Cigarette butt-derived carbons have ultra-high surface area and unprecedented hydrogen storage capacity†
L. Scott Blankenship and
Robert Mokaya
and
Robert Mokaya *
*
University of Nottingham, University Park, Nottingham NG7 2RD, UK. E-mail: r.mokaya@nottingham.ac.uk
First published on 24th October 2017
Abstract
Discarded cigarette filters, in the form of cigarette butts, are a major waste disposal and environmental pollution hazard due to mainly containing cellulose acetate which is non-biodegradable; 5.8 trillion cigarettes are smoked worldwide per annum generating >800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons of cigarette butts. Apart from causing litter, cigarette butts contain contaminants such as toxic heavy metals, which can leach into waterways, potentially causing harm to both humans and wildlife. In an effort to turn dangerous waste into high value products, this study explores the valorisation of discarded smoked cigarette filters/butts. We show that porous carbons derived from cigarette butts, via sequential benign hydrothermal carbonisation and activation, are super porous and have ultra-high surface area (4300 m2 g−1) and pore volume (2.09 cm3 g−1) arising almost entirely (>90%) from micropores. The carbons also have uncharacteristically high oxygen content associated with O-containing functional groups (COOH, C–OH and C
000 metric tons of cigarette butts. Apart from causing litter, cigarette butts contain contaminants such as toxic heavy metals, which can leach into waterways, potentially causing harm to both humans and wildlife. In an effort to turn dangerous waste into high value products, this study explores the valorisation of discarded smoked cigarette filters/butts. We show that porous carbons derived from cigarette butts, via sequential benign hydrothermal carbonisation and activation, are super porous and have ultra-high surface area (4300 m2 g−1) and pore volume (2.09 cm3 g−1) arising almost entirely (>90%) from micropores. The carbons also have uncharacteristically high oxygen content associated with O-containing functional groups (COOH, C–OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), and show anomalous behaviour with respect to the effect of activation temperature on porosity, the latter being ascribable to the chemical mix present in cigarette butts and their hydrochar products. Due to the combined effects of high surface area, high microporosity and an oxygen-rich nature, the carbons exhibit unprecedentedly high hydrogen storage capacity of 8.1 wt% excess uptake, and 9.4 wt% total uptake at −196 °C and 20 bar, rising to total uptake of 10.4 wt% and 11.2 wt% at 30 and 40 bar, respectively. The hydrogen storage capacity is the highest reported to date for any porous carbons and attains new levels for porous materials in general. This work also raises the question on whether valorisation can solve the intractable cigarette butt problem.
O), and show anomalous behaviour with respect to the effect of activation temperature on porosity, the latter being ascribable to the chemical mix present in cigarette butts and their hydrochar products. Due to the combined effects of high surface area, high microporosity and an oxygen-rich nature, the carbons exhibit unprecedentedly high hydrogen storage capacity of 8.1 wt% excess uptake, and 9.4 wt% total uptake at −196 °C and 20 bar, rising to total uptake of 10.4 wt% and 11.2 wt% at 30 and 40 bar, respectively. The hydrogen storage capacity is the highest reported to date for any porous carbons and attains new levels for porous materials in general. This work also raises the question on whether valorisation can solve the intractable cigarette butt problem.
Broader contextThis manuscript not only addresses an intractable environmental pollution problem – cigarette butts – and also offers new insights on a valorisation route to the best performing hydrogen storage materials to date as part of the drive towards the anticipated Hydrogen Economy wherein efficient storage and transportation of hydrogen are key to exploitation of hydrogen as an energy source. Discarded cigarette filters, in the form of cigarette butts, are a major waste disposal and environmental pollution hazard due to the fact that they are mainly composed of cellulose acetate which is non-biodegradable. The problem is huge; 5.8 trillion cigarettes are smoked worldwide per annum generating >800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons of cigarette butts. Apart from causing unsightly litter, cigarette butts contain contaminants such as toxic heavy metals, which can leach into waterways, potentially causing harm to both humans and wildlife. In an effort to turn such undesirable and dangerous waste into high value products, this study explores the valorisation of discarded smoked cigarette filters/butts. We show that porous carbons derived from cigarette butts, via sequential benign hydrothermal carbonisation and activation, are super porous with ultra-high surface area (4300 m2 g−1) and pore volume (2.09 cm3 g−1), and exhibit unprecedentedly high hydrogen storage capacity. This work, therefore, not only raises the interesting question of whether valorisation can solve the intractable cigarette butt problem but also offers hydrogen storage materials that attain new levels for porous materials in general. 000 metric tons of cigarette butts. Apart from causing unsightly litter, cigarette butts contain contaminants such as toxic heavy metals, which can leach into waterways, potentially causing harm to both humans and wildlife. In an effort to turn such undesirable and dangerous waste into high value products, this study explores the valorisation of discarded smoked cigarette filters/butts. We show that porous carbons derived from cigarette butts, via sequential benign hydrothermal carbonisation and activation, are super porous with ultra-high surface area (4300 m2 g−1) and pore volume (2.09 cm3 g−1), and exhibit unprecedentedly high hydrogen storage capacity. This work, therefore, not only raises the interesting question of whether valorisation can solve the intractable cigarette butt problem but also offers hydrogen storage materials that attain new levels for porous materials in general.
|
1. Introduction
Hydrogen is an attractive energy source especially for motor vehicles due to its high gravimetric energy capacity as well as the fact that it does not produce any CO2 emissions.1–7 Hydrogen may be used to produce energy via a hydrogen fuel cell, which is a redox cell wherein the chemical energy of hydrogen is converted to electrical energy through use of an oxidising agent (usually oxygen).7,8 An alternative to using a hydrogen fuel cell in a car is to burn the hydrogen in a manner similar to that of a combustion engine.9,10 Targets, for the year 2020, set by the United States Department of Energy (DOE) for hydrogen-powered cars include gravimetric energy of 1.8 kW h kg−1 (5.5 wt% H2), and energy density of 1.3 kW h L−1 (0.040 kg L−1).11 Solid state materials are considered to be a viable route to achieving the required level of hydrogen storage.12–14 Storage materials currently under investigation can be divided into two main groups, the first of which store hydrogen via chemisorption, including, for example, metal hydrides,15 while the second group use physisorption and are typically highly porous materials with a high surface area such as carbons,16–30 metal organic frameworks (MOFs)31–37 and covalent organic frameworks (COFs).38–40 It is now well established that the hydrogen storage capacity of porous solid state materials is dependent on surface area (with high area favouring good uptake) and the presence of micropores.16–59 In terms of porosity, optimised hydrogen storage materials should possess high surface area that arises from micropores.The ongoing search for new materials is a key factor in attempts to achieve the hydrogen storage targets required to make the mooted Hydrogen Economy a reality.12–40 The search for new hydrogen stores targets materials that have new or improved properties, are easier or cheaper to prepare, and that are sustainable. Improved properties can be engendered by tailored design based on knowledge of what is required for hydrogen storage, while sustainability may be achieved via valorisation routes with respect to the starting materials. As part of our on-going search for carbon materials with optimised properties for hydrogen storage, we have recently shown that cellulose-based precursors are good starting materials for such porous carbons.20,22 This is of particular interest, with respect to valorisation of waste materials, because a cellulose derivate (i.e., cellulose acetate) is the principal component of cigarette filters.60,61 The import of this connection is that discarded cigarette filters, in the form of cigarette butts, are currently a major waste disposal and environmental pollution hazard because cellulose acetate, though photodegradable (in the presence of TiO2) is not biodegradable.62–67 The number of cigarettes smoked worldwide per annum tops 5.8 trillion and generates in the order of 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons of cigarette butts.63,68 For example, disposed cigarette filters (butts) make up the majority, by mass, of litter found on beaches.69 A further hazard are the contaminants, especially toxic heavy metals, present in cigarette filters, which leach into waterways, potentially causing harm to both humans and wildlife.70–72 It is therefore clear that cigarette butts need to be taken out of the environment. It would be highly desirable if the cigarette butts, rather than being disposed of, are converted to a high value product via a valorisation process. Given that they are mainly composed of cellulose acetate, the aforementioned factors make cigarette butts waste a particularly attractive starting material for valorisation to porous (e.g., activated carbons). This would be in line with the current trend to move away from coal-based carbonaceous precursors to biomass-derived or waste-based starting materials for activated carbon synthesis.16
000 metric tons of cigarette butts.63,68 For example, disposed cigarette filters (butts) make up the majority, by mass, of litter found on beaches.69 A further hazard are the contaminants, especially toxic heavy metals, present in cigarette filters, which leach into waterways, potentially causing harm to both humans and wildlife.70–72 It is therefore clear that cigarette butts need to be taken out of the environment. It would be highly desirable if the cigarette butts, rather than being disposed of, are converted to a high value product via a valorisation process. Given that they are mainly composed of cellulose acetate, the aforementioned factors make cigarette butts waste a particularly attractive starting material for valorisation to porous (e.g., activated carbons). This would be in line with the current trend to move away from coal-based carbonaceous precursors to biomass-derived or waste-based starting materials for activated carbon synthesis.16
There has previously been some interest in the use of cigarette filters as starting material for the formation of porous carbons. Polarz et al. carbonised cellulose acetate from cigarette filters to generate lowly porous (surface area of 262 m2 g−1 and pore volume of 0.21 cm3 g−1) non-graphitic carbons.73 Chen et al. prepared p6mm mesostructured carbonaceous materials with surface area of 526 m2 g−1, pore volume of 0.59 g cm3 g−1 and pore width of 5.1 nm via an evaporation-induced self-assembly method using cigarette filters as matrix scaffold.74 Lee et al. prepared micro–mesoporous carbon with surface area of 573 m2 g−1 from carbonisation of cigarette filters, while samples carbonised under ammonia had higher surface area of 1634 m2 g−1.75 However, as far as we are aware, there has so far been no report on preparation of activated carbons from cigarette filters or waste cigarette butts. In this study we explore the preparation of activated carbons from cigarette filters and discarded cigarette butts and assess the hydrogen uptake capability of the resulting carbons. This study unravels some unexpected and anomalous trends between activating conditions and the properties, especially porosity, of the resulting activated carbons and how this affects hydrogen uptake capacity. We discuss the findings in terms of how the chemical mix present in cigarette butts affects the activation process.
2. Experimental section
2.1 Materials synthesis
Used cigarette filters/butts were collected to form the precursor for the S (smoked) group of carbons. Unused (or ‘fresh’) filters that were used to generate the F group of carbons were obtained from purchased cigarettes (of similar brand as S group) following separation of the filter from the cigarette. In both cases, the wrapping paper was removed from the filter, care being taken to avoid contamination with tobacco or ash. The cigarette filters were then ground in a spice grinder to produce a fluffy white or yellow-brown mass, for F and S filters, respectively. The ground cigarette filters were then hydrothermally carbonised by heating a mixture of the filters and water (at a ratio of 1 g filter to 10 mL water) in a stainless steel autoclave to 250 °C at a ramp rate of 5 °C min−1, holding at 250 °C for 2 h and then cooling to room temperature at a ramp rate of 5 °C min−1. The resulting carbonaceous matter (hydrochar) was recovered and dried at 112 °C. The hydrochars were denoted as FF-hydrochar (fresh filters) or SF-hydrochar (smoked filters).For the activation a KOH/hydrochar ratio of 4 was used. A ground and homogenous KOH/hydrochar mixture, in alumina boats, was placed in a horizontal furnace and heated under nitrogen to 600, 700 or 800 °C at a ramp rate of 3 °C min−1. Samples were held at the target temperature for 1 h and then allowed to cool under nitrogen. The generated activated carbons were recovered by filtration and washed in 2 M HCl with stirring at room temperature and then with deionised water until washings had neutral pH. The samples were dried in an oven at 112 °C. The activated carbons were designated as FF-4T (group F, fresh filters) or SF-4T (group S, smoked filters) where 4 is the KOH/hydrochar ratio and T is the activation temperature (600, 700 or 800 °C).
2.2 Materials characterisation
Thermogravimetric analysis (TGA) was performed using a TA Instruments SDT Q600 analyser under flowing air conditions (100 mL min−1). Powder XRD analysis was performed using a PANalytical X’Pert PRO diffractometer with Cu-Kα light source (40 kV, 40 mA) with step size of 0.02° and 50 s time step. CHN elemental analysis was performed using an Exeter Analytical CE-440 Elemental Analyser. Inorganic (metal) content was determined via ICP-OES analysis using a Perkin Elmer Optima 2000 DV ICP-OES analyser. Temperature programmed desorption (TPD) was performed using a Hiden CATLAB system with He as carrier gas. Infra-red spectroscopy (FTIR) spectra was obtained using a Bruker Alpha FTIR spectrometer. Porosity analysis and determination of textural properties was performed via nitrogen sorption using a Micromeritics 3FLEX sorptometer. Prior to analysis (at −196 °C), the carbons were degassed under vacuum at 200 °C for 12 h. The apparent surface area (hereinafter referred to simply as surface area) was calculated using the Brunauer–Emmett–Teller (BET) method applied to adsorption data in the relative pressure (P/P0) range 0.04–0.22. The total pore volume was determined from the nitrogen uptake at close to saturation pressure (P/P0 ≈ 0.99). The micropore surface area and micropore volume were determined via t-plot analysis. Non-local density functional theory (NL-DFT) was applied to nitrogen adsorption isotherms to determine pore size distribution.2.3 Hydrogen uptake measurements
Hydrogen uptake capacity of the carbons was measured by gravimetric analysis with a Hiden XEMIS Intelligent Gravimetric Analyser using 99.9999% purity hydrogen additionally purified by a molecular sieve filter. Prior to analysis, the carbon samples were dried for 24 h at 80 °C and then placed in the analysis chamber and degassed at 200 °C and 10−10 bar for 4–6 h. The hydrogen uptake measurements were performed at −196 °C (in a liquid nitrogen bath) over the pressure range of 0 to 40 bar.3. Results and discussion
3.1 Elemental composition and nature of carbon
The starting materials in this study, fresh (FF) or smoked (SF) cigarette filters were first converted to hydrochar (FF-hydrochar and SF-hydrochar, respectively) via hydrothermal carbonisation. Given the nature of cigarette filters (or butts for smoked cigarettes) with respect to their chemical composition, wherein the main component is cellulose acetate along with a host of other additives including metals, we analysed the cigarette butt derived hydrochars and compared them to cellulose acetate-derived hydrochar.22 Firstly, we determined the carbon content of the FF and SF hydrochars as shown in Table 1 and Table S1 (ESI†). The C and H content of the fresh FF-hydrochar, at 63.6 and 4.2 wt%, respectively, is slightly lower than that of the smoked SF-hydrochar (68.5 and 5.7 wt%, respectively), meaning that the former has a higher oxygen content (32.2 wt%) compared to 24.8 wt% for the latter. It is also noteworthy that the SF-hydrochar contains some N while the FF-hydrochar appears to be N-free. The presence of N in the SF-hydrochar may be explained by nicotine that is trapped in the filters during smoking, while for the fresh un-smoked cigarettes such trapping is absent. Comparison with CA-hydrochar derived from cellulose acetate (Table S1, ESI†) shows, in general, a similar C, H, O content to that of the cigarette filter or butt derived hydrochars, which is consistent with the fact that cellulose acetate is the main component of cigarette filters. As shown in Table 1, activation of both the FF and SF hydrochars results in an increase in the C content, with the increase being generally greater at higher activation temperature. On the other hand, the H content decreases significantly, while the oxygen content shows a less drastic reduction. This means that the cigarette filter or butt derived activated carbons possess higher oxygen content (16–31 wt%) than is normally observed for activated carbons, including those derived from cellulose (4.4–11.5 wt%) but similar to those derived from cellulose acetate.16,20,22 The SF series of activated carbons also retain some N content of between 0.4 and 1 wt%.| Sample | C [%] | H [%] | N [%] | O [%] | (O/C)a | (H/C)a |
|---|---|---|---|---|---|---|
| a Atomic ratio. | ||||||
| FF-hydrochar | 63.6 | 4.2 | 0 | 32.2 | 0.380 | 0.793 |
| SF-hydrochar | 68.5 | 5.7 | 1.0 | 24.8 | 0.272 | 1.000 |
| FF-4600 | 66.9 | 1.9 | 0 | 31.2 | 0.350 | 0.340 |
| FF-4700 | 78.3 | 0.5 | 0 | 21.2 | 0.203 | 0.077 |
| FF-4800 | 82.5 | 0.4 | 0 | 17.1 | 0.156 | 0.058 |
| SF-4600 | 70.5 | 0.6 | 1.1 | 27.8 | 0.296 | 0.102 |
| SF-4700 | 77.6 | 0.7 | 0.4 | 21.3 | 0.206 | 0.108 |
| SF-4800 | 82.8 | 0.3 | 0.5 | 16.4 | 0.149 | 0.044 |
The presence and amount of O in the activated carbons was probed using temperature programmed desorption (TPD). The TPD was performed after thermal evacuation of the carbons at 150 °C, and thus probed the nature of O-functional groups that are stable under such conditions. The TPD profiles for the evolution of CO2 and CO for the two sets of activated samples are shown in Fig. 1. Desorption of CO2 occurs in the temperature range 150–600 °C, and it is noticeable that the temperature of maximum desorption slightly shifts to higher values as activation temperature rises. Desorption of CO is observed within the temperature range of 300 and 900 °C with desorption maxima in the range 600–750 °C. The TPD profiles are consistent with the expectation that the CO2 arises from O-containing functional groups that are thermally less stable, including carboxylic acids, lactones and anhydrides, while the CO is from thermally more stable moieties such as carbonyls, quinonic or phenolic groups.22,76–78 In general the TPD profiles are similar to those that have previously been reported for activated carbons.22,76–78 The high oxygen content of the present carbons (16–31 wt%) may be evidenced by a comparison of their CO2 and CO TPD profiles to those of an activated carbon derived from cellulose (designated as C-4700) that has oxygen content of 7.1 wt%.20 Such a comparison (Fig. S1, ESI†) shows that much higher CO2 and CO is evolved from the SF-4T carbons compared to sample C-4700, which is consistent with the greater oxygen content of the former. The O-functional groups on the activated carbons were also probed with FTIR. The IR spectra (Fig. S2, ESI†) of the SF-hydrochar and representative activated carbon (sample SF-4600) show several bands arising from O-functional groups, including the following; C–OH stretch band at ca. 3430 cm−1, C–OH bend band at ca. 1625 cm−1, C–O vibration at 1380 cm−1, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak at 1710 cm−1.22,79–82 The spectra of sample SF-4600 does not, however, exhibit C–H peaks at ca. 2850 and 1450 cm−1 that are observed for the SF-hydrochar, which is in agreement with the much lower H content of the activated carbon (Table 1). The IR spectra offers further evidence for a high content of oxygen functional groups, which is consistent with the O content of the present carbons.
O peak at 1710 cm−1.22,79–82 The spectra of sample SF-4600 does not, however, exhibit C–H peaks at ca. 2850 and 1450 cm−1 that are observed for the SF-hydrochar, which is in agreement with the much lower H content of the activated carbon (Table 1). The IR spectra offers further evidence for a high content of oxygen functional groups, which is consistent with the O content of the present carbons.
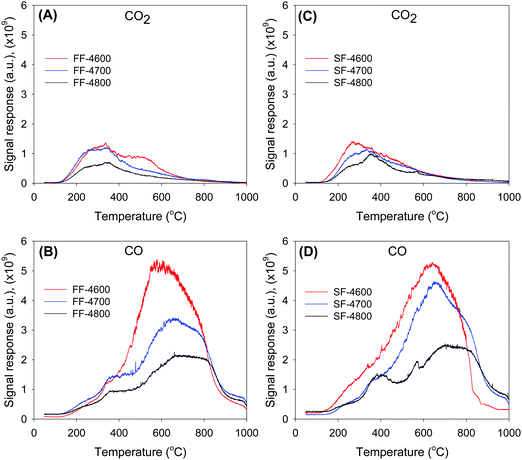 | ||
| Fig. 1 Evolution of CO2 (A and C) and CO (B and D) under TPD conditions for activated carbons derived from fresh cigarette filters (A and B) and smoked cigarette butts (C and D). | ||
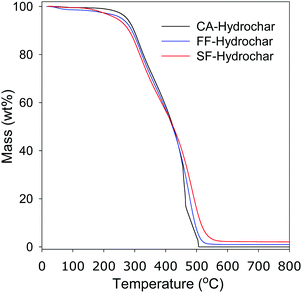
Given that cigarettes are known to contain a mix on non-carbonaceous additives, including metals, we performed thermogravimetric analysis of the FF and SF hydrochars in an attempt to gauge the amount of inorganic residue they contain. Fig. 2 shows the TGA curves for the FF and SF hydrochars, and CA-hydrochar obtained from pure cellulose acetate. All three hydrochars are stable below 200 °C, after which carbon combustion occurs in a single mass-loss event that is completed at ca. 550 °C. It is interesting to note that the CA-hydrochar was completely burnt off and no residue was left at 600 °C, while the FF-hydrochar and SF hydrochar had residual mass of ca. 1 and 2–2.5 wt%, respectively. We attribute the residual mass to inorganic matter (likely in the form of metal oxides) arising from metal additives that are known to be present in smoked cigarettes filters/butts.70–72 The smoked SF-hydrochar has higher residual mass as more of the additives are transferred to and trapped in the filters (or butts) when the cigarette is smoked.70–72 We performed powder XRD analysis of the hydrochars in an effort to establish the presence of crystalline inorganic matter as would be indicated by sharp peaks that are uncharacteristic of porous carbon frameworks. The XRD patterns in Fig. 3 show no sharp peaks for the CA-hydrochar, while some peaks are observed in the patterns of the FF and SF hydrochar, with the peaks being more prominent for the SF-hydrochar. Thus the XRD patterns are consistent with the presence of non-carbonaceous matter in the FF and SF hydrochars with the later having greater amounts, which is in agreement with the TGA data in Fig. 2. Elemental analysis to determine the metal content of the SF-hydrochar, via ICP-OES, detected (i.e., close to or above 10 ppm) the presence of several metals, including Al, Ba, Cu, Fe, Ca, Na, K, Mg, Mn, and Zn. The most prevalent metals (i.e., close to or greater than 0.1 wt%) were Ca, K, Mg Na, and Al. This findings are consistent with previous reports on the additives present in cigarette butts.70–72Inter alia, the presence of metals (especially K, Na and Ca) in the hydrochars can be expected to influence their activation. This premise is based on the fact that the activating process, for example with KOH, arises from the action of K as activating agent. Indeed, the interesting mix of metal additives present in cigarette filters or butts was part of the motivation for using them as starting materials for activated carbons. We however note that the activated carbons were themselves free of metals or crystalline inorganic residues as indicated by the absence of sharp peaks in their XRD patterns (Fig. S3, ESI†). The extensive post activation washing process appears to remove all inorganic matter.
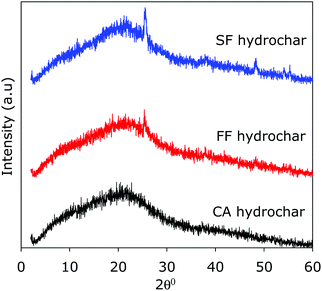 | ||
| Fig. 3 Powder XRD patterns of hydrochar derived from fresh (FF-hydrochar) and smoked (SF-hydrochar) cigarette filters, and from cellulose acetate (CA-hydrochar). | ||
3.2 Porosity
The porosity of the FF and SF series of activated carbons was probed by nitrogen sorption analysis. The nitrogen sorption isotherms of the FF series of activated carbons are given in Fig. 4A. All three FF-4T samples exhibit type I isotherms that are characteristic of microporous materials.83 The extent of porosity generated, as measured by the amount of nitrogen adsorbed, increases as activation temperature rises from 600 to 800 °C, which is the normal trend usually observed.16,20,26,84–94 The shape of the isotherms indicates that the pore size increases as activation temperature rises, which is the expected trend in line with the fact that increase in overall porosity in activated carbons is usually accompanied with isotherm changes arising from increase in the amount of nitrogen adsorbed, widening of the adsorption knee and increase in pore size.16,20,26,84–94Fig. 4B shows the pore size distribution (PSD) curves of the FF-4T carbons, and as expected the pore size distribution broadens as activation temperature rises. The pore size maxima obtained from the PSD curves is summarised in Table 2. Although the small micropores of size ≤1.2 nm are retained for all the FF-4T carbons, there is a gradual broadening of PSD to larger micropores of size up to 2 nm as activation temperature rises.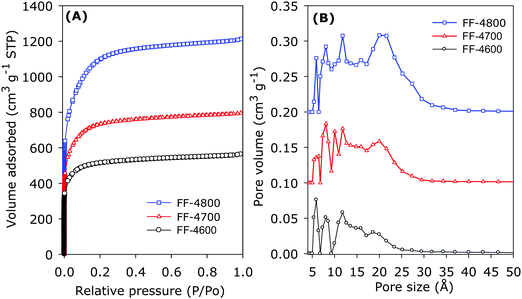 | ||
| Fig. 4 (A) Nitrogen sorption isotherms and (B) pore size distribution curves of FF series of activated carbons derived from fresh cigarette filters. See Experimental section for sample designation. | ||
| Sample | Surface areaa (m2 g−1) | Pore volumeb (cm3 g−1) | Pore sizec (Å) | H2 uptaked,e (wt%) | |||
|---|---|---|---|---|---|---|---|
| 1 bar | 20 bar | 30 bar | 40 bar | ||||
| a The values in the parenthesis refer to: micropore surface area. b Micropore volume. c Pore size distribution maxima obtained from NLDFT analysis. d Gravimetric (wt%) H2 uptake at −196 °C and various pressures (i.e., 1, 20, 30 and 40 bar). e The values in parenthesis are excess H2 uptake. | |||||||
| FF-4600 | 1970 (1512) | 0.86 (0.59) | 6/8/12/18 | 2.0 | 5.2 (4.6) | 5.6 (4.8) | 5.9 (4.9) |
| FF-4700 | 2803 (1901) | 1.23 (0.73) | 6/8/12/20 | 3.2 | 7.2 (6.4) | 7.8 (6.6) | 8.3 (6.7) |
| FF-4800 | 4113 (2075) | 1.87 (0.79) | 6/8/12/21 | 3.0 | 8.2 (7.0) | 9.1 (7.2) | 9.7 (7.3) |
| SF-4600 | 4310 (3867) | 2.09 (1.71) | 6.4/8/12/19 | 4.0 | 9.4 (8.1) | 10.4 (8.3) | 11.2 (8.4) |
| SF-4700 | 2512 (2019) | 1.20 (0.91) | 6/8/12.5/20 | 2.7 | 6.8 (6.0) | 7.4 (6.2) | 7.8 (6.3) |
| SF-4800 | 2393 (1810) | 1.09 (0.70) | 6.4/8/12/21 | 3.0 | 6.6 (5.9) | 7.2 (6.1) | 7.6 (6.2) |
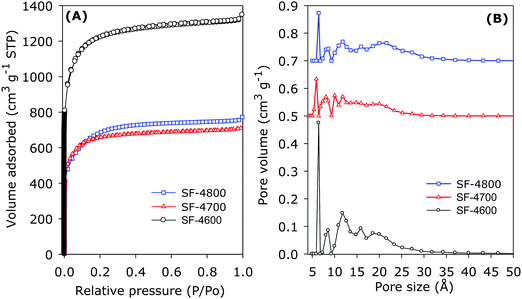
The nitrogen sorption isotherms of the SF-4T series of samples prepared from cigarette butts are shown in Fig. 5A. All the isotherms are type I and typical for microporous materials. However, the isotherms show an unusual, even anomalous, trend with respect to the effect of activation temperature. The extent of porosity generated, as measured by the amount of nitrogen adsorbed, decreases as activation temperature rises from 600 to 800 °C, which is the reverse of what is observed for FF-4T samples as described above (Fig. 4), and is the opposite of what is known to occur for activated carbons.16,20,26,84–94 For the SF-4T carbons, sample SF-4600 has the highest amount of adsorbed nitrogen. However, the shape of the isotherms suggests that the pore size increases as activation temperature rises. Thus for SF-4T samples, the increase in overall porosity is not in tandem with widening of the adsorption knee and increase in pore size.16,20,26,84–94 The PSD curves shown in Fig. 5B indicate that the pore size distribution broadens as activation temperature rises, but according to the isotherms, this change is accompanied by a decrease rather than increase in overall porosity. The increase in pore size of SF-4T carbons at higher activation temperature is clear from the pore size maxima obtained from the PSD curves as summarised in Table 2. Small micropores of size ≤1.2 nm are retained for all the SF-4T carbons in a manner similar to the FF-4T carbons, along with a gradual increase in the proportion of larger micropores of size up to 2 nm as activation temperature rises. The observed anomalous behaviour (i.e., highest porosity at 600 °C, which then decreases for samples activated at higher temperature), may be explained by the presence of significant amounts of metal additives in the SF-hydrochar as evidenced above by TGA, XRD and elemental analysis data. It is likely that the metal additives (namely, K, Ca, Na, Mg, etc.) retained in the hydrochar act as ‘extra’ activating agents in addition to the KOH thus resulting in what may be considered as ‘overactivation’ of the carbons prepared at temperatures higher than 600 °C. The main factors that determine the extent of activation of any carbon precursor under our activation regime are KOH/carbon ratio (i.e., amount of activating agent) and activation temperature. Increase in the KOH/carbon ratio and/or temperature causes greater activation. The two factors need to be judiciously combined to optimise the porosity generated. If the amount of activating agent, temperature or a combination of both is too high, then the activation conditions can be too severe and beyond the optimum conditions, which, in a sense, leads to ‘overactivation’ and a decrease in the porosity generated. Thus, there is a point beyond which the carbon becomes over activated leading to a decrease rather than increase in overall porosity (as indicated by apparent surface area and pore volume) – although the pore size can still rise. We postulate that for SF-4T carbons, activation temperature of 700 and 800 °C leads to over activation due to the ‘added’ activating effect of the metal additives already present in the SF-hydrochar. On the other hand, at 600 °C, the mix of activating agents (KOH and metal additives) is just right to generate what may be described as optimal porosity. The lower amount of metal additives in the FF-hydrochar means that the anomalous behaviour is not observed. The effect of metal additives was evidenced by the fact that simple heating of the non-porous SF-hydrochar at 600–800 °C for 1 h in the absence of KOH generated porous carbon (Fig. S4, ESI†) with apparent surface area and pore volume, at 800 °C, of up to 983 m2 g−1 and 0.49 cm3 g−1, respectively. Similar heating of the CA-hydrochar (which contains no metal additives) resulted in hardly any increase in porosity.
The textural properties of both FF-4T and SF-4T series of carbons are given in Table 2. For The FF-4T carbons, the apparent surface area rises from 1970 m2 g−1 for sample FF-4600 to 2803 m2 g−1 for FF-4700 and to a high of 4113 m2 g−1 for FF-4800. We note that sample FF-4800 has one of the highest surface areas ever reported for activated carbons.16,88–96 The pore volume follows a similar trend, rising from 0.86 cm3 g−1 for sample FF-4600 to 1.23 cm3 g−1 for FF-4700 and 1.87 cm3 g−1 for CA-4800. Regarding the proportion of microporosity, the usual trend (decrease in microporosity at high activation temperature) is observed; the proportion of micropore surface area is 77% for FF-4600, and decreases to 68% for FF-4700 and 51% for FF-4800. For micropore volume, the proportions are 69%, 60%, and 42% for FF-4600, FF-4700 and FF-4800, respectively.
For the SF-4T carbons, the apparent surface area is highest at 4310 m2 g−1 for sample SF-4600, and then decreases to 2512 m2 g−1 for SF-4700 and 2393 m2 g−1 for SF-4800, which is consistent with our ‘‘overactivation’’ proposal. Sample SF-4600 has the highest apparent surface area ever reported for activated carbons.16,88–96 We are unaware of any reports of higher surface area (above 4300 m2 g−1) for activated carbons. We attribute this high apparent surface area to an optimal mix of KOH, the metal additives in the SF-hydrochar and an ideal activation temperature. The pore volume of the SF-4T carbons follows the same trend as surface area, being 2.09 cm3 g−1 for sample SF-4600, and decreasing to 1.20 cm3 g−1 for SF-4700 and 1.09 cm3 g−1 for SF-4800. It is noteworthy that despite the very high apparent surface area of sample SF-4600, it still exhibits an extremely high micropore surface area of 3867 m2 g−1, which is 90% of the total surface area. This is the highest micropore surface area ever reported for an activated carbon.16,88–96 Furthermore, the micropore volume that arises from micropores is also very high at 82% of total pore volume for sample SF-4600. The proportion of microporosity decreases at higher activation temperature in line with our over activation proposal.
3.3 Hydrogen storage
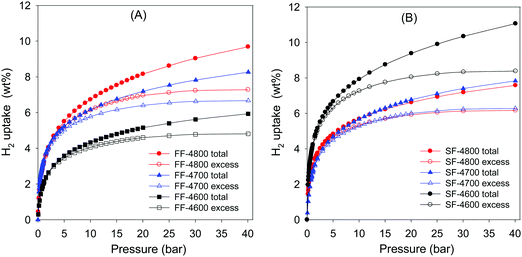
As described above, all the carbons derived from fresh cigarette filters or used cigarette butts have high oxygen content, and some of the carbons also exhibit surface area that is amongst the highest ever reported for activated carbons. In addition, the highest surface area carbons also have very high microporosity with a significant proportion of porosity arising from small micropores of size <1 nm. Given that hydrogen storage in porous materials, and in particular porous carbons, is favoured by the presence of high surface area arising from micropores, we assessed the hydrogen uptake properties of the FF-4T and SF-4T samples. Our assessment conditions (i.e., storage capacity at −196 °C and pressure of 0–40 bar) were chosen due to the fact that cryo-storage under such conditions is currently considered as viable for low pressure vehicular hydrogen storage.7,97–99 For context, we note that, currently, apart from activated carbons,16–24,41,43,88–92,100 the best carbon-based hydrogen storage materials also include carbide-derived carbons (CDCs),28,47,57 and zeolite templated carbons.21,25,52The hydrogen uptake measured by a Hiden XEMIS analyser determined the excess hydrogen storage capacity from which we calculated the total storage capacity using established procedures (see ESI,† Appendix 1, for details on how excess and total hydrogen uptake were obtained). In our discussion, we refer to both the excess and total uptake as wt%, which are based on a dry material (carbon) basis. Fig. 6 shows the excess and total hydrogen uptake isotherms at −196 °C, and the uptake at 1, 20, 30 and 40 bar is summarised in Table 2. We first note that the hydrogen uptake isotherms in all cases showed no hysteresis (Fig. S5, ESI†), which indicates that the hydrogen sorption process into the present activated carbons is reversible, which is similar to previous reports.16–24,41,43,88–92,100 The hydrogen storage capacity at 1 bar ranges between 2 and up to 4 wt% (for sample SF-4600). An uptake of 4 wt% at 1 bar is impressive and at the top end of what has previously been reported for carbons.16–24,41,43,88–92,100 A combination of the high apparent surface area, high microporosity in sample SF-4600 contributes to the high hydrogen uptake.
At 20 bar, the excess hydrogen uptake of FF-4T samples varies between 4.6 and 7.0 wt%, and increases with activation temperature in line with the rise in surface area. Excess hydrogen uptake (at 20 bar) of 6.4 wt% (FF-4700) and 7.0 wt% (FF-4800) is higher than most previously reported porous carbons.16–24,41,43,88–92,100 The corresponding total uptake at 20 bar for FF-4T samples is 5.2 wt% (FF-4600), 7.2 wt% (FF-4700) and 8.2 wt% for FF-4800. For context, we note that amongst the best reported hydrogen uptake values under such conditions (−196 °C and 20 bar) are; 7.03 wt%,46 and 7.3 wt%,88 for polypyrrrole-derived activated and compactivated carbons, respectively, 7.08 wt% for a carbon that was both physically and chemically activated,41 8.1 wt%, for cellulose acetate derived activated carbons,22 7.1 wt%, for a sawdust-derived compactivated carbon,88 and 7.3 wt% for a zeolite templated carbon.25 Amongst the SF-4T carbons, the hydrogen uptake at 20 bar is similar to that of the FF-4T series except for sample SF-4600 which has significantly higher excess and total uptake of 8.1 wt% and 9.4 wt%, respectively. Such uptake is unprecedented for porous carbons and far exceeds values reported to date.22,25,41,46,88 The very high hydrogen uptake is attributable to the equally unprecedentedly high total and micropore surface area of sample SF-4600. As shown in Fig. 6 and Table 2, the superior hydrogen uptake performance of SF-4600 is maintained at higher pressure, and the sample achieves total uptake of 10.4 wt% and 11.2 wt% at 30 and 40 bar, respectively.
It is well acknowledged that the weak interaction between adsorbed hydrogen and carbon surfaces can be improved via functionalization or doping with various heteroatoms.46,52,54,58,59,101 Although the presence of O-functional groups (and thus an oxygen-rich surface) is not considered as doping, some studies suggest that they may afford an enhancing effect on atomic hydrogen adsorption.101–104 In this regard, it was claimed that the hydrogen storage capacity of metal-doped carbons is enhanced due to the presence of oxygen functional groups,101 and that oxygen-rich pillared graphene boron nitride have improved hydrogen storage capacity.104 Regarding the adsorption of molecular hydrogen, which is more relevant to the present study, some previous studies have arrived at contradictory conclusions. Agarwal et al.105 and Bleda-Martınez and co-workers,106 claimed that high oxygen content causes an increase in hydrogen storage capacity while Huang et al.,107 Schimmel et al.,108 and Llorens and Pera-Titus109 observed no positive effects. The conflicting claims may be explained by the fact that these previous studies105–109 involved carbons for which both the oxygen content and textural properties varied to the extent that changes in one (oxygen content) caused variations in porosity (surface area, pore size and pore volume), for example via O-functional groups blocking micropores or limiting the space available for storage of hydrogen.110,111 Such variation in both oxygen content and textural properties limits the ability to independently determine the effect of either variable. The ambiguity may be eliminated by considering how large,22 rather than small,112 variations in oxygen content influence hydrogen uptake in carbons with similar pore size or porosity. We have recently shown that whilst porosity is the main determinant of hydrogen storage capacity, it is also the case that at any given level of porosity, oxygen-rich carbons exhibit enhanced gravimetric hydrogen storage uptake compared to relatively oxygen-poor samples of similar porosity.22 In the present study, for completeness, we compared the hydrogen uptake of sample SF-4800 to that of a cellulose-derived activated carbon (designated as C-4700),20 which has similar porosity (Fig. S5 and Table S2, ESI†) but much lower oxygen content (ESI,† Fig. S1). Such a comparison eliminates any ambiquities and can clarify on the effect of oxygen content. The total surface area and pore volume of the two samples is identical and their pore size is very similar (Table S2 and Fig. S5, ESI†). The main difference is that sample SF-4800 has a much higher oxygen content of 16.4 wt% compared to 7.1 wt% for C-4700. Despite the similarity in porosity, the O-rich sample (SF-4800) has much higher hydrogen uptake (Fig. S6, ESI†); at 1 bar the uptake of SF-4800 (3.0 wt%) is higher than for C-4700 (2.5 wt%). At 20 bar the excess uptake of SF-4800 is 5.9 wt%, which is 20% higher than 4.9 wt% for C-4700. We tentatively ascribe the higher hydrogen storage capacity of the SF-4800 sample to a higher oxygen content, which is consistent with previous finding on the interaction between H and O-rich carbon surfaces.104,113,114
The gravimetric hydrogen uptake of the best performing carbons (FF-4800 and SF-4600), at pressures of up to 30 bar, is better than that of MOF materials that are currently the best hydrogen stores.34,36,37 This is depicted in Fig. 7, and the relevant data is summarised in Table S3 (ESI†). This is despite the fact that the MOFs possess much higher surface area (Table S3, ESI†). At 20 bar, the excess hydrogen storage capacity of sample SF-4600 (8.1 wt%) is higher than that of high surface area MOFs such as NOTT-112 (6.9 wt%),34 NU-100 (6.8 wt%)36 and MOF-210 (6.4 wt%),37 which are the current record holders for gravimetric hydrogen storage in porous materials under cryogenic conditions. The total hydrogen uptake of SF-4600 (9.4 wt%), at 20 bar, is also higher than that of NOTT-112 (7.8 wt%),34 NU-100 (8.5 wt%)36 and MOF-210 (8.4 wt%).37 We postulate that the superior hydrogen storage capacity of sample SF-4600, despite having lower apparent surface area, may be explained in part by the high oxygen content and that an oxygen-rich surface is attractive for adsorption of molecular hydrogen, and thus compensates for the lower surface area compared to the MOFs. At 30 bar, the hydrogen uptake capacity of SF-4600 still outperforms that of the MOFs (Fig. 7 and Table S3, ESI†). In addition to their impressive storage capacity, the present carbons also offer all the advantages of carbons such as easy processing, high mechanical and chemical stability along with ease of preparation and low cost, in addition to the valorisation and environmental protection aspects associated with using cigarette butts as starting materials.
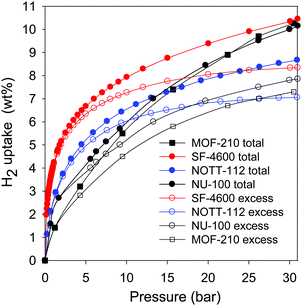 | ||
| Fig. 7 Excess and total gravimetric hydrogen uptake of activated carbon (SF-4600) derived from smoked cigarette butts compared to the best benchmark high surface area metal organic frameworks (MOFs), namely, NOTT-112,34 NU-100,36 and MOF-210.37 | ||
4. Conclusions
Hydrothermal carbonisation of cigarette filters and discarded (i.e., smoked) cigarette butts yields hydrochar, which when activated generates oxygen-rich (with oxygen functional groups such as COOH, C–OH and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) porous carbons that have extremely high apparent surface area (up to 4300 m2 g−1) with most of the surface area (up to 3867 m2 g−1), i.e. 90%, arising from micropores. The carbons derived from smoked cigarette butts show anomalous behaviour with respect to the effect of activation temperature on their porosity, i.e., highest surface area and pore volume at 600 °C, which then decreases for samples activated at higher temperature. The anomalous trend is explained by the presence of significant amounts of metal additives in the cigarette butt-derived hydrochar used as starting material; the metal additives (K, Ca, Na, Mg, etc.) themselves act as activating agent in addition to the added KOH. Due to the combined effects of high surface area, high microporosity and an oxygen-rich nature, the carbons exhibit unprecedentedly high hydrogen storage capacity of 8.1 wt% excess uptake, and 9.4 wt% total uptake at −196 °C and 20 bar, rising to total uptake of 10.4 wt% and 11.2 wt% at 30 and 40 bar, respectively. Our findings offer new insights on the valorisation of a major waste disposal and environmental pollution hazard (discarded cigarette butts) to attractive energy materials with, in the present case, the highest hydrogen uptake capacity reported to date for any carbons or porous materials in general. The findings therefore have direct relevance to an environmental pollution issue and offer a new research direction in the search for carbon-based sustainable energy storage materials, and not least raise the interesting question of whether valorisation can solve the intractable cigarette butt problem.
O) porous carbons that have extremely high apparent surface area (up to 4300 m2 g−1) with most of the surface area (up to 3867 m2 g−1), i.e. 90%, arising from micropores. The carbons derived from smoked cigarette butts show anomalous behaviour with respect to the effect of activation temperature on their porosity, i.e., highest surface area and pore volume at 600 °C, which then decreases for samples activated at higher temperature. The anomalous trend is explained by the presence of significant amounts of metal additives in the cigarette butt-derived hydrochar used as starting material; the metal additives (K, Ca, Na, Mg, etc.) themselves act as activating agent in addition to the added KOH. Due to the combined effects of high surface area, high microporosity and an oxygen-rich nature, the carbons exhibit unprecedentedly high hydrogen storage capacity of 8.1 wt% excess uptake, and 9.4 wt% total uptake at −196 °C and 20 bar, rising to total uptake of 10.4 wt% and 11.2 wt% at 30 and 40 bar, respectively. Our findings offer new insights on the valorisation of a major waste disposal and environmental pollution hazard (discarded cigarette butts) to attractive energy materials with, in the present case, the highest hydrogen uptake capacity reported to date for any carbons or porous materials in general. The findings therefore have direct relevance to an environmental pollution issue and offer a new research direction in the search for carbon-based sustainable energy storage materials, and not least raise the interesting question of whether valorisation can solve the intractable cigarette butt problem.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to Dr Isabel Vicente and Dr Gual Gozalbo Aitor for assistance with TPD measurements. This work was supported by the Engineering and Physical Sciences Research Council [grant number EP/M000567/1] for the Nottingham Gas Adsorption Analysis Suite. RM thanks the Royal Society for a Research Grant, and for a Royal Society Wolfson Research Merit Award.References
- J. A. Turner, Science, 1999, 285, 687 CrossRef CAS PubMed.
- C.-J. Winter, Int. J. Hydrogen Energy, 2009, 34, S1–S52 CrossRef CAS.
- J. Andrews and B. Shabani, Int. J. Hydrogen Energy, 2012, 74, 1184 CrossRef.
- P. Moriarty and D. Honnery, Int. J. Hydrogen Energy, 2009, 34, 31 CrossRef CAS.
- L. Schlapbach and A. Züttel, Nature, 2001, 414, 353 CrossRef CAS PubMed.
- G. W. Crabtree, M. S. Dresselhaus and M. V. Buchanan, Phys. Today, 2004, 57, 39 CrossRef CAS.
- U. Eberle, B. Muller and R. von Helmot, Energy Environ. Sci., 2012, 5, 8780 Search PubMed.
- R. P. O'Hayre, S.-W. Cha, W. Colella and F. B. Prinz, Fuel cell fundamentals, John Wiley & Sons, New York, 2006 Search PubMed.
- A. Cho, Science, 2004, 305, 964 CrossRef CAS PubMed.
- R. von Helmolt and U. Eberle, J. Power Sources, 2007, 165, 833 CrossRef CAS.
- http://energy.gov/eere/fuelcells/hydrogen-storage, (accessed June 2017).
- D. Pukazhselvan, V. Kumar and S. K. Singh, Nano Energy, 2012, 1, 566 CrossRef CAS.
- P. Jena, J. Phys. Chem. Lett., 2011, 2, 206 CrossRef CAS.
- A. W. C. van den Berg and C. O. Arean, Chem. Commun., 2008, 668 RSC.
- I. P. Jain, P. Jain and A. Jain, J. Alloys Compd., 2010, 503, 303 CrossRef CAS.
- M. Sevilla and R. Mokaya, Energy Environ. Sci., 2014, 7, 1250 CAS.
- J. Burress, M. Kraus, M. Beckner, R. Cepel, C. Wexler and P. Pfeifer, Nanotechnology, 2009, 20, 204026 CrossRef PubMed.
- J. Juan-Juan, J. P. Marco-Lozar, F. Suarez-Garcia, D. Cazorla-Amoros and A. Linares-Solano, Carbon, 2010, 48, 2906 CrossRef CAS.
- C. Wang and S. Kaskel, J. Mater. Chem., 2012, 22, 23710 RSC.
- M. Sevilla, A. B. Fuertes and R. Mokaya, Energy Environ. Sci., 2011, 3, 1400 Search PubMed.
- Z. Yang, Y. Xia and R. Mokaya, J. Am. Chem. Soc., 2007, 129, 1673 CrossRef CAS PubMed.
- T. S. Blankenship, N. Balahmar and R. Mokaya, Nat. Commun., 2017 DOI:10.1038/s41467-017-01633-x.
- G. Sethia and A. Sayari, Carbon, 2016, 9, 289 CrossRef.
- M. Jordá-Beneyto, F. Suárez-García, D. Lozano-Castelló, D. Cazorla-Amorós and A. Linares-Solano, Carbon, 2007, 45, 293 CrossRef.
- E. Masika and R. Mokaya, Energy Environ. Sci., 2014, 7, 427 CAS.
- N. Balahmar, A. M. Lowbridge and R. Mokaya, J. Mater. Chem. A, 2016, 4, 14254 CAS.
- M. de la Casa-Lillo, F. Lamari-Darkrim, D. Cazorla-Amoros and A. Linares-Solano, J. Phys. Chem. B, 2002, 106, 10930 CrossRef CAS.
- M. Sevilla, R. Foulston and R. Mokaya, Energy Environ. Sci., 2010, 3, 223 CAS.
- N. Alam and R. Mokaya, Energy Environ. Sci., 2010, 3, 1773 CAS.
- E. Poirier, R. Chahine and T. K. Bose, Int. J. Hydrogen Energy, 2001, 26, 831 CrossRef CAS.
- S. S. Kaye, A. Dailly, O. M. Yaghi and J. R. Long, J. Am. Chem. Soc., 2007, 129, 14176 CrossRef CAS PubMed.
- H. W. Langmi, J. W. Ren, B. North, M. Mathe and D. Bessarabov, Electrochim. Acta, 2014, 128, 368 CrossRef CAS.
- L. J. Murray, M. Dincă and J. R. Long, Chem. Soc. Rev., 2009, 38, 1294 RSC.
- Y. Yan, X. Lin, S. Yang, A. J. Blake, A. Dailly, N. R. Champness, P. Hubberstey and M. Schroder, Chem. Commun., 2009, 1025 RSC.
- S. Y. Ding and W. Wang, Chem. Soc. Rev., 2013, 42, 548 RSC.
- O. K. Farha, A. O. Yazaydın, I. Eryazici, C. D. Malliakas, B. G. Hauser, M. G. Kanatzidis, S. T. Nguyen, R. Q. Snurr and J. T. Hupp, Nat. Chem., 2010, 2, 944 CrossRef CAS PubMed.
- H. Furukawa, N. Ko, Y. B. Go, N. Aratani, S. B. Choi, E. Choi, A. O. Yazaydin, R. Q. Snurr, M. O’Keeffe, J. Kim and O. M. Yaghi, Science, 2010, 329, 424 CrossRef CAS PubMed.
- S. S. Han, H. Furukawa, O. M. Yaghi and W. A. Goddard, J. Am. Chem. Soc., 2008, 130, 11580 CrossRef CAS PubMed.
- H. Furukawa and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 8875 CrossRef CAS PubMed.
- E. Klontzas, E. Tylianakis and G. E. Froudakis, Nano Lett., 2010, 10, 452 CrossRef CAS PubMed.
- H. Wang, Q. Gao and J. Hu, J. Am. Chem. Soc., 2009, 131, 7016 CrossRef CAS PubMed.
- A. Almasoudi and R. Mokaya, J. Mater. Chem., 2012, 22, 146 RSC.
- B. Panella, M. Hirscher and S. Roth, Carbon, 2005, 43, 2209 CrossRef CAS.
- Y. Xia and R. Mokaya, J. Phys. Chem. C, 2007, 111, 10035 CAS.
- E. Masika and R. Mokaya, J. Phys. Chem. C, 2012, 116, 25734 CAS.
- M. Sevilla, R. Mokaya and A. B. Fuertes, Energy Environ. Sci., 2011, 4, 2930 CAS.
- Y. Gogotsi, R. K. Dash, G. Yushin, T. Yildirim, G. Laudisio and J. E. Fischer, J. Am. Chem. Soc., 2005, 127, 16006 CrossRef CAS PubMed.
- N. Alam and R. Mokaya, Microporous Mesoporous Mater., 2011, 142, 716 CrossRef CAS.
- O. K. Farha, I. Eryazici, N. C. Jeong, B. G. Hauser, C. E. Wilmer, A. A. Sarjeant, R. Q. Snurr, S. T. Nguyen, A. Ö. Yazaydın and J. T. Hupp, J. Am. Chem. Soc., 2012, 134, 15016 CrossRef CAS PubMed.
- C. Robertson and R. Mokaya, Microporous Mesoporous Mater., 2013, 179, 151 CrossRef CAS.
- H. Tanaka, H. Kanoh, M. Yudasaka, S. Iijima and K. Kaneko, J. Am. Chem. Soc., 2005, 127, 7511 CrossRef CAS PubMed.
- Y. Xia, G. S. Walker, D. M. Grant and R. Mokaya, J. Am. Chem. Soc., 2009, 131, 16493 CrossRef CAS PubMed.
- A. Almasoudi and R. Mokaya, J. Mater. Chem. A, 2014, 2, 10960 CAS.
- M. Sevilla, A. B. Fuertes and R. Mokaya, Int. J. Hydrogen Energy, 2011, 36, 15658 CrossRef CAS.
- N. Alam and R. Mokaya, Microporous Mesoporous Mater., 2011, 144, 140 CrossRef CAS.
- Z. Yang, Y. Xia, X. Sun and R. Mokaya, J. Phys. Chem. B, 2006, 110, 18424 CrossRef CAS PubMed.
- G. Yushin, R. Dash, J. Jagiello, J. E. Fischer and Y. Gogotsi, Adv. Funct. Mater., 2006, 16, 2288 CrossRef CAS.
- Y. Xia, R. Mokaya, D. M. Grant and G. S. Walker, Carbon, 2011, 49, 844 CrossRef CAS.
- E. Masika, R. A. Bourne, T. W. Chamberlain and R. Mokaya, ACS Appl. Mater. Interfaces, 2013, 5, 5639 CAS.
- P. Wexler, Encyclopedia of Toxicology, Four-Volume Set, ACADEMIC Press INC, 2005 Search PubMed.
- E. Lassner, W.-D. Schubert, E. Lüderitz and H. U. Wolf, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, 2000, DOI: DOI:10.1002/14356007.a27_229.
- H. Moriwaki, S. Kitajima and K. Katahira, Waste Manage., 2009, 29, 1192 CrossRef CAS PubMed.
- E. A. Smith and T. E. Novotny, Tob. Control, 2011, 20, i2–i9 CrossRef PubMed.
- B. Harris, Tob. Control, 2011, 20, i10–i16 CrossRef PubMed.
- T. E. Novotny, S. N. Hardin, L. R. Hovda, D. J. Novotny, M. K. McLean and S. Khan, Tob. Control, 2011, 20, i17–i20 CrossRef PubMed.
- E. Slaughter, R. M. Gersberg, K. Watanabe, J. Rudolph, C. Stransky and T. E. Novotny, Tob. Control, 2011, 20, i25–i29 CrossRef PubMed.
- J. Puls, S. A. Wilson and D. Hölter, J. Polym. Environ., 2011, 19, 152 CrossRef CAS.
- http://www.tobaccoatlas.org/topic/cigarette-use-globally/(Accessed June 2017).
- T. E. Novotny, K. Lum, E. Smith, V. Wang and R. Barnes, Int. J. Environ. Res. Public Health, 2009, 6, 1691 CrossRef PubMed.
- J. W. Moerman and G. E. Potts, Tob. Control, 2011, 20, i30–i35 CrossRef PubMed.
- F. Y. Iskander, T. L. Bauer and D. E. Klein, Analyst, 1986, 3, 107 RSC.
- P. Bell and C. L. Mulchi, Tob. Sci., 1990, 34, 32 Search PubMed.
- S. Polarz, B. Smarsly and J. H. Schattka, Chem. Mater., 2002, 14, 2940 CrossRef CAS.
- A. Chen, Y. Li, Y. Yu, Y. Li, L. Zhang, H. Lv and L. Liu, RSC Adv., 2015, 5, 107299 RSC.
- M. Lee, G. P. Kim, H. Don Song, S. Park and J. Yi, Nanotechnology, 2014, 25, 345601 CrossRef PubMed.
- M. C. Tellez-Juarez, V. Fierro, W. Zhao, N. Fernandez-Huerta, M. T. Izquierdo, E. Reguera and A. Celzard, Int. J. Hydrogen Energy, 2014, 39, 4996 CrossRef CAS.
- J. L. Figueiredo, M. F. R. Pereira, M. M. A. Freitas and J. J. M. Orfao, Carbon, 1999, 37, 1379 CrossRef CAS.
- J. L. Figueiredo and M. F. R. Pereira, Catal. Today, 2010, 150, 2 CrossRef CAS.
- M. Sevilla and A. B. Fuertes, Carbon, 2009, 47, 2281 CrossRef CAS.
- J. Romanos, M. Beckner, T. Rash, L. Firlej, B. Kuchta, P. Yu, G. Suppes, C. Wexler and P. Pfeifer, Nanotechnology, 2012, 23, 015401 CrossRef CAS PubMed.
- Y. Zhao, W. Ran, J. He, Y. Song, C. Zhang, D. B. Xiong, F. Gao, J. Wu and Y. Xia, ACS Appl. Mater. Interfaces, 2015, 7, 1132 CAS.
- H. Wang and Y. H. Hu, Ind. Eng. Chem. Res., 2011, 50, 6132 CrossRef CAS.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquerol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603 CrossRef CAS.
- H. M. Coromina, D. A. Walsh and R. Mokaya, J. Mater. Chem. A, 2016, 4, 280 CAS.
- H. M. Coromina, B. Adeniran, R. Mokaya and D. A. Walsh, J. Mater. Chem. A, 2016, 4, 14586 CAS.
- N. Balahmar, A. Al-Jumialy and R. Mokaya, J. Mater. Chem. A, 2017, 5, 12330 CAS.
- B. Adeniran and R. Mokaya, Chem. Mater., 2016, 28, 994 CrossRef CAS.
- B. Adeniran and R. Mokaya, Nano Energy, 2015, 16, 173 CrossRef CAS.
- W. Sangchoom and R. Mokaya, ACS Sustainable Chem. Eng., 2015, 3, 1658 CrossRef CAS.
- B. Adeniran and R. Mokaya, J. Mater. Chem. A, 2015, 3, 5148 CAS.
- B. Adeniran, E. Masika and R. Mokaya, J. Mater. Chem. A, 2014, 2, 14696 CAS.
- A. Almasoudi and R. Mokaya, Microporous Mesoporous Mater., 2014, 195, 258 CrossRef CAS.
- N. Balahmar, A. C. Mitchell and R. Mokaya, Adv. Energy Mater., 2015, 5, 1500867 CrossRef.
- M. Sevilla, W. Sangchoom, N. Balahmar, A. B. Fuertes and R. Mokaya, ACS Sustainable Chem. Eng., 2016, 4, 4710 CrossRef CAS.
- J. He, J. W. F. To, P. C. Psarras, H. Yan, T. Atkinson, R. T. Holmes, D. Nordlund, Z. Bao and J. Wilcox, Adv. Energy Mater., 2016, 6, 1502491 CrossRef.
- A. S. Jalilov, Y. Li, J. Tian and J. M. Tour, Adv. Energy Mater., 2017, 7, 1600693 CrossRef.
- D. A. Gomez-Gualdron, Y. J. Colón, X. Zhang, T. C. Wang, Y. Chen, J. T. Hupp, T. Yildirim, O. K. Farha, J. Zhang and R. Q. Snurr, Energy Environ. Sci., 2016, 9, 3279 CAS.
- http://energy.gov/sites/prod/files/2015/05/f22/fcto_myrdd_storage.pdf, accessed Jan. 2017.
- U. Eberle and R. von Helmolt, Energy Environ. Sci., 2010, 3, 689 CAS.
- M. Jorda-Beneyto, D. Lozano-Castello, F. Suarez-Garcıa, D. Cazorla-Amoros and A. Linares-Solano, Microporous Mesoporous Mater., 2008, 112, 235 CrossRef CAS.
- Z. Wang, F. H. Yang and R. T. Yang, J. Phys. Chem. C, 2010, 114, 1601 CAS.
- L. Wang, F. H. Yang, R. T. Yang and M. A. Miller, Ind. Eng. Chem. Res., 2009, 48, 2920 CrossRef CAS.
- Q. Li and A. D. Lueking, J. Phys. Chem. C, 2011, 115, 4273 CAS.
- F. Shayeganfar and R. Shahsavari, Langmuir, 2016, 32, 13313 CrossRef CAS PubMed.
- R. K. Agarwal, J. S. Noh, J. A. Schwarz and R. Davini, Carbon, 1987, 25, 219 CrossRef.
- M. J. Bleda-Martınez, J. M. Perez, A. Linares-Solano, E. Morallon and D. Cazorla-Amoros, Carbon, 2008, 46, 1053 CrossRef.
- C. C. Huang, H. M. Chen, C. H. Chen and J. C. Huang, Sep. Purif. Technol., 2010, 70, 291 CrossRef CAS.
- H. G. Schimmel, G. Nijkamp, G. J. Kearley, A. Rivera, K. P. De Jong and F. M. Mulder, Mater. Sci. Eng., B, 2004, 108, 124 CrossRef.
- J. Llorens and M. Pera-Titus, Colloids Surf., A, 2009, 350, 63 CrossRef CAS.
- M. Georgakis, G. Stavropoulos and G. P. Sakellaropoulos, Int. J. Hydrogen Energy, 2007, 32, 1999 CrossRef CAS.
- H. Takagi, H. Hatori, Y. Yamada, S. Matsuo and M. Shiraishi, J. Alloys Compd., 2004, 385, 257 CrossRef CAS.
- M. C. Tellez-Juarez, V. Fierro, W. Zhao, N. Fernandez-Huerta, M. T. Izquierdo, E. Reguera and A. Celzard, Int. J. Hydrogen Energy, 2014, 39, 4996 CrossRef CAS.
- Z. Wang, F. H. Yang and R. T. Yang, J. Phys. Chem. C, 2010, 114, 1601 CAS.
- L. Wang, F. H. Yang, R. T. Yang and M. A. Miller, Ind. Eng. Chem. Res., 2009, 48, 2920 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of calculation of total hydrogen uptake, and three tables and six figures. See DOI: 10.1039/c7ee02616a |
| This journal is © The Royal Society of Chemistry 2017 |