble Sec on L3), a single rail track (Tetuan on L2) and new stations with platform screen doors (PSD's) separating the single rail track from the platform (Onze de Setembre and Llefià on L10).
Individual particles collected in the grids were then characterised using field scanning electron microscopy. The size and shape of individual particles were observed using (i) a (FESEM) J-7100F Jeol Scanning Electron Microscope via an energy dispersive X-ray microanalysis system (EDS Inca series 250, Oxford Instruments) after being carbon-coated using a 208HR Sputter Coater (Cressington, UK) and an MTM20 Thickness Controller (Cressington, UK). The microscope working distance was 10 mm, with an accelerating voltage of 15 kV; and (ii) a TEM (Jeol, JEM 1220, Tokyo, Japan), coupled with an energy-dispersive X-ray (EDX) spectrometer. TEM-grids were attached to air sample cassettes (SKC Inc., USA, inlet diameter 1/8 in. and filter diameter 25 mm).
Results and discussion
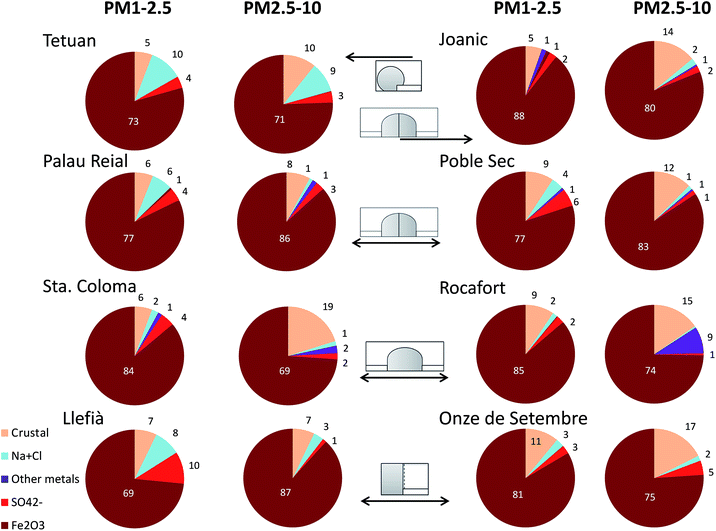
A total of 865 single particles (randomly selected) distributed among the 8 different subway platforms were analysed under the SEM. The following elements were identified in the particles: Na, Zn, Mg, Al, Si, S, Cl, K, Ca, Ba, Ti, Cr, Mn, Fe, Cu, Mo, Pd, P and Mo. These elements were grouped into 5 categories including crustal (the sum of Mg, Al, SI, K, Ca, Ti, and P), sea salt (Na + Cl), sulphates, hematite (calculated assuming all iron present as hematite, as reported by Moreno et al., and identified by XRD analysis on PM2.5 filters) and other metals (Zn, Ba, Cr, Mn and Cu).10Considering the average chemical composition of all single particle analyses for each subway platform, hematite was by far the most common component in all PM2.5–10 and PM1–2.5 samples (Fig. 1), ranging between 70 (Llefià new station) and 88% (Joanic) in the PM1–2.5 composition, and between 69 (Santa Coloma) and 87% (Llefià) in the PM2.5–10 samples. After hematite, in the PM2.5–10 fraction the second chemical component had a crustal composition (Si, Al, Mg, K and Ti) although in a much lower proportion (from 7% in Llefià to 19% in Santa Coloma), followed by marine aerosols (up to 9% in Tetuan), other metals (Zn, Ba, and Cu, up to 9% in Rocafort), and sulphates (up to 5% in Onze de Setembre). It should be noted that as samples were carbon coated for the use of high resolution microscopy, carbonaceous particles in general (including organics) are not considered in this classification, although they were presumably present in all samples and sourced from the graphite catenaries and brake pads.
An additional approach is to consider the number of particles, instead of particle average composition, identified in each sample. In this case the relative contribution of each of the five main groups of particles between the fine and coarse fractions is very variable depending on the station (Table 1). In this case Fe-rich particles were again the most common independent of the type of line or station, varying from 49% (Onze de Setembre) to 95% (Rocafort) in the finer particles, and from 37% (Onze de Setembre) to 89% (Poble Sec) in the coarser particles. These Fe-rich particles are typically present with other particles including silicates (especially in the coarse fraction) and sodium salts (more frequent in the finer size). Aluminium and magnesium silicates (crustal particles, mostly sourced from the ballast) were also identified in all stations and were more common in the coarser size as expected, especially in Tetuan (Table 1), whereas fine mineral particles were predominant in the Palau Reial station. Similarly Na salts (halite and other salts) were very frequent in fine and coarse samples from Tetuan, Onze de Setembre and Llefià, but were not identified in Santa Coloma. Salt particles were more abundant in the coarse fraction in the cases of Joanic, Rocafort and Onze de Setembre; however, the rest of the stations showed a preference for finer particles. This could be related to the abundant presence of other salt species in these samples in addition to the marine salt, as many Na-rich particles (without Cl) were observed attached to Fe-rich particles in the PM1–2.5 samples. Similarly, sulphate particles were more abundant in the finer size fraction especially in the case of Llefià where gypsum (calcium sulphate) was the most common (14% in the PM1–2.5 samples), whereas in the other stations sulphur was more commonly associated with metals such as Ba (especially in the coarse fraction of Onze de Setembre in the new line) or Zn. Such particles rich in Ba, and sometimes Zn, are emitted by brakes and were recognised in all sites except in the new station of Llefià, being particularly abundant in Santa Coloma (24% fine samples). This is in agreement with the high concentrations of Ba analysed in PM2.5 filters collected on the platform of this station.2 Cu-rich particles were abundant in the Joanic samples, especially in the finer size fraction, and attributed to sourcing from the use of copper catenary. Copper-bearing particles, although much less common, were also identified in all other conventional stations (although not in the new PSD line). Given the fact that such stations operate using a graphite catenary, the origin of the copper is most likely to be from the composition of the lateral brakes used in these lines. No clear differences can be observed in the particle composition according to the design of the stations, indicating that their sources are similar independent of the age, depth, number of rail tracks or presence of platform screen doors in the stations.
| PM1–2.5/PM2.5–10 | Crustal | Na + Cl | SO42− | Fe2O3 | Other metals |
|---|---|---|---|---|---|
| Tetuan | 0.11 (4%) | 1.44 (32%) | PM2.5–10 (<1%) | 0.94 (63%) | 1.58 (1%) |
| Joanic | PM2.5–10 (2%) | PM2.5–10 (9%) | 1.53 (6%) | 1.37 (74%) | 1.15 (9%) |
| Palau Reial | 9.16 (16%) | 2.89 (6%) | 0.48 (2%) | 0.79 (64%) | 0.53 (12%) |
| Poble Sec | 1.06 (4%) | 1.32 (9%) | — | 0.89 (83%) | PM1–2.5 (5%) |
| Rocafort | 0.43 (4%) | PM2.5–10 (<1%) | — | 1.11 (91%) | 0.43 (4%) |
| Santa Coloma | PM2.5–10 (2%) | — | — | 1.17 (71%) | 0.78 (27%) |
| Onze de Setembre | 0.78 (14%) | 0.68 (33%) | PM1–2.5 (3%) | 1.31 (43%) | 0.78 (7%) |
| Llefià | 0.45 (3%) | 2.24 (15%) | 6.29 (9%) | 0.75 (73%) | — |
The distributions shown in Table 1 reflect the dominant heterogeneity of particle sizes between stations depending on their composition, with the exception of Fe-rich particles that are equally frequent for both PM sizes. There is no clear explanation for mineral particles being more frequent in the finer size fraction in some of the stations, these particles come mostly from the presence of granite ballast holding the rail tracks and the resuspension of dust deposited on the platforms; however the presence or absence of ballast does not seem to influence the relative concentration of these particles in the studied stations (Palau Reial, Joanic, Poble Sec and Rocafort have ballast). Equally calcium sulphate (gypsum) and sea salt particles are distributed differently depending on the station. Although metal particles are preferentially concentrated in the finer size fraction in some stations, the opposite occurs in others suggesting in this case a possible nugget effect.10
The differences in size distribution for PM chemical components in the subway system compared to outdoor ambient air may be related to the differences in the formation processes and later alterations of subway airborne particles. Outdoor particles in Barcelona are dominated by mineral dust, secondary inorganic compounds and traffic related PM (48, 16 and 14% in PM2.5–10, and 27, 23 and 23% in PM2.5), followed by particles from fuel oil, industries and sea salt.14 In the subway environment, particles are mainly generated by mechanical friction between surfaces that can reach high temperatures, and can be subsequently strongly altered and/or milled by the constant movement of trains. In addition, the chemical composition of single particles in the subway environment is complex with most of them being present as agglomerates with smaller particles being attached to larger ones. In the PM1–2.5 fraction we detected in most cases that the majority of the Fe particles present a coating made up of salts (Na, Cl, S, Mg, Ca,…) and small particles of mineral matter (including Ca-carbonates, clay minerals and quartz).
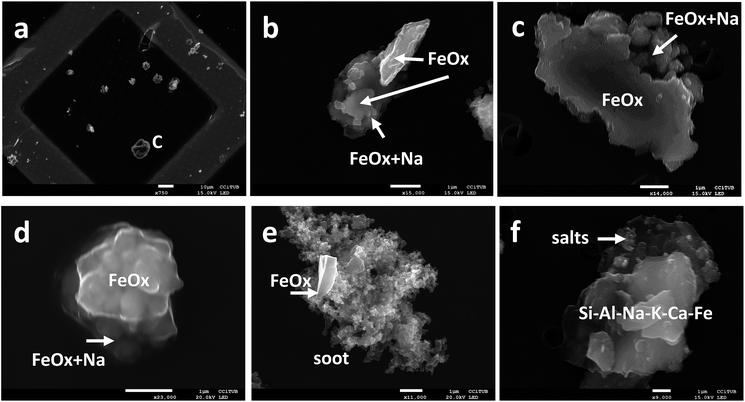
Detailed analysis using a back scattered electron detector (BSED) coupled with an Everhart Thornley Detector (ETD) for secondary electrons, as well as TEM-DRX analysis, showed that an important proportion of particles comprise a nucleus made of metallic Fe that is coated by hematite (Fe2O3), with a bright signal with BSED (indicative of a high density material), and being itself coated with a low BSED signal (indicative of a low density material) that clearly defines an external layer or crust. EDX analysis demonstrated that these external crustal layers are composed of salts and mineral matter. Fig. 2 shows a random typical grid area where particles were analysed (Fig. 2a) and provides examples of particles showing a high Fe-oxide core with an external rim richer in salts (Fig. 2b–d). The presence of a mixture of iron metal covered by various oxides has already been recorded in previous studies in subway particles, being interpreted as a record of a progressive oxidation of iron splinters and flakes to magnetite, maghemite and hematite.5,10–13,15 Unaltered Fe-oxide particles have also been observed either isolated or attached to other particles such as carbonaceous soot (Fig. 2e) emitted by traffic sources, most probably from the outside ambient air or diesel emissions during tunnel nightworks.
In the PM2.5–10 fraction we observed a similar behaviour for mineral particles, which are also typically surrounded by salts (Fig. 2f, with Na–S–Ca–Cl) and Fe-rich (from wheel and rail wear) particles.
We interpret these structural and chemical patterns to be the result of the emission of mainly PM2.5 (or smaller) train and rail wear primary particles, which were mostly made up originally of metallic Fe (Fig. 3a) and were rapidly oxidised (Fe2O3, Fig. 3b). The morphologies of these particles, generally planar with few spherical forms, suggest a mechanical origin by the friction of rails, wheels and brakes rather than condensation of Fe vapour.10 However during the friction that caused the emission of abrasion products high temperatures would have been reached, promoting the subsequent rapid oxidation of the native metal particle.9,11,16 A film of water condensing on the cooled particle surface (Fig. 3c) then formed a liquid coating in which soluble (Na–Cl–S–Ca–Mg) and insoluble (calcite, CaCO3 and a variety of aluminium silicates and quartz phases, as well as tiny FePM) phases were present (Fig. 3d and e). Evaporation of this film left the Fe-rich particles coated by an external salt and mineral matter crust (Fig. 3f). A similar layered structure resulting in the addition of a mineral coating was also observed by close examination of silicate PM in our samples. A graphic illustration of the progressive modification of the original PM, both metallic and non-metallic, is provided in Fig. 3. Other metal particles present in the subway environment (e.g. Zn, Ba, Cr, Mn or Cu) can equally be altered similarly to Fe oxides.
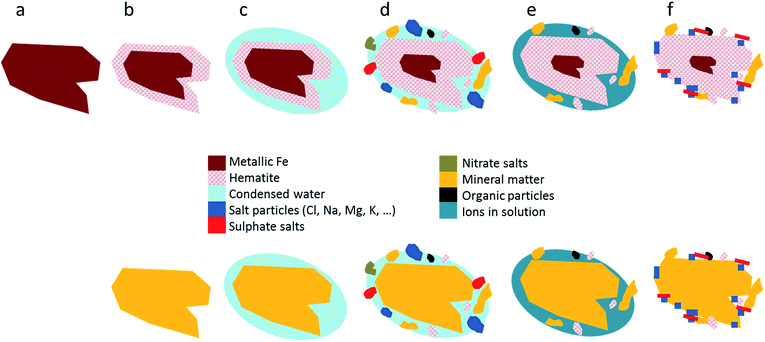 | ||
| Fig. 3 Schematic process from “a” to “f” of Fe-rich (top) and mineral (bottom) particle alteration and formation of new smaller particles attached to them. See text for discussion. | ||
Conclusions
The processes involved in the generation of airborne particles in the subway air and their subsequent alteration are very different from those happening in other environments. The immense majority of the particles are produced by the movement of trains, with the mechanical friction between wheels, rails and brakes, with the elements Na, Zn, Mg, Al, Si, S, Cl, K, Ca, Ba, Ti, Cr, Mn, Fe, Cu, Mo, Pd, P and Mo being detected by EDX coupled to electron microscopy. The main components observed under the SEM can be grouped as crustal (Mg, Al, SI, K, Ca, Ti, and P), sea salts (Na and Cl), sulphates, hematite (calculated assuming all iron as hematite) and other metals (Zn, Ba, Cr, Mn and Cu).After studying PM collected on 8 different subway platforms the average chemical composition of these samples is clearly and predictably dominated by hematite in both coarse and fine fractions for all types of stations (up to 88%), followed by a crustal component (up to 19% in the coarse fraction), marine aerosols (up to 9% coarse fraction), other metals (up to 9% coarse fraction), and sulphates (up to 5% coarse fraction), not considering carbon as a component as samples had to be coated with this element for their observation in the SEM and TEM.
In any case, the relative contribution of each of the chemical components between the fine and coarse fractions is very variable depending on the station, with no clear differences according to the design of the stations, indicating that their sources are similar independent of the age, depth, number of rail tracks or presence of platform screen doors. The lack of similar size distribution for PM chemical components in the subway system compared to outdoor ambient air may be related to the differences in the formation processes and later alterations.
Close microscopic observation of the Fe-rich airborne particles in the subway environment reveals that most of them possess a coating made up of salts (Na, Cl, S, Mg, Ca,…) and small particles of mineral matter (including Ca-carbonates, clay minerals and quartz). The morphologies of these particles are generally planar with few spherical forms, conforming with a mechanical origin from the friction produced between rails, wheels and brakes rather than condensation of Fe vapour. However during the friction that caused the emission of abrasion products high temperatures were reached and water could condense on the surface forming a water coating in which soluble and insoluble phases are adsorbed, and evaporation of this water leaves an external salt and mineral matter rim coated on the particles.
Acknowledgements
This study was supported by the Spanish Ministry of Economy and Competitiveness and FEDER funds (METRO CGL2012-33066), the IMPROVE LIFE Project (LIFE13 ENV/ES/000263), the European Union Seventh Framework Programme (FP7/2007–2013) under grant agreement no. 315760 HEXACOMM and the Korean Ministry of Environment through “The Eco-Innovation project”. Support from Generalitat de Catalunya 2014 SGR33 is also acknowledged.References
- T. Moreno, N. Pérez, C. Reche, V. Martins, E. de Miguel, M. Capdevila, S. Centelles, M. C. Minguillón, F. Amato, A. Alastuey, X. Querol and W. Gibbons, Subway platform air quality: assessing the influences of tunnel ventilation, train piston effect and station design, Atmos. Environ., 2014, 92, 461–468 CrossRef CAS.
- V. Martins, T. Moreno, M. C. Minguillón, B. L. Van Drooge, C. Reche, F. Amato, E. De Miguel and X. Querol, Origin of inorganic and organic components of PM2.5 in subway stations of Barcelona, Spain, Environ. Pollut., 2016, 208, 125–136 CrossRef CAS PubMed.
- L. G. Murruni, V. Solanes, M. Debray, A. J. Kreiner, J. Davidson, M. Davidson, M. Vázquez and M. Ozafrán, Concentrations and elemental composition of particulate matter in the Buenos Aires underground system, Atmos. Environ., 2009, 43, 4577–4583 CrossRef CAS.
- S. Abbasi, A. Jansson, U. Sellgren and U. Olofsson, Particle emissions from rail traffic: a literature review, Crit. Rev. Environ. Sci. Technol., 2013, 43, 2511–2544 CrossRef CAS.
- M. H. Jung, H. R. Kim, Y. J. Park, D. S. Park, K. H. Chung and S. M. Oh, Genotoxic effects and oxidative stress induced by organic extracts of particulate matter (PM10) collected from a subway tunnel in Seoul, Korea, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2012, 749, 39–47 CrossRef CAS.
- K. Midander, K. Elihn, A. Wallén, L. Belova, A.-K. B. Karlsson and I. O. Wallinder, Characterisation of nano- and micron-sized airborne and collected subway particles, a multi-analytical approach, Sci. Total Environ., 2012, 427–428, 390–400 CrossRef CAS PubMed.
- V. Mugica-Álvarez, J. Figueroa-Lara, M. Romero-Romo, J. Sepúlveda-Sánchez and T. López-Moreno, Concentrations and properties of airborne particles in the Mexico city subway system, Atmos. Environ., 2012, 49, 284–293 CrossRef.
- X. Querol, T. Moreno, A. Karanasiou, C. Reche, A. Alastuey, M. Viana, O. Font, J. Gil, E. de Miguel and M. Capdevila, Variability of levels and composition of PM10 and PM2.5 in the Barcelona metro system, Atmos. Chem. Phys., 2012, 12, 5055–5076 CAS.
- M. Loxham, M. J. Cooper, M. E. Gerlofs-Nijland, F. R. Cassee, D. E. Davies, M. R. Palmer and D. A. H. Teagle, Physicochemical characterization of airborne particulate matter at a mainline underground railway station, Environ. Sci. Technol., 2013, 47, 3614–3622 CrossRef CAS PubMed.
- T. Moreno, V. Martins, X. Querol, T. Jones, K. BéruBé, M. C. Minguillón, F. Amato, M. Capdevila, E. de Miguel, S. Centelles and W. Gibbons, A new look at inhalable metalliferous airborne particles on rail subway platforms, Sci. Total Environ., 2015, 505, 367–375 CrossRef CAS PubMed.
- S. Kang, H. Hwang, Y. Park, H. Kim and C. U. Ro, Chemical compositions of subway particles in Seoul, Korea determined by a quantitative single particle analysis, Environ. Sci. Technol., 2008, 42, 9051–9057 CrossRef CAS PubMed.
- B.-W. Kim, H.-J. Jung, Y.-C. Song, M.-J. Lee, H.-K. Kim, J.-C. Kim, J.-R. Sohn and C. U. Ro, Characterization of summertime aerosol particles collected at subway stations in Seoul, Korea using Low-Z Particle Electron Probe X-ray Microanalysis. Asian J, Atmos. Environ., 2010, 4, 97–105 CrossRef.
- H.-J. Eom, H.-J. Jung, S. Sobanska, S.-G. Chung, Y.-S. Son, J.-C. Kim, Y. Sunwoo and C.-U. Ro, Iron speciation of airborne subway particles by the combined use of energy dispersive electron probe X-ray microanalysis and Raman microspectrometry, Anal. Chem., 2013, 85, 10424–10431 CrossRef CAS PubMed.
- C. Reche, T. Moreno, F. Amato, M. Viana, B. van Drooge, H. C. Chuang, K. Berube, T. Jones, A. Alastuey and X. Querol, A multidisciplinary approach to characterise exposure risk and toxicological effects of PM10 and PM2.5 samples in urban environments, Ecotoxicol. Environ. Saf., 2012, 78, 327–335 CrossRef CAS PubMed.
- L. D. Knibbs, T. Cole-Hunter and L. Morawska, A review of commuter exposure to ultrafine particles and its health effects, Atmos. Environ., 2011, 45, 2611–2622 CrossRef CAS.
- I. Salma, Air pollution in underground railway systems, in Air Quality in Urban Environments, Royal Society of Chemistry, 2009, pp. 65–84 Search PubMed.
| This journal is © The Royal Society of Chemistry 2017 |