 Open Access Article
Open Access ArticleDevelopment of algae biorefinery concepts for biofuels and bioproducts; a perspective on process-compatible products and their impact on cost-reduction
Lieve M. L.
Laurens
 *a,
Jennifer
Markham
*a,
Jennifer
Markham
 a,
David W.
Templeton
a,
David W.
Templeton
 a,
Earl D.
Christensen
b,
Stefanie
Van Wychen
a,
Earl D.
Christensen
b,
Stefanie
Van Wychen
 a,
Eric W.
Vadelius
a,
Melodie
Chen-Glasser
a,
Tao
Dong
a,
Eric W.
Vadelius
a,
Melodie
Chen-Glasser
a,
Tao
Dong
 a,
Ryan
Davis
a and
Philip T.
Pienkos
a
a,
Ryan
Davis
a and
Philip T.
Pienkos
a
aNational Bioenergy Center, National Renewable Energy Laboratory, 15013 Denver West Parkway, Golden, Colorado, USA. E-mail: Lieve.Laurens@nrel.gov
bTransportation and Hydrogen Systems Center, National Renewable Energy Laboratory, 15013 Denver West Parkway, Golden, Colorado, USA
First published on 10th July 2017
Abstract
Identifying and addressing critical improvements in biomass, bioproduct and biofuel productivity is a priority for the nascent algae-based bioeconomy. Economic and sustainability principles should guide these developing improvements and help to unravel the contentious water–food–energy–environment nexus that algae inhabit. Understanding the biochemistry of the storage carbon metabolism of algae to produce biofuels and bioproducts can bring to light the key barriers that currently limit the overall carbon efficiency and the photosynthetic efficiency, and ultimately guide productivity and commercial viability in the context of limiting resources. In the analysis reported here, we present different potential pathways for a conceptual algae biorefinery framework, with each pathway addressing one of the main identified barriers to future deployment. We highlight the molecular identification, in the form of an extensive literature review, of potential bioproducts that may be derived directly from both biomass and fractions produced through a conversion pathway, for three important commercially-relevant genera of algae, Scenedesmus, Chlorella and Nannochloropsis. We establish a relationship between each of the potential bioproducts, describe relevant conversion and extraction processes, and discuss market opportunities with values and sizes as they relate to commercial development of the products.
Broader contextCell biomass from algae, in particular phototrophic microalgae in the context of the work described here, for bioenergy applications is highly topical, where tremendous opportunities are met with equal if not greater challenges for commercialization. Thanks to their unprecedented biological photosynthetic carbon assimilation potential, microalgae are heralded as the most efficient form of biomass production and thus carry enormous potential to contribute to a clean energy future. Economic barriers deter many promising commercial ventures, while many of these can be overcome with the correct conceptual and technical framework for maximizing the value from algal biomass. For years, fuel-only pathways from algae have been deemed unviable, and thus the market introduction of other higher-value components of the cells was, and still is, critical. Fundamental biochemical principles and biomass composition underpin the potential yields of individual products in the biomass and integrate the discussion with highly topical conversion pathways. In this context, we provide a unique perspective on developing bioproducts from microalgae, to drive the bioenergy narrative towards a more realistic framework around algae bioenergy. This approach is critical in the global R&D framework. Simultaneously placing the biorefinery discussion in the context of the large-scale farms that are envisioned for bioenergy production from algae is needed to impact and create markets commensurate with the volumes produced in a demonstrated and implemented fractionation pathway. We conclude that a path towards successful commercialization needs to address major research barriers and be placed in the correct economic and sustainability context. Examples of areas that are covered in this review are applications for products such as polyunsaturated fatty acids, polysaccharides and amino acids as high value bio-derived polymers. Thanks to the enormous market and the opportunity to replace often-toxic synthesis routes with bio-derived polymer alternatives, these materials have the potential to change the global dynamics around sustainably sourced commodity chemical products. The unique perspective of our team highlights the potential technical and perhaps even commercial feasibility of algal biomass. For the first time, we discuss the biorefinery concept in a context of a demonstrated and modeled conversion pathway. We have used well-documented and validated techno-economical process modeling to, for the first time, calculate the magnitude of the impact that the composition of the biomass exerts on the calculated fuel costs, while our extensive market analysis of bioproducts and biopolymers presented here provides a reference framework for future discussion. We hope this work will eventually pave the way for a viable photosynthesis-driven algal biochemical technology framework. |
1 Introduction
Supporting a future bioeconomy that includes photosynthetic microalgae as a key player necessitates exploration of the opportunities and challenges of pursuing a route towards biofuels and bioproducts. Of specific interest are the technical and economic hurdles to market deployment for algae-derived biofuels. One path to drive down the cost of biofuels is to reduce the cost of biomass production (i.e. cultivation/harvesting). Recent techno-economic analysis work has demonstrated that reducing the costs to a level that would enable biofuel economical viability is exceedingly difficult.1,2 Another path is identified through the development of high value bioproducts, ultimately increasing the inherent value of algal biomass through different conversion or upgrading pathways. The goals of research towards successful bioproduct pathways include identifying issues at the interface between production and conversion processes, discovering novel compounds, and establishing a link with scaled conversion process characteristics and respective market opportunities for different bioproducts. This discussion focuses primarily on established lipid extraction or biochemical processing or fractionation processes for algal biomass conversion, but does not include hydrothermal liquefaction, as this process does not easily lend itself to the development of bioproducts.3–8 In this context, a biorefinery is defined as a facility in which algal biomass can be sustainably processed into a spectrum of bio-based products (food, animal feed, chemicals, and materials) and bioenergy products (biofuels, biogas, power and/or heat).Though there are challenges associated with the production of fuels from algae,9 there is room for algae to contribute to a future bioeconomy, aiding in the transition to energy independence and energy security. To move the field forward, a rationale is needed to allow for a different focus on the value of biomass, providing a better link with biomass production costs and detailed biomass composition, as a means to resolve the potential conflict between maximizing biofuel yields and maximizing potential revenue to provide a better sense of the most viable path to commercialization. A focus on intrinsic biomass value can provide a framework to identify critical factors for economic development and deployment of algal biofuels, alongside biomass productivity, compositional characteristics, and conversion efficiency.
As promising bioproducts are discovered and considered through techno-economic modeling, a higher value can be assigned to the biomass, thereby alleviating pressure on increasing the productivity of the biomass to reach aggressive cost targets. Identifying the potential products also lays the groundwork for future strain and process development, with an overall goal of at least matching the cost of petroleum fuels and petroleum-derived products.
The current literature on the generation and exploitation of bioproducts from algae (and even terrestrial feedstock) biorefineries remains highly conceptual and not tied to a particular conversion pathway, rather describing a process that is agnostic of conversion pathways.5–8,10–13 Often these reports are based on hypothetical assumptions of biomass composition and intact separations of each of the fractions.8 In this review, we build on a demonstrated fractionation approach that has great flexibility and was shown to be more economically viable compared to the more traditional lipid extraction.3,14 We also explore how biomass composition and associated fractionation techniques can increase the value of biomass, improving the overall economics of the algal biorefinery concept and ultimately allowing for successful biofuel economics.
2 Algal biomass composition dynamics
A large focus of this review is on products derived from three important genera of photosynthetic microalgae, Chlorella, Scenedesmus and Nannochloropsis. These genera contain examples of species with varying macromolecular biochemistry and are used throughout projects pursued globally for algae bioenergy applications and in particular as the focus of projects currently funded by the US Department of Energy's Bioenergy Technologies Office (BETO) (including productivity modeling and resource availability and allocation, such as the Biomass Assessment Tool (BAT) and national consortia like the Algae Testbed Public-Private Partnership, ATP3).15–19 For each of the three algae genera, the biomass composition can be divided into three major fractions: lipids, proteins and carbohydrates. Each of these fractions has a molecular compositional make up that is specific to the species and growth phase (e.g. the lipid fraction, for example, may include varying levels of triacylglycerides (TAGs), phospholipids, sulfolipids, free fatty acids (FFAs), hydrophobic proteins, pigments, and other non-saponifiable lipids), which will ultimately guide the products that can be derived for valorization. Bioproducts recovered in an algae biorefinery approach are by definition highly dependent on the composition of the algal biomass, which is not static as often assumed, but highly dynamic and dependent on both the strain and the physiological environment of the algae culture.20,21The dynamics of biomass component accumulation are illustrated in Fig. 1 and Table 1 and indicate distinct accumulation profiles over the course of cultivation that include nitrogen depletion for increased lipid yields. The compositional data was collected in our laboratory using the reference methods previously described.14,20 The data covers primary biomass components (protein, lipid, carbohydrates) as well as a breakdown into respective constituents (e.g. fermentable and non-fermentable carbohydrates, polyunsaturated fatty acids (PUFA), sterols and pigments) as shown in Table 1. The constituent components include targets that can be used for high-value product applications that are relevant to the later discussion. In Table 1, three time points representing the early, mid and late stages of a growth cycle are summarized for the same three species as in Fig. 1, though different samples, and show a detailed and distinct compositional profile, with some components inversely correlating to increasing lipid content and other components showing a non-linear, independent accumulation pattern.
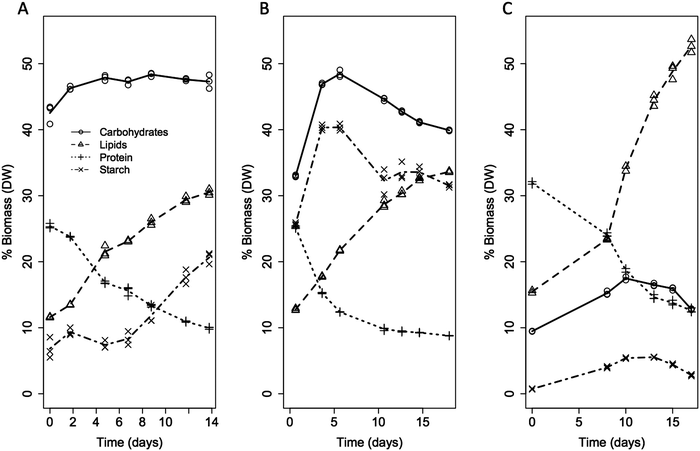 | ||
| Fig. 1 Illustration of the dynamic biomass composition in algae for each of the three strains; Scenedesmus acutus (A), Chlorella vulgaris (B), Nannochloropsis granulata (C). Further discussion around early, mid and late stages in cultivation can be superimposed here, with early stage representing fully nutrient replete conditions and approximately 6–8 days and 15–21 days of nutrient depletion respectively for mid and late stages of growth in outdoor flat panel photobioreactors in Phoenix, AZ, in early Spring.20 | ||
| Metrica (%DW) | Scenedesmus | Chlorella | Nannochloropsis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Early | Mid | Late | Early | Mid | Late | Early | Mid | Late | |
| a Biomass composition shown here was measured on representative samples per previously published methodology developed in our laboratory, ref. 20, and http://www.nrel.gov/bioenergy/microalgae-analysis.html. b Values for fermentable carbohydrates are based on a typical yeast (S. cerevisiae) ethanol fermentation process and includes glucose and mannose, ‘other carbohydrates’ include uronic acids (where detected), rhamnose, arabinose, galactose and ribose. Sugar utilization patterns will vary with the fermentative organism. c Glycerol was calculated based on the FAME to fatty acid conversion and release of glycerol in the aqueous phase, this may only be a Scenedesmus specific phenomenon, but is presented for all three species as the potential yield. d Non-FAME lipids include unsaponifiable components beyond the listed sterols and chlorophyll, e.g. other pigments, hydrocarbons, and polar lipid head groups, that are known to contribute to the lipid fraction but are not measured as fatty acids (estimated based on a detailed mass spectrometry lipidomics analysis for these species, NREL unpublished data). e Mass closure is the summative account of individual constituents listed, the remaining difference from 100% refers to ‘other’ cell mass as an unknown component of the biomass that has not yet been quantified and includes among others, unknown fractions of the cell wall, e.g. algaenan and other unknown minor contributing components or hydrolysis-resistant polymeric carbohydrates. f Data for HHV was measured on the same representative biomass samples for each harvest scenario as described in Table 1; standard bomb calorimetry methodology was used for this measurement. | |||||||||
| Ash | 5.6 | 2.3 | 2.1 | 4.7 | 2.1 | 2.6 | 14.2 | 13.6 | 5.1 |
| Ferm carbsb | 20.9 | 46.3 | 37.9 | 5.8 | 36.7 | 23.6 | 4.6 | 8.0 | 7.6 |
| Mannitol | ND | ND | ND | ND | ND | ND | 4.0 | 2.1 | 2.2 |
| Other carbohydrates | 3.4 | 1.6 | 1.3 | 5.9 | 5.0 | 3.5 | 2.9 | 1.5 | 2.1 |
| Glycerolc | 0.7 | 2.9 | 4.5 | 1.4 | 2.5 | 4.5 | 1.4 | 2.8 | 6.4 |
| Protein | 34.5 | 12.8 | 8.9 | 40.2 | 13.2 | 12.7 | 32.7 | 23.1 | 9.4 |
| Lipids total (as FAME) | 6.6 | 26.5 | 40.9 | 13.0 | 22.1 | 40.5 | 12.3 | 25.6 | 57.3 |
| Lipids (<2 unsat FAME) | 3.1 | 17.1 | 33.4 | 7.0 | 15.5 | 35.0 | 6.2 | 16.1 | 43.0 |
| PUFA (>2 unsat FAME) | 3.5 | 9.4 | 7.5 | 6.0 | 6.6 | 5.5 | 6.2 | 9.5 | 14.3 |
| Sterols | 0.9 | 0.7 | 0.4 | 0.2 | 0.4 | 0.3 | 0.4 | 0.6 | 0.2 |
| Chlorophyll (33% of MW as phytol) | 3.0 | 1.2 | 1.2 | 5.8 | 2.4 | 2.1 | 3.0 | 1.8 | 0.3 |
| Non-FAME lipidsd | 4.1 | 2.8 | 1.3 | 3.8 | 1.7 | 1.5 | 3.8 | 3.3 | 1.2 |
| Nucleic acids | 4.1 | 1.5 | 1.0 | 4.6 | 1.1 | 0.9 | 4.6 | 1.1 | 0.9 |
| Mass closuree | 83.8 | 98.6 | 99.5 | 85.4 | 87.2 | 92.2 | 83.9 | 83.5 | 92.7 |
| Biomass energy content, HHV,f in ×103 BTU per lb (and MJ kg−1) | 9.2 (21.3) | 10.1 (23.4) | 11.1 (25.9) | 9.2 (21.5) | 9.4 (21.8) | 10.8 (25.2) | 9.2 (21.4) | 10.1 (23.5) | 13.2 (30.6) |
In this latter category are the carbohydrates, in particular the storage and structural polysaccharides. For example, starch and other high-molecular weight polymers follow distinct trends for each of the species. Over the course of nutrient depletion, cell biomass shows storage carbohydrates (such as starch as shown in Fig. 1) peaking (at over 50% of the biomass) prior to the maximum lipid content accumulation for Chlorella, while Nannochloropsis shows a similar peak in the storage carbohydrates but at much lower levels, with the majority of the metabolic energy storage funneled into lipids. Scenedesmus exhibits a seemingly parallel accumulation of lipids and starch, with the majority of carbohydrates associated with a storage polysaccharide, primarily composed of glucose and mannose.
The measured biomass energy content (as higher heating value, HHV, via standard bomb calorimetry analysis) is also shown in Table 1 alongside the biochemical composition. The caloric content of algal biomass ranges between 9170 and 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 160 BTU per lb (or between 21.3 and 30.6 MJ kg−1), which is similar to what has been described before22 and primarily driven by the biomass composition.
160 BTU per lb (or between 21.3 and 30.6 MJ kg−1), which is similar to what has been described before22 and primarily driven by the biomass composition.
Even though the compositional shifts are typically associated with longer cultivation time and thus lower biomass averaged productivity rates, the potential for additional value derived from different components will ultimately need to be weighed against the extra time needed to maximize lipid yields.24 As an example of the cost impacts from biomass composition when considered in isolation, the calculated minimum fuel-selling price (MFSP) is included in Table 2 for the exact same compositional scenarios presented in Table 1.
| Metric (%DW) | Scenedesmus | Chlorella | Nannochloropsis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Early | Mid | Late | Early | Mid | Late | Early | Mid | Late | |
| a MFSP = minimum fuel selling price, following techno-economic analysis modeling methodologies described in detail before.3 GGE and LGE = gallons/liters of gasoline equivalent, respectively (based on adjusting total fuel yields by heating values of the resulting fuels, in this case ethanol and renewable diesel). All modeled costs are based on a previously-documented process for fractionation of algal biomass with fermentation of sugars to ethanol, extraction of lipids from fermentation stillage and conversion to hydrocarbon fuels and relegation of residual components to anaerobic digestion;3,14,25 all calculated costs are based on a targeted algal biomass feedstock price of $494 per ton AFDW delivered to the biorefinery facility.1 | |||||||||
| MFSP in $ per GGE (and $ per LGE) in 2014 $a | 11.4 (3.0) | 5.9 (1.6) | 5.1 (1.3) | 12.6 (3.3) | 6.8 (1.8) | 5.3 (1.4) | 10.5 (2.8) | 6.5 (1.7) | 4.4 (1.2) |
| Uncertainty (±25% TCI) in $ per GGE (and $ per LGE) in 2014 $ | 0.6 (0.2) | 0.4 (0.1) | 0.4 (0.1) | 0.7 (0.2) | 0.4 (0.1) | 0.3 (0.1) | 0.6 (0.2) | 0.4 (0.1) | 0.3 (0.1) |
MFSP is a metric based on established calculations and techno-economic analysis (TEA) modeling methodologies that is used to set cost targets and track progress towards achieving those targets based on underlying technical attributes of an integrated process, and we use this metric in this review to quantify the impact of composition. The underlying calculations follow TEA modeling methodologies and underlying assumptions that are described in detail elsewhere.3 Generally, the TEA methods are consistent with an engineering feasibility-level analysis, with stated uncertainties of ±25% around the estimated total capital investment (TCI) costs, which translate to MFSP ranges on the order of ±$0.3–$0.7 per GGE for the cases considered here (shown in the bottom row of Table 2). All modeled costs are based on a well-documented process for fractionating algal biomass with fermentation of hydrolyzed sugars to ethanol, extraction of lipids from the fermentation stillage for conversion to hydrocarbon fuels, and relegation of residual components to anaerobic digestion (as described in Fig. 2B and recently published literature3,14,25). We emphasize here that the MFSP values are based on a fixed target algal biomass feedstock price of $494 per ton AFDW delivered to the biorefinery facility as calculated and described before.1 The biomass feedstock cost is a function of productivity, with a fixed biomass cost implying that productivity remains constant throughout nutrient depletion. This is, as noted, an aspirational target that has not yet been achieved in a validated outdoor cultivation process, but is the goal of many strain/cultivation improvement strategies.
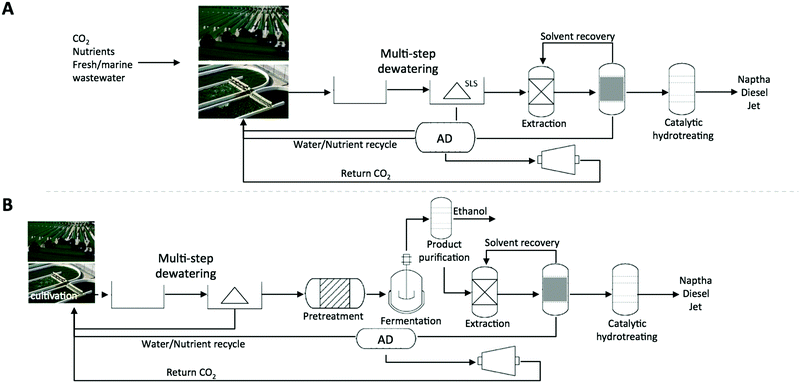 | ||
| Fig. 2 Illustration of two algae conversion pathways including fuel upgrading currently under development: (A) base-case algal lipid extraction and upgrading (ALU) approach; algae are grown in open ponds, or photobioreactors, or hybrid systems after which the algal cell mass is harvested by a multistep dewatering process, and then either dried or processed wet to extract lipids, which are further upgraded via hydrotreating to renewable diesel, jet fuel, or via transesterification to FAME biodiesel; all residual cell mass is anaerobically digested, with the produced biogas used for heat and power generation to support facility operations;38 (B) current base-case of the combined algal processing (CAP) pathway,25 where biofuels are derived from both the carbohydrates (after dilute acid pretreatment and fermentation to ethanol) and lipids remaining with the fermentation stillage, extracted and further upgraded to renewable diesel or jet fuel, or FAME biodiesel. | ||
The focus of presenting the data in this table is to reflect the impact on fuel production costs strictly as a function of composition irrespective of the cultivation time, i.e. as a reflection of varying fuel yields and biogas yields/nutrient cycles from anaerobic digestion. The results indicate that the composition and the associated energy content has a dramatic impact on the calculated fuel cost, which is primarily driven by the combined fuel yield from lipids and ethanol, with a smaller cost benefit from anaerobic digestion of the protein residues. The MFSP presented establishes a “base case” focused on lower-value commodity fuel products and relatively low-value use of the protein, and does not include any potential credits from higher-value bioproducts that could instead be pursued.
Beyond the base case, a full cost sensitivity analysis that examines reasonable minima and maxima for input variables is outside the scope of this review. However, in an effort to address the uncertainty of the model, we ran a sensitivity analysis around an increase or decrease of 25% for the TCI for the conversion facility. The ±25% range in the TCI sprouts from the factored approach used in previous TEAs.3 Future analyses will need to not only understand the base case, but also consider uncertainties surrounding specific parameters such as algae productivity, continuous growth at commercial scale, CO2 siting and sourcing, nutrient cost, and dewatering efficiency. As this analysis uses a set algae feedstock price, the uncertainty of these parameters cannot be quantified. Literature sources in both algae TEA and life-cycle analysis (LCA) have examined uncertainty parameters around algae cultivation and biorefining.26–28 In all, these sources often use a Monte Carlo approach with probability distribution functions to determine the probability of a specific outcome, in this case an MFSP.
The purpose of the following sections is to elaborate both on the fractionation pathway as well as on the potential use for such potential products identified in algae to support the future bioeconomy. In future communications, the calculated cost impact of components or products identified here on the MFSP will be reported alongside experimental demonstration of the purification and upgrading routes. Even though the TEA calculations ultimately will define the boundary conditions around commercial feasibility and help guide and prioritize R&D, additional analyses around the sustainability of process operations and products identified here should be carried out. Many of the chemical products discussed here carry relatively high greenhouse gas (GHG) emissions attributed to their standard production processes, and thus the fractionation approach to isolate and/or synthesize those products from algal biomass may offer significant GHG benefits through this integrated biorefinery concept by displacing energy- or GHG intensive processes to arrive at the same ultimate functional product.
3 Fractionation of algal biomass to maximize valorization pathways
In order to valorize components in algal biomass to their maximum extent, a conversion process depends on fractionation of the biomass to individual constituents or on sequential processes that do not impact the quality of the substrates for subsequent steps. Each of the respective fractions, generated in a minimally destructive process, could support their own route to products. The processes described in the diagram shown in Fig. 2 illustrate two parallel pathways of algal biomass conversion, either focused on algal oil extraction and isolation (algal lipid extraction and upgrading, ALU) as had previously been the focus for numerous algal biofuel processes (Fig. 2A) or on whole biomass fractionation (through a combined algal processing pathway, CAP) designed to take full advantage of the composition of the biomass (Fig. 2B).14,25,29 Even though the focus on a fractionation process leaves us with a narrow discussion, the modular implementation of any of the steps in the process allows us to valorize the individual components. A detailed and critical review of alternative conversion pathways is relevant to this discussion but outside the scope of this work. A recent critical review of the fundamental principles around lipid extraction and the respective contribution of different process configurations, including novel lipid extraction technologies, has recently been published.107 The fractionation process includes a dilute acid pretreatment of algal biomass, during which the carbohydrates are solubilized to monomeric sugars available for subsequent fermentation. If the fermentation step produces ethanol as one example (among other options), the ethanol may be distilled from the fermentation broth and the still-bottoms subjected to hexane extraction, followed by upgrading the extracted oils to a renewable diesel blendstock.3,14 The insoluble residue remaining after fermentation and lipid extraction is an enriched protein fraction, which is available for additional product development. Each of the three isolated fractions can be (partially or completely) diverted for the production of bioproducts. This approach not only increases the overall fuel fraction obtained from the biomass, but also allows for the implementation of a modular approach to the valorization of each of the fractions. Pursuing the recovery of high-quality and potentially high value products replaces a lipid-extraction-only approach (Fig. 2A). The initial demonstration and theoretical calculations include fermentative routes to fuels, including renewable diesel and ethanol; however, there is no reason to discount the option of diverting a fraction of each of these streams (e.g. a subset of the lipids or fermentable sugars) to high value alternative products, as long as the cost-impact can be modeled accurately and the respective process steps are not compromised. In the following discussion, we explore options that are compatible with such slipstreams, implemented as the next stage of fractionation, supporting maximal biomass utilization.By comparison to lipid extraction technologies, a thermochemical approach where the whole algal biomass is subjected to, for example, hydrothermal liquefaction (HTL), a high-temperature and pressure conversion process, to produce a green crude oil, is more destructive and may reduce the opportunities for valorizing high-value components beyond nutrient recycling from the aqueous phase. Typically, a hydrothermal liquefaction process of algae yields four main outputs. Gas is emitted after the hydrothermal liquefaction process, while an aqueous, organic and solid phase are present after phase separation.30 Though the composition of the gas depends on the reaction conditions, it is mostly composed of CO2, allowing for recycling to algae cultivation. The aqueous phase contains nitrogen, phosphorus, and many organic compounds.30 Recycling these nutrients for algae cultivation is feasible, however only at high dilutions and they have been shown to sometimes negatively impact the algae growth.31,32 The solid residue, often referred to as biochar, has a wider variety of uses. Biochar in general has been proposed to have water purification uses and soil amendment properties or can be burned for energy production. Biochar from wood sources has been used to remove lead and fluoride from water.33,34 Biochar added to agricultural soil can reduce the loss of inorganic nitrogen and phosphorus during crop growth.35 In terms of high value products, the HTL oils from algae can result in the crystallization of hydroxyapatite (HAp).30 HAp is a calcium orthophosphate (Ca10(PO4)6(OH)2) and is primarily used as a bio-medical replacement for bone, as well as a catalyst to form butanol and acrylic acid.30 In addition, HAp has been shown to be an effective heterogeneous catalyst for the production of butanol and acrylic acid.36,37 Thus, it is feasible to collect high value products from a HTL conversion process, however, in addition to a limited set of chemical feedstocks, there remain questions on the process integration and species and pathway dependencies that need to be solved in future iterations of HTL development.
In the following sections, we will introduce options for products from algal biomass beyond fuel that are exceeding “niche” market volume applications, based on our knowledge of the above three species of algae and their representative compositional profiles and their compatibility and potential to be integrated in a fractionation pathway as described here. The goal is to identify opportunities for such bioproducts in addition to traditional food or feed applications from algae, to better align low cost biomass production and quality of input streams (e.g. municipal wastewater as a nutrient source) with market demands. Future work needs to include detailed mapping of some of the major high-value components against the cultivation and dynamic compositional shifts as well as experimental demonstration of some of the major pathways toward isolation and conversion of bioproducts associated with a corresponding quantitative economic valorization of the biomass and the respective products.
The initial motivation for developing a conversion or fractionation approach was to create three different potential fuel streams: ethanol from fermenting the released carbohydrates, renewable diesel or jet fuel blendstock from the lipid fraction through hydrotreating and isomerization and finally, mixed alcohols (isobutanol, isopentanol, and others) from the protein fraction.3,14,24,39 The first two fuel fractions (ethanol and renewable diesel or jet fuel blendstock) have been accounted for and demonstrated recently in a combined and integrated process.3,14 The reports indicate a potential for 35% reduction in the overall minimum fuel selling price by combining both fuel fractions relative to a renewable diesel-only pathway.3,14
4 Microalgae-based feedstocks for commodity bioproducts
Moving beyond strictly (high-volume but low-value) fuel opportunities from fractionated biomass components, we next consider higher-value product opportunities primarily based on applications in excess of small niche markets. The concept of developing a biorefinery using algal biomass relies on a compatible cultivation system and in particular a scale that is compatible with the respective markets that are targeted. For example, if commodity markets such as fuels are envisioned for one aspect of the biorefinery, then bioproducts from the same biomass will be produced at similarly large volumes and their use and markets must be considered to match the produced quantities, in order to avoid saturating any one particular market.The major drivers behind successful biorefineries are focused on identifying means to achieve targeted levels of algal biomass productivity and composition and conversion efficiencies, all identified as critical factors for economic development of algal biofuels. By integrating the dynamic algal biomass composition with downstream process characteristics, options are generated for the development of commercially-relevant products derived from lipid, carbohydrate or protein fractions.3,40
There are typically three criteria that are useful to consider in the context of developing a viable biorefinery concept when introducing bioproduct options; the envisioned product developed should be either (i) identical to an existing chemical, fuel or other product, where the primary driver would be the price of the bio-derived product, (ii) identical in functional performance, where price is still a primary driver, but the bio-derived nature of new commodity products may render the products more commercially attractive, or (iii) potentially an entirely new material with unique and useful, functional performance characteristics.41 This last criterion is perhaps the most difficult to pursue because of the unpredictable nature of the potential market volume and price targets, however, the potential for a large number of the novel products in algae to form the basis of new materials is high. A large number of products can be identified in algal biomass, as shown by the list in Table 3, which organizes the bioproducts by their approximate concentration in algal biomass and their projected market size. According to the DOE National Algal Biofuels Technology Roadmap, good bioproduct candidates produced along with fuels could sell for approximately $0.67–$2.2 kg−1 at a volume of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 1
000 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 T per year.41
000 T per year.41
| Source | wt% | Product | US market sizea (T) | Price ($ T−1) | Maximum feedstockb (T farm−1 year−1) | Ref. |
|---|---|---|---|---|---|---|
a Where available, 3 or 5 year average US market size (metric tonnes, T) of consumption and price is used.
b Product yield based on the listed biomass composition (using the high end of the ranges shown) on a 5000 acre (2023 ha) algae farm with total biomass production of 184![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 T per farm per year, assuming a projected 25 g m−2 d−1 productivity.1,3
c Market value for fuel additives is difficult to estimate because of a multitude of products and applications.
d Market sizes are shown ranging between succinic acid and adipic acid, specifically, the market volume for succinic acid as a final product is fairly small, but has the potential to be well over 2 MM tons per year when including potential derivative products that may be made from succinic acid.6
e Market sizes are shown ranging between succinic acid and adipic acid, specifically, the market volume for succinic acid as a final product is fairly small, but has the potential to be well over 2 MM tons per year when including potential derivative products that may be made from succinic acid.6
f North American consumption market size for only bio-plastics focused on packaging materials, as opposed to the 300 600 T per farm per year, assuming a projected 25 g m−2 d−1 productivity.1,3
c Market value for fuel additives is difficult to estimate because of a multitude of products and applications.
d Market sizes are shown ranging between succinic acid and adipic acid, specifically, the market volume for succinic acid as a final product is fairly small, but has the potential to be well over 2 MM tons per year when including potential derivative products that may be made from succinic acid.6
e Market sizes are shown ranging between succinic acid and adipic acid, specifically, the market volume for succinic acid as a final product is fairly small, but has the potential to be well over 2 MM tons per year when including potential derivative products that may be made from succinic acid.6
f North American consumption market size for only bio-plastics focused on packaging materials, as opposed to the 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 T production capacity in light of the global 1.62 MT capacity.
g Solely based on US production of 2-ethylhexanol (2-EH) as a non-phthalate plasticizer.67 000 T production capacity in light of the global 1.62 MT capacity.
g Solely based on US production of 2-ethylhexanol (2-EH) as a non-phthalate plasticizer.67
|
||||||
| Fatty acids | 10–45 | Hydrocarbon fuel products | 209![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
920 | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 069 069 |
44 and 45 |
| Omega-3-fatty acids | 3–6 | Polyols | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 430 430![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2500 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 076 076 |
46–49 |
| 3–6 | Polyurethane | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4980 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 076 076 |
50–54 | |
| 3–6 | Nutraceuticals | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
80–160 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 076 076 |
55–57 | |
| Hydroxy-, branched chain fatty acids, fatty alcohols | ∼1 | Surfactants | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2280 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 076 076 |
58 and 59 |
| ∼1 | Fuel additives | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
—c | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 076 076 |
||
| Sterols | 2–4 | Surfactants | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2280 | 7384 | 58 and 59 |
| 2–4 | Phytosterol nutra/pharma-ceuticals | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
7384 | 43, 55 and 60 | |
| Phytol | 3–4 | Surfactants | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2280 | 7384 | 58 and 59 |
| Glycerol | 2–6 | Di-acids (e.g. succinic acid) | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–2 000–2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000d 000d |
1550–3400 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 076 076 |
6, 61 and 62 |
| Fermentable sugars (glucose, mannose) | 10–45 | Fuel ethanol | 209![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
780 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 365 365 |
44 and 45 |
| 10–45 | Di-acids (e.g. succinic acid) | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–2 000–2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000e 000e |
1550–3400 | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 069 069 |
6 and 62 | |
| Mannitol | 3–6 | Polyether polyols | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2500 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 076 076 |
48 and 49 |
| Starch | 5–40 | Polylactic acid (PLA) polymers (bioplastics) | 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000f 000f |
2204 | 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840 840 |
63 and 64 |
| Protein | 19–40 | Thermoplastics | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1900 | 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840 840 |
63 and 64 |
| Amino acids/peptides | 19–20 | Polyurethane | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4980 | 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840 840 |
50–54 |
| Amino acids/peptides | 19–20 | Plasticizers | 353![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g 000g |
1850 | 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840 840 |
65–67 |
4.1 Products, yields and markets
The projected costs of biofuels are calculated based on a biorefinery operation that is scaled to a 5000 acre (2023 ha) farm.1 It is assumed that with productivity projection approximating 25 g m−2 d−1 the annual biomass yield per farm will be approximately 184![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 metric tonnes, T, per year. Based on the chemical composition of the biomass, extrapolations can be made for yields of any given product, when produced alongside fuels and projections can be made on the corresponding market size compatibility (Table 3).
600 metric tonnes, T, per year. Based on the chemical composition of the biomass, extrapolations can be made for yields of any given product, when produced alongside fuels and projections can be made on the corresponding market size compatibility (Table 3).
The list we compiled serves as an example and is not meant to be comprehensive; several additional compounds can be found in different strains and many remain to be discovered. Where possible, a market volume and average values over recent historical ranges are shown, though some are missing. For example fuel additive prices are either not well understood or not known because of the multitude of products that can be made, each commanding its own market value based on their molecular properties. Additionally, because of the varied prices and end products for nutraceuticals markets, it is difficult to understand the U.S. market based on mass. In 2010 the U.S. nutraceutical market was worth $50.4 billion and accounted for 33.5% of the rest of the world market.42 Of the full nutraceutical market, phytosterol based products account only for a small percentage, estimated at $300 million (49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 299 tons) globally.43 Assuming that phytosterols are used for nutraceuticals and have the same market distribution, the U.S. market size would be approximately 17
299 tons) globally.43 Assuming that phytosterols are used for nutraceuticals and have the same market distribution, the U.S. market size would be approximately 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 T year−1.43
000 T year−1.43
Where possible, products have been selected to represent petrochemical replacements (such as oleochemicals) with a significant commercial impact and global markets capable of supporting multiple algae farms of 5000 acre (2023 ha). We calculate that even if the products proposed could only capture 10% of their respective markets, it would be possible for our proposed scheme to sell all of the products produced by multiple farms without greatly impacting supply and demand and thus market value price.
A highly topical example of a conflict between niche and commodity market products is that of nutraceutical fatty acids (e.g. omega-3 polyunsaturated fatty acids, PUFA) present in algal oils, which are known to play an important role in reducing cardiovascular diseases, regulating membrane fluidity, and electron and oxygen transport, as well as thermal adaptation and are therefore recommended as nutritional supplements in human and animal food and feed rations.68 These fatty acids in the food supplement market are worth about between $30 and $100 kg−1, however, the total market size is only in the range of 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 T year−1. It would only take 6% of the total algal biomass produced, based on a single farm's output, to achieve full market saturation (Table 3). This same projection is true for several other higher value, but smaller market bioproducts. However, in light of uncertain future markets, it is possible that the availability of these current niche products may become commodity products and applications could change and thus demand much larger market shares, but also at a lower price point. To stay relevant to a large-scale biorefinery approach in context of a fuel production scenario, prospective bioproduct value calculations should be carried out relative to fuel-scale production.
000 T year−1. It would only take 6% of the total algal biomass produced, based on a single farm's output, to achieve full market saturation (Table 3). This same projection is true for several other higher value, but smaller market bioproducts. However, in light of uncertain future markets, it is possible that the availability of these current niche products may become commodity products and applications could change and thus demand much larger market shares, but also at a lower price point. To stay relevant to a large-scale biorefinery approach in context of a fuel production scenario, prospective bioproduct value calculations should be carried out relative to fuel-scale production.
The products listed in Table 3 can be separated into groups relating to their applications. For example, products with applications in food ingredient and additive markets (including nutraceuticals) are shown to have relatively small market sizes (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 T) but can command an extremely high unit price ($30
000 T) but can command an extremely high unit price ($30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–$100
000–$100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 T−1). Algae-derived products, present at smaller volumes, such as PUFAs, pigments, anti-oxidants, cosmetics or bioactive peptides for food, nutraceutical and pharmaceutical applications represent options for high value recovery from algal biomass. However, some of the scenarios may not allow for integration with a farm-based biorefinery described here due to very strict purity and process control requirements.4,69,70
000 T−1). Algae-derived products, present at smaller volumes, such as PUFAs, pigments, anti-oxidants, cosmetics or bioactive peptides for food, nutraceutical and pharmaceutical applications represent options for high value recovery from algal biomass. However, some of the scenarios may not allow for integration with a farm-based biorefinery described here due to very strict purity and process control requirements.4,69,70
We will focus on identifying components of the biomass that can serve as feedstocks for the development of large-market commodity products, and not on minor components that could be considered final products and are reviewed elsewhere.4,69 A second large market segment covers products that may displace petrochemicals (e.g. polyurethane replacements, bioplastics and surfactants), which each have a large potential market (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–40
000–40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 T). Producing replacements for petrochemical products in the surfactant and biopolymer realm simplifies constraints around the strict control over cultivation environment (e.g. use of wastewater or flue gas prior to conversion) compared with food and feed applications of the biomass. In particular the presence of metals or toxins from wastewater or flue gas utilization ending up in the biomass is potentially less critical to petrochemical replacement applications, compared to a potentially highly detrimental impact on feed applications, though other factors may play a role, such as ash, salts, and other impurities.71–73
000 T). Producing replacements for petrochemical products in the surfactant and biopolymer realm simplifies constraints around the strict control over cultivation environment (e.g. use of wastewater or flue gas prior to conversion) compared with food and feed applications of the biomass. In particular the presence of metals or toxins from wastewater or flue gas utilization ending up in the biomass is potentially less critical to petrochemical replacement applications, compared to a potentially highly detrimental impact on feed applications, though other factors may play a role, such as ash, salts, and other impurities.71–73
4.2 Feed markets
Food and feed market applications for whole algal biomass are commensurate with the commodity production levels estimated from the farms described here, though their application faces numerous challenges. It is estimated that the global feed industry market approximates 980![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 T year−1, with 96% allocated to livestock and 4% to aquaculture; a more detailed breakdown of the market distribution is given in Table 4.74 In addition, the global production of feed has increased every year for the past five years.74 Aquaculture feed production has seen a 1.8% increase in demand corresponding to a rise in demand for aquaculture itself, as natural sources of marine resources are exhausted and more people need the nutrition provided by omega-3 fatty acids.74 Similarly, fish need feed that support fatty acid production to maintain a healthy nutritional balance.75 Microalgae are currently used as feed for the larva of fish and crustaceans, and have potential as a feed source for adult species due to their nutritional properties.75,76 As for aquaculture, demand for livestock feed has also increased.74
000 T year−1, with 96% allocated to livestock and 4% to aquaculture; a more detailed breakdown of the market distribution is given in Table 4.74 In addition, the global production of feed has increased every year for the past five years.74 Aquaculture feed production has seen a 1.8% increase in demand corresponding to a rise in demand for aquaculture itself, as natural sources of marine resources are exhausted and more people need the nutrition provided by omega-3 fatty acids.74 Similarly, fish need feed that support fatty acid production to maintain a healthy nutritional balance.75 Microalgae are currently used as feed for the larva of fish and crustaceans, and have potential as a feed source for adult species due to their nutritional properties.75,76 As for aquaculture, demand for livestock feed has also increased.74
| Total | All livestock | Poultry | Pig | Ruminant | Aquaculture | |
|---|---|---|---|---|---|---|
| Production (106 tonnes) | 980 | 939 | 439 | 256 | 196 | 41 |
| Percentage | 100% | 96% | 45% | 27% | 20% | 4% |
| China (106 tonnes) | 183 | 158.2 | 65 | 85 | 8.2 | 18 |
| USA (106 tonnes) | 173 | 146 | 82 | 24 | 40 | 11 |
Algal biomass could also offer a supplement to the existing feed produced for livestock consumption and comprise anywhere from 7–20% of feed composition depending on the species.75,77–79 Some algal biomass feeds may have greater nutritional quality than the currently used soy biomass.80 On the other hand, for livestock, high levels of algal biomass in the diet can lead to reduced digestibility and higher feed intake, as the cell wall prevents access to proteins and other cell components.81–83 The use of lipid-extracted algae may mitigate these problems. Studies on algae digestibility and organic matter digestibility for ruminants indicated that certain processing pathways cause an increase in digestibility with the addition of algae.78
Unfortunately, the use of lipid-extracted algae may reduce nutritional benefits, as MUFAs, PUFAs, and carotenoids are removed from the biomass. The crude protein and gross energy are reduced for lipid extracted algae, indicating that it may take more lipid-extracted algae than whole algae to replace portions of feed.84 A more in-depth discussion of using algal biomass and protein-rich residues as feed additives is included in a later section specifically dedicated to protein content and amino acid composition. Approximately 30% of the global algae production contributed to the animal feed industry in 2004.75,77 Extrapolating the production of algae for food and fuel products has the potential to impact global energy, resources, land use and availability and greenhouse gas emissions.85,86 Therefore, the impact on resource demand and availability (including land and nutrient use) needs to continuously be assessed alongside a detailed study of the quality of algal biomass for any of these applications. Recently, a resource study concluded that through contribution of algae to food production alongside fuels, a form of land use intensification is implemented and this can aid the maximal utilization of resources and thus aid the route to commercialization.87
The contentious food–water–energy nexus that algae occupy has room for much further discussion, though it is outside the scope of this work. Much of the continued discussion here will therefore not focus on food or feed product applications. Furthermore, the highly specialized and targeted markets for these products would either rapidly saturate when scaling an algae farm for fuel production or are currently mostly uncharted territory for the introduction of algal biomass at scale. The nutritional impact and like-for-like substitution of algae products in food and feed rations is an area that is actively studied in the literature but not yet implemented at scale.75,77–79
In order for algae to be implemented in the food and feed markets, they must be approved by relevant government organizations. In the U.S., Food and Drug Administration (FDA) approval for food products includes a necessary approval of the manufacturing process, which could limit some technology or feedstock options (e.g. wastewater or flue gas) from being implemented in conjunction with feed production.88
4.3 Biobased plastics
Bioplastics is another example of a commodity product with large market opportunities that can be produced from all three major components: lipids, protein and carbohydrates. Biobased plastics are a small, but growing, segment of the enormous plastics market. The global consumption of bioplastics in 2013 was already 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 620
620![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 T and this is projected to grow to over 2
000 T and this is projected to grow to over 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 T by 2020.63,64 Renewable sources of fermentable sugars and polysaccharides such as starch, cellulose, lignin, chitosan and protein, can be used to produce such plastics, and this is discussed later.89,90 The price for polysaccharide-derived plastics is currently assumed to be consistent with petroleum-based plastics. This may change if a premium can be assigned to bio-sourced products or a performance benefit can be found. At this point, each market segment, e.g. catering products, diapers, and packaging, has its own market value and required quality properties and it is out of the scope of this review article to discuss the details of these markets. Common bioplastics currently produced or researched include polylactic acid (PLA), polyhydroxyalkanoates (PHA), cellulose esters, starch and protein plastics (often from plant or animal proteins).63,89,90 Several researchers have described blending whole algae as a filler material for different types of plastics. Whole algae has been mixed in various proportions with polypropylene (PP),91 polyvinyl chloride (PVC),92 polyethylene (PE),93,94 blends of algae and starch,95 and various other polymers.96 An alternative biologically-derived polymer is poly-β-hydroxybutyrate (PHB), a storage polymer that can be used to produce high-quality biodegradable plastics.97 PHBs can be natively produced by cyanobacteria,98 though examples exist where eukaryotic algae, such as Phaeodactylum tricornutum99 and Chlamydomonas reinhardtii,100 have been transformed to produce PHB.
000 T by 2020.63,64 Renewable sources of fermentable sugars and polysaccharides such as starch, cellulose, lignin, chitosan and protein, can be used to produce such plastics, and this is discussed later.89,90 The price for polysaccharide-derived plastics is currently assumed to be consistent with petroleum-based plastics. This may change if a premium can be assigned to bio-sourced products or a performance benefit can be found. At this point, each market segment, e.g. catering products, diapers, and packaging, has its own market value and required quality properties and it is out of the scope of this review article to discuss the details of these markets. Common bioplastics currently produced or researched include polylactic acid (PLA), polyhydroxyalkanoates (PHA), cellulose esters, starch and protein plastics (often from plant or animal proteins).63,89,90 Several researchers have described blending whole algae as a filler material for different types of plastics. Whole algae has been mixed in various proportions with polypropylene (PP),91 polyvinyl chloride (PVC),92 polyethylene (PE),93,94 blends of algae and starch,95 and various other polymers.96 An alternative biologically-derived polymer is poly-β-hydroxybutyrate (PHB), a storage polymer that can be used to produce high-quality biodegradable plastics.97 PHBs can be natively produced by cyanobacteria,98 though examples exist where eukaryotic algae, such as Phaeodactylum tricornutum99 and Chlamydomonas reinhardtii,100 have been transformed to produce PHB.
5 Lipid composition and extraction towards lipid-based bioproducts
The value of algal biomass is in part derived from the lipid fraction and respective composition, among which the fatty acids play a major role in determining both the fuel properties as well as hydrotreating metrics. The lipids of algae are relatively complex mixtures of polar, neutral and acidic molecules (a summary of lipid types found in algae is shown in Table 5 and the references therein), which again are dynamic in their respective contribution to the extractable lipid fraction depending on the physiological status of the algal cells. Depending on the strain, microalgae can show similarities in lipid class production to terrestrial oil producers; however their lipid classes tend to be far more speciated (Table 5).101–103 In fact the diversity of triglycerides found in Chlorella, Scenedesmus and Nannochloropsis species is an order of magnitude more diverse. For example, a total of ∼400 individual triglycerides were found in algae, relative to ∼20–30 individual TAGs found in terrestrial oily feedstocks such as canola and soy oils (NREL unpublished data). There may be potential for microalgal oils to be used as a substitute for plant oils for oleochemical synthesis and ultimately replace and potentially expand opportunities based on novel product parameters derived from unique triglyceride compositions. Triglycerides can be hydrolyzed into fatty acids and glycerol, with both components contributing to the oleochemical industry. Valuable products that are present in, or derived from, algal oils comprise fatty acids, including fatty acid esters, fatty acid ethoxylates, soaps, fatty amines and fatty alcohols.104–107 In addition, a multitude of pigments can be found in algae, which most uniquely associate with the respective species and function to maximize light energy capture in the light harvesting apparatus. For example, the carotenoids in microalgae, in particular astaxanthin, lutein/zeaxanthin, canthaxanthin and β-carotene in Nannochloropsis, currently encompass a growing market as natural additives in food and feed.70,108 Even though the native biomass lipid composition may vary, the final composition of the oils after a fractionation processing approach has been demonstrated to impact, for example, the free fatty acid content of the oils, while reducing the phospholipid concentration.107| Category | Class |
|---|---|
| Glycerolipids | Triacylglycerides (TAG)109,110 |
| Diacylglycerides (DAG)109,110 | |
| Monoacylglycerides (MAG)110 | |
| Glycerophospholipids | Phosphatidylethanolamine (PE)109 |
| Phosphatidylcholine (PC)109 | |
| Phosphatidylsulfocholine (PSC) | |
| Phosphatidic acid (PA) | |
| Phosphatidylserine (PS) | |
| Phosphatidylglycerol (PG)109 | |
| Phosphatidylinositol (PI)109 | |
| Glycolipids | Monogalactosyldiacylglycerol (MGDG)109 |
| Digalactosyldiacylglycerol (DGDG)109 | |
| Sulfolipids | Sulfoquinovosylmonoacylglycerol (SQMG)111 |
| Sulfoquinovosyldiacylglycerol (SQDG)109 | |
| Betaine lipids | Diacylglyceryltrimethylhomoserine (DGTS)109 |
| Diacylglycerylhydroxymethyltrimethyl-β-alanine (DGTA) | |
| Diacylglyceryl carboxyhydroxymethylcholine (DGCC) | |
| Hydrocarbons | Terpenoids |
| Isoprenoids | |
| Alkanes | |
| Phytol | |
| Sterols (as steryl esters, and steryl glycosides) | Cholesterol112 |
| Cholestanol112 | |
| Brassicasterol112 | |
| Ergostenol112 | |
| Pollinastanol113 | |
| Clionasterol112 | |
| Stigmasterol113 | |
| Fucosterol113 | |
| Wax esters109 | |
| Fatty acyls | Straight chain fatty acids (FA)109 |
| Branched chain fatty acids | |
| Hydroxy fatty acids (OHFA)109,114 | |
| Hydrocarbons | Terpenoids13 |
| Isoprenoids109 | |
| Alkanes109 | |
| Phytol109 | |
| Carotenoids | Carotene109 |
| Xanthophyll109 | |
| Vitamins | Tocopherol109 |
| Tocotrienol |
5.1 Fatty acid composition
A number of fuel metrics and co-product routes are defined by the fatty acid profile of algae. Representative profiles are shown in Table 6 based on measured data and literature values. Among the most valuable fatty acids are the polyunsaturated fatty acids (PUFAs), defined as fatty acids that contain more than two double bonds along the acyl chain. Microalgae produce a series of unique PUFAs such as docosapentaenoic acid (DHA, 22:6, in Schizochytrium limacinum),115 eicosapentaenoic acid (EPA, 20:5 n-6, in Nannochloropsis and Phaeodactylum sp.),68,116,117 arachidonic acid (ARA, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 n-6, for example in Porphyridium purpureum),118–120 γ-linolenic acid (GLA, 18
4 n-6, for example in Porphyridium purpureum),118–120 γ-linolenic acid (GLA, 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 n-6, in Spirulina platensis),121 and α-linolenic acid (ALA, 18
3 n-6, in Spirulina platensis),121 and α-linolenic acid (ALA, 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 n-3 in Spirulina platensis and Chlorella),121,122 all of which have been widely used as nutraceuticals and have been shown to provide an advantage to feed quality when mixed with traditional feeds.70,77,123 It has been reported that highly unsaturated fatty acids occur more frequently in polar lipid fractions, especially phospholipids.124 Phospholipids can range from 8–47% of the total fraction of algal oil depending on species and growth conditions.125
3 n-3 in Spirulina platensis and Chlorella),121,122 all of which have been widely used as nutraceuticals and have been shown to provide an advantage to feed quality when mixed with traditional feeds.70,77,123 It has been reported that highly unsaturated fatty acids occur more frequently in polar lipid fractions, especially phospholipids.124 Phospholipids can range from 8–47% of the total fraction of algal oil depending on species and growth conditions.125
| Scenedesmus acutus | Chlorella vulgaris | Nannochloropsis granulata | Linseed128 | Soybean129 | Fish75 | |
|---|---|---|---|---|---|---|
| Myristic acid, C14:0 | 1.3 | 1.1 | 5.4 | 0 | 0 | 7.5 |
| Palmitic acid, C16:0 | 18.4 | 11.5 | 15.6 | 5.1 | 10.6 | 18.0 |
| Palmitoleic acid, C16:1 n-9 | 3.6 | 0.7 | 19.4 | 0 | 0 | 0 |
| Stearic acid, C18:0 | 1.3 | 1.1 | 0.3 | 4.3 | 4.1 | 3.6 |
| Oleic acid, C18:1 n-9 | 5.9 | 3.5 | 5.2 | 15.8 | 23.0 | 7.7 |
| Linoleic acid, C18:2 n-6 | 14.1 | 11.4 | 4.1 | 16.5 | 54.5 | 1.2 |
| Linolenic acid, C18:3 n-3 | 31.5 | 34.9 | 0 | 58.3 | 7.2 | 0.3 |
| Arachidic acid, C20:0 | 1.0 | 0 | 0 | 0 | 0.3 | 0.2 |
| Arachidonic acid, C20:4 n-6 | 0 | 0 | 6.1 | 0 | 0 | 1.0 |
| Eicospentaenoic acid, C20:5 n-3 | 0 | 0 | 38.7 | 0 | 0 | 0.4 |
| Behenic acid, C22:0 | 1.9 | 0 | 0 | 0 | 0 | 0 |
| Erucic acid, C22:1 n-9 | 1.2 | 0.8 | 0 | 0 | 0 | 0.1 |
| Lignoceric acid, C24:0 | 1.6 | 1.1 | 0 | 0 | 0 | 0 |
The implication of removing a slipstream of material from for example the fuel-bound lipid fraction has the potential to provide additional benefits by improving the hydrotreating conditions of the oils. The cost of hydrogen was the third largest variable cost identified in recent TEA modeling reports for conversion of the lipid fraction into diesel fuel and thus removing polyunsaturated fatty acids prior to hydroprocessing could have a significant economic impact.3,126 Hydroprocessing of triglyceride or free fatty acid oil streams involves hydrogenation of double bonds and removal of oxygen by either hydrodeoxygenation, decarboxylation or decarbonylation reactions to reduce the oxygen content. These reactions produce a high cetane number diesel blendstock consisting of C15 to C19 normal alkanes derived from the predominantly C16 to C20 fatty acids.127 For example, in a hypothetical system where decarboxylation or decarbonylation reactions represent a minor proportion of the overall conversion process, hydroprocessing of a fully saturated FFA requires 3 moles of H2 per mole of FFA. Hydro-processing a triple-unsaturated FFA such as linolenic acid would require 6 moles of H2, a 100% increase. A more practical example can be made for hydroprocessing of Nannochloropsis oil with 46.6% of the fatty acids being C20:5 (Table 6). Removal of all of the polyunsaturated fatty acids thus reduces the hydrogen requirement during hydrotreating by a calculated 41%, assuming that all oxygen is removed by hydrogenation. This percentage reduction could be even larger if a significant fraction of oxygen removal occurred by decarboxylation. There is thus an overall process benefit to removing the highly unsaturated fatty acids from the fuel-bound lipids, in addition to the value that can be derived from product upgrading.
5.2 Oleochemicals from algal oils
Oleochemicals are chemicals derived from oils and fats that are similar to and could potentially replace petrochemicals. These oleochemical products can be triglycerides, FFAs, FAMEs, fatty alcohols and fatty amines as well as glycerol, derived from high-triglyceride content plant-derived feedstocks. An overview of the complexity of the microalgal lipid fraction is shown in Tables 4 and 5.The chainlength distribution of the fatty acids that make up the lipids will help define the particular oleochemical application. Fatty acyl chains of 8–12 carbons are ideal for surfactant synthesis, 12–18 carbons are typically slated for diesel, solvents or cosmetics applications, while longer chains, e.g. 18–22 carbon are used as lubricants. Biopolymers derived from lipids ideally use the fraction with fatty acyl chains longer than 22 carbons.
Phospholipids can make up the majority of the lipid composition of algae that are harvested from fully nutrient replete environments. These molecules are known to be surface active and are used as emulsifiers in food, cosmetic, and pharmaceutical applications.
Chemical transformations applied in oleochemistry, such as epoxidation and ozonolysis,130–132 might give rise to new opportunities for novel products derived from phospholipids.8 For example, cosmetics, pharmaceuticals, nutraceuticals, paints, lubricants, surfactants and polymer additives are common products that can be derived from algal oils.124 Any target application will have to take into account the dynamic composition shifts as described above, where the lipid composition with respect to the relative molecular composition varies dramatically with the cultivation conditions of the biomass.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700
700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons, with the largest end use market for surfactants being household cleaning detergents (Table 3).58,59 Specialty surfactants are higher-priced, low-volume products used in a broad range of industrial and personal care market applications, often with applications in the fuel-additives business with annual demand estimated at 1
000 tons, with the largest end use market for surfactants being household cleaning detergents (Table 3).58,59 Specialty surfactants are higher-priced, low-volume products used in a broad range of industrial and personal care market applications, often with applications in the fuel-additives business with annual demand estimated at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons or 26% of the total US surfactant market.58,59
000 tons or 26% of the total US surfactant market.58,59
Surfactants are traditionally produced from petrochemical (synthetic) feedstocks or oleochemical (natural) feedstocks. The current estimates of the U.S. surfactant production are approximately 40% derived from petrochemical and 60% from oleochemical feedstocks.58 The basic petrochemical feedstocks are ethylene and benzene which are derived from crude oil and converted to surfactant intermediates ethylene oxide (EO), linear alkylbenzene (LAB) and detergent alcohols. The most common oleochemical feedstocks are seed oils, such as palm, coconut or tallow. In general it is assumed that the chain length of the predominant fatty acyl chains defines the surfactant properties, with the shorter chains found in palm and coconut oils becoming prime feedstocks for surfactants. Algal oils may be suitable, however the complexity and dynamic nature of the lipid composition will play a role in the fraction of contaminants present in the final feedstock, which could impact the quality of the resulting surfactants (Tables 4 and 5).
Biodegradability has become an important factor in the environmental acceptance of a surfactant, which was behind most of the recent development of surfactants from natural products. Many natural raw materials incorporate special structures in the surfactant that may reveal new and unexpected functional properties, which can lead to good substitutes for the traditional surfactants. Fatty acids, monoglycerides and glucosides are natural raw materials that have been used for many years in the production of surfactants.133–135 Sterol-based surfactants are a more novel class of raw materials from a natural origin and present a possible large-market and high-value application for unsaponifiable lipids that are undesirable in the fuel fraction.135
It is possible that natural glycolipids, containing hydrophilic headgroups, primarily galactose or rhamnose, linked to a glycerol backbone along with two fatty acyl chains, can form surfactants.133 Alternatively, sugar-based surfactants can be produced by selective glycosylation of long chain hydrophobic lipids.133 The majority of the synthetic analogues of natural membrane glycolipids can form liquid crystalline phases at temperatures significantly higher than room temperature. This imposes a severe limitation in exploiting sugar-based surfactants in many technical applications. A new approach to depress the Krafft eutectic temperature (TK, temperature, below which no micelles are formed because surfactant solubility, is also referred to as the Critical Micelle Concentration or CMC) of the surfactants is therefore necessary to fully realize their technical potential. Sugar-based surfactants with isoprenoid-type hydrophobic chains are a new class of surfactants that largely overcome the high TK problem inherent in the conventional sugar-based surfactants.
Biobased surfactants synthesized by ethoxylation of bio-based fatty components to form non-ionic surfactants and lubricants, are becoming popular alternatives to traditional petroleum-based products.134–138 The bioderived surfactants are gaining traction in the oil and gas fields as drilling fluid additives, as well as industrial cleaners. Biodegradability in oil field applications is becoming important as non-ionic surfactants play a large role as demulsifiers and defoamers and are being used in very high volumes. The estimated volumes of these non-ionic surfactants, often polyethoxylates of fatty amines, fatty alcohols and alkylphenols (e.g. petrochemical-derived nonylphenol ethoxylate) are estimated to exceed 346![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons per year.105
000 tons per year.105
Isoprenoids are derivatives of terpenes and include sterols as well as phytol, the hydrocarbon side-chain on chlorophyll molecules. Phytol is a large contributor to the hydrolyzed lipid fraction, and the single largest contributor to the unsaponifiable lipids (between 40% and >80%, Table 7) and a great potential target for the development of highly valuable surfactants.135,136 The glycosylated phytol surfactants can be prepared based on alcoholysis and Koenigs–Knorr beta-selective glycosylation.139 Alternatively, phytol can also be converted to ethoxylated non-ionic surfactants, some of which are currently commercialized by Dow and Proctor and Gamble.140,141 Similarly, the sterol's alcohol functionality can be used for ethoxylation, which renders highly valuable properties to the derived surfactant molecule.135 The large hydrophobic, planar four-ring structure group can provide good packing properties at emulsion interfaces. Commercial ethoxylated sterols are available such as for example Generol R E5 (BASF), as an ethoxylated mixture of phytosterols. The wide range of microalgal sterols will likely affect surfactant properties and the influence of the different structures is yet to be determined and this is an area under active investigation.
| Scenedesmus acutus | Chlorella vulgaris | Nannochloropsis granulata | |
|---|---|---|---|
| Hexadecane | 0.3 | 0.2 | |
| 8-Heptadecene | 1 | ||
| Heptadecane | 0.4 | 0.5 | |
| Trimethyl 2-pentadecanone | 0.4 | 0.2 | 0.1 |
| n-Hexadecanoic acid | 0.7 | 0.5 | 0.3 |
| Phytol | 68.5 | 82.1 | 41.1 |
| Phytol acetate | 1.6 | 1.6 | 0.2 |
| 9-Tricosene (z) | 1.2 | 0.2 | |
| 7-Methyl (z,8,10 dodecadienal) | 0.4 | ||
| Eicosadiene | 0.2 | ||
| α-Tocopherol | 0.5 | ||
| Cholesterol | 0.4 | 27.5 | |
| Brassicasterol | 0.7 | 0.4 | 0.9 |
| Unknown sterol | 0.6 | 1.8 | |
| Ergosterol | 10.9 | ||
| Campesterol | 0.8 | 0.3 | 0.7 |
| Stigmasterol | 1.2 | 0.5 | 0.6 |
| Gamma-ergosterol | 5.2 | 0.7 | |
| Stigmast-7,16 dien-3-ol | 12.9 | ||
| β-Sitosterol | 3.7 | ||
| Fucosterol | 4.3 | ||
| Unknown sterol 2 | 1.5 | ||
| Stigmast-7-en-3-ol | 2.9 | ||
| Unknown hydrocarbon | 3.6 | ||
| Unknown hydrocarbon | 1.8 |
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) located along the fatty acid chains. Epoxidation is a commercially important reaction in organic synthesis since the high reactivity of oxirane rings allows facile transformation to the desired functionality.143–146 Epoxidized oils are natural, nontoxic, non-corrosive and biodegradable, making them ideal substitutes for phthalates and other plasticizers derived from petroleum.
C) located along the fatty acid chains. Epoxidation is a commercially important reaction in organic synthesis since the high reactivity of oxirane rings allows facile transformation to the desired functionality.143–146 Epoxidized oils are natural, nontoxic, non-corrosive and biodegradable, making them ideal substitutes for phthalates and other plasticizers derived from petroleum.
Vegetable oils are widely used as plasticizers in the form of epoxidized oils because of the high number of carbon–carbon double bonds, as in the algae-derived polyunsaturated fatty acids, which make them a good target for manipulation into high-value products.146,147 Epoxidized oils are also compatible with polyvinylchloride (PVC), and as stabilizers for resins to improve the flexibility, elasticity and stability of polymers towards heat and UV radiation. Epoxides can also be used as high-temperature lubricants, and the polyols obtained through ring opening to polyols can be employed as low-temperature lubricants.148,149 The quality of these epoxides is directly related to the amount of epoxy groups per molecule, expressed as an oxirane number. Epoxides with higher oxirane values and lower iodine values (indicative of level of unsaturation of the oils) are considered high-quality plasticizers.148
Even if there is an adequate amount of epoxidized vegetable oil available at the time,147 only those vegetable oils with a relatively high iodine value or high content of unsaturated fatty acids especially soybean and linseed oils (Table 3) are chosen to produce functional epoxides.150 Even though epoxidation of algal oils has been demonstrated, the purification of a highly unsaturated feedstock by selecting specific lipid molecular components or manipulating the feedstock's chemical composition, e.g. level of unsaturation, has not been experimentally shown. Manipulation of these properties could allow for testing the influence of composition on the polymer performance parameters.
The conversion of fatty acids (often converted to FAMEs prior to epoxidation) into polyols is a two-step chemical process that involves epoxidizing carbon–carbon double bonds and subsequently ring opening of the oxirane (epoxy) functional group either by an alcohol or carboxylic acid.151 The synthesis and characterization of polyurethane coatings from vegetable oil-based polyols has been intensively investigated, producing a series of vegetable oil-based polyols with a constant hydroxyl functionality of 2.7 and residual unsaturation ranging from 0.6 to 3.7 double bonds per triglyceride.152,153
If identical like-for-like substitutions were the target for the algal polyols relative to plant-based polyols, then synthesis of algal lipid-based epoxies and polyols would require precise control of the overall oxirane and hydroxyl functionalities given the high concentration of highly unsaturated double bonds in algal oil. Alternatively, entirely novel polymers can be formed based on the novel functional properties derived from unusual fatty acids in algae and likely novel processes may have to be developed. The fatty acid distribution of algal oil from Chlorella, Scenedesmus and Nannochloropsis, relative to more traditional vegetable oil feedstocks for epoxidation is listed in Table 3. The double bonds on the higher concentration and highly unsaturated C20:5 fatty acids in Nannochloropsis oils have a higher probability of reacting than the double bonds on the low concentration and low unsaturation C16:1, C18:1, C18:2, and C18:3 fatty acids. Initial calculations of functionality based on the fatty acid profile of enriched algal oil indicate that the C20:5 fatty acid will have a much higher functionality than the C16:1, C18:1, and C18:2 fatty acids. Furthermore, the C14:0 and C16:0 will have no OH oxirane functionality due to the absence of carbon–carbon double bonds. The higher functionality of the C20:5 fatty acids in algal oils relative to those of lower unsaturation (i.e. C16:1, C18:1, and C18:2) becomes more skewed as the overall hydroxyl functionality of the conversion increases from 2.3 to 3.0. Based on these calculations, the target functionality range for algal fatty acid-based epoxies and polyols is 2.3 and greater. Below the functionality of 2.3, a higher percentage of free fatty acids (∼22%) will have a functionality of less than 2.0, which would act as chain terminators during polymerization and plasticizers in the final polymers.
Water contamination is difficult to avoid with fuel transportation and storage. A major problem caused by water contamination is microbial growth.157,159 Gasoline and diesel storage tanks can become contaminated with water either due to entrained water picked up during pipeline transport separating out of solution with colder temperatures or due to humid air entering the storage tank. The interface between fuel and water is a point of microbial growth, which can lead to tank corrosion and filter plugging. To prevent these problems, tank bottoms are drained, but the use of biocides is also effective in preventing microbial growth. Biocide formulations are diverse, but one class of compounds, quaternary ammonium salts, has potential to be synthesized from algae products, in particular phosphatidylethanolamines. Although a pathway to deconstruct phospholipids extracted from algae has not yet been demonstrated, a feasible pathway would be to hydrolyze these compounds to break them down into glycerin, free fatty acids, phosphatidic acid, and choline. Phospholipase hydrolysis is an example of such a deconstruction pathway.
Other surface-active molecules used as fuel additives are corrosion inhibitors. Corrosion inhibition is important for fuel transportation through pipelines, fuel storage, and for engine lubrication.157,158 Water entrained in fuels or lubricants in contact with metal surfaces leads to corrosion, which causes engine wear and in extreme cases can cause pipeline and storage tank leakage. Corrosion inhibitors are surfactant materials that attach to metal surfaces with a polar head group while creating a protective layer with a hydrophobic chain. Corrosion inhibitor additives are made from numerous chemical classes, which include carboxylic acids, carboxylates and esters or amine salts of alkenyl succinic acids, which can either be isolated from lipid extracts or directly produced by fermentation of the sugars (e.g. succinic acid fermentations).160
Surfactant molecules are also used as friction modifiers in lube oils and fuels and to control injector, combustion chamber, and valve deposits for both gasoline and diesel engines.157,158 These compounds create a barrier on metal surfaces, similar to corrosion inhibitors, preventing metal on metal contact and reducing wear. Some common functionalities of these surfactants include carboxylic acids, amines, amides and esters and can be derived from the short-chain fatty acids found in algae. In general, additive formulas demonstrated for use in fuel include a mixture of polymerized carboxylic acids of carbon chain length 13 to 18 and alkenyl succinic acid with alkenyl groups from 8 to 18 carbons.161 Algal lipids, being rich in unsaturated fatty acids may require hydrogenation to produce saturated carbon chains for use in corrosion inhibitor formulations. It is reasonable to assume that corrosion inhibitor and friction modifier formulations could be demonstrated with algae derived products.
Deposit control additives (DCAs) can be effective at reducing deposit formation and mitigating increasing fuel consumption and pollutant emissions.155,157 In the US, gasoline marketers are required to use an EPA certified DCA as part of the Clean Air Act.162 A wide range of DCAs have been certified by the EPA for use in gasoline and a large number of products are also suitable for use with diesel fuel. Some of the common chemical functional groups utilized as DCA include polyalkyl amines, polyether amines, polyalkylsuccinimides, polyisobutylene amines, quaternary ammonium salts, and ester amines. Fatty amines and other nitrogen functionalities that can be isolated form algal lipids have potential as precursors for DCA synthesis.
To increase safety and mitigate the risk of static dissipation during diesel fillings at terminals, antistatic additives are added to the diesel fuel.163 Polyamines and polysulfone copolymers are effective antistatic additives at low concentrations.164 Oxygenates such as alcohols and ethers are also effective at dissipating static.157 There is potential for any of these products to be synthesized from compounds extracted from algae for use as static dissipater additives.
Another consequence of severe hydrotreating for reduced desulfurization is the reduced lubricity of diesel fuels due to the removal of other heteroatomic molecular species that impart lubricity. Modern diesel engines rely on the fuel to provide lubrication to engine parts, therefore a minimum amount of lubricity is required.163 Lubricity additives are generally based on carboxylic acids, amides and esters.156,157 Increased demand on diesel engine combustion has necessitated the use of ignition improvers (cetane number improvers) to assist in reducing engine emissions.156 Compounds typically utilized include alkyl nitrates and ether nitrates. It has been demonstrated that additives can be derived from triglycerides, which act simultaneously as lubricity enhancers and ignition improvers.165
Production of fuel and lubricant additives from algae is one potential avenue to increase the petroleum offset and economic viability of an algae biorefinery platform. Synthesis of these compounds from algae has not yet been demonstrated; however, there are many applications of surfactant and detergent compounds with potential for production from fatty acids and phospholipids found in algae extracts, often after pretreatment.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300
300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 T year−1 (Table 3).166 Glycerol is most often produced as a coproduct from lipid conversion (e.g. biodiesel production from triglyceride-rich oils). Glycerol forms the backbone of saponifiable lipids, and is left behind when the constituent fatty acids are converted to fatty acid methyl esters (FAME) to make biodiesel. After washing out from the fuel fraction, glycerol is available in crude form at a low cost ($170 T−1).167
000 T year−1 (Table 3).166 Glycerol is most often produced as a coproduct from lipid conversion (e.g. biodiesel production from triglyceride-rich oils). Glycerol forms the backbone of saponifiable lipids, and is left behind when the constituent fatty acids are converted to fatty acid methyl esters (FAME) to make biodiesel. After washing out from the fuel fraction, glycerol is available in crude form at a low cost ($170 T−1).167
In at least one genus of algae, Scenedesmus, endogenous lipases in the cell biomass hydrolyze a large fraction of the cell-lipids to free fatty acids immediately upon harvest and this extends during the initial phases of biomass storage.168 This endogenous hydrolysis of lipids prior to an extraction process will cause glycerol to be soluble in water and be present in the aqueous fraction of the hydrolyzate, where it can form a co-substrate for the fermentation organism for downstream conversion. It is thought that the lipases in Scenedesmus are activated upon cell damage during or after harvest and storage of the biomass. This is a phenomenon that is species-dependent and only recently has been documented in the literature as a demonstrated storage effect on T-isochrysis.168 The presence of high levels of free fatty acids in Scenedesmus and in Chlorella has been reported before and it is likely the result of similar, storage-induced lipolysis.169 Alternatively, in the case of Chlorella or Nannochloropsis, where the lipids are most often detected as intact TAGs (NREL unpublished data), the glycerol would be released upon conversion to hydrocarbon fuel and thus, if a hydrotreating process is selected, glycerol would be converted to propane, and no longer be available for conversion. The concentration of lipid-derived glycerol can be up to 4% of the biomass and linearly increases with the lipid content, based on the theoretical calculation that the glycerol backbone makes up ∼10% of the weight of an average triglyceride molecule.
If glycerol can be recovered at high purity from any part of the process, it can serve as a feedstock for short-chain dicarboxylic acids such as acrylic acid, short-chain hydrocarbons or polyethylene glycol, which all command a much higher market value compared to crude glycerol (between $1550 and $3400 T−1, Table 3). The short chain hydrocarbons can be produced through aqueous-phase reforming.170 Propylene glycol can be derived from glycerol via an acetol intermediate, after which it can be used as an antifreeze product.171 Acrylic acid can be produced through the conversion of glycerol and other α- or β-hydroxy carboxylic acids, which displaces production from petroleum.172 Acrylic acid polymerizes or readily combines with other unsaturated monomers such as acrylamides, styrene and butadiene to form homo- or co-polymers and can be used to manufacture plastics, coatings, adhesives, elastomers, polishes and paints.173 Acrylic acid esters are considered superabsorber polymers or detergents, and are produced through acrolein as an intermediate from glycerol dehydration using a sub- and supercritical water reaction.174 Biological conversion of glycerol could be a cost effective carbon source and can yield 1,3-propanediol, succinic acid, polyhydroxyalkanoates, 3-hydroxypropionaldehyde, citric acid, 3-hydroxypropionic acid, butanol, and propionic acid.11,104
6 Carbohydrate composition, isolation and routes to bioproducts
Microalgal carbohydrates present an opportunity for the production of a readily convertible sugar stream for upgrading to a variety of fuels and biobased chemicals (including sugar-based surfactants from glycosylation139), and as stand-alone value-added products. The need for a more integrated, economical, and holistic approach to the use of sustainable energy resources has researchers and industry looking more closely at non-fuel uses for renewable feedstock streams. The most promising candidates for valorization – mainly from sugars or their derivatives – have been highlighted before.175 We focus here on the potential for upgrading and utilizing microalgal sugars as value-added, viable bioproducts. In the context of the conversion process described above, it is likely that the carbohydrate fraction of the algal feedstock will end up as soluble monomeric components in the aqueous phase, which lends itself well to biological fermentation-based upgrading.3,14The carbohydrate composition found in Nannochloropsis is mainly composed of glucose, which accounts for approximately 68% of the neutral monosaccharides, followed by galactose at 20%.176 Of the remaining 6 neutral monosaccharides measured, ∼8% was mannose followed by 4% as ribose, and trace amounts of rhamnose, fucose, arabinose, and xylose. Approximately 20% of the total carbohydrate fraction was identified as the sugar alcohol D-mannitol, thought to be directly synthesized from photo-assimilated fructose-6-phosphate.102,176Nannochloropsis exhibits a unique carbon storage metabolism. The storage carbohydrate is found mainly in the form of β-1,3-glucan, with the occasional β-1,6-branch point (laminarin), thus markedly departing from most plant storage carbon metabolism, which uses α-1,4-glucans (glycogen or starch classified based on their secondary structure and crystallinity).177 Laminarin is instead polydisperse, consisting of a minor G-series with polymers containing only glucose residues, and a more abundant M-series with glucans terminated with a 1-linked mannitol residue.178 Both laminarin and mannitol are interchangeable storage components as are sucrose and starch in higher plants. However, the biochemical route, which connects mannitol and laminarin, is currently not well understood, as is the reason why the majority of laminarin chains are terminated by a mannitol residue at their reducing end.178
The carbohydrate composition in Chlorella and Scenedesmus is typical of green algae, with glucose and galactose representing the primary neutral monomers. Scenedesmus also contains a not insignificant fraction of mannose and Chlorella, arabinose. Both species also have contributions of fucose, rhamnose, xylose, and ribose. The polysaccharides common to these two species are similar to those found in higher plants, e.g. starch and cellulose. However, the exact polymeric structures have not been fully described in the literature. There are reports on the presence of both glucomannan and arabinomannan storage polysaccharides in Scenedesmus and Chlorella, respectively.179–181
6.1 Monosaccharide utilization
Glucose, one of the most abundant sugars found in the Nannochloropsis, Chlorella and Scenedesmus strains explored here, can be utilized in a variety of processes to produce value-added products, beyond fermentation to ethanol. Routes to glucose valorization through bacterial or fungal (including yeast) fermentation of glucose to high-value compounds such as 1,4 diacids (e.g. succinic acid), 3-hydroxypropionic acid, itaconic acid, glutamic acid, adipic and muconic acid and sorbitol have recently been described in the literature.62,160 Each of these products becomes a feedstock for subsequent upgrading to final products such as solvents, polyesters, nylon and equivalents, adjustment of food and beverage pH, fabrics, inks, paints, carpet fibers, plastics, adhesives, superabsorbent polymers, personal care products (contact lenses), rubber (tires), flavor augmenters, sweeteners, de-icers, and abrasion resistant coatings.175,182 In brief, beyond the biological fermentative pathways, there are a range of chemical upgrading routes that can be applied to glucose, e.g. chemical dehydration to form 2,5 furandicarboxylic acid (FDCA) and levulinic acid, which can be used in the production of plastic polymers, fabrics, nylon, carpet fibers, fuel ingredients, solvents, polyesters, and herbicides. Similarly, chemical oxidation of glucose to glucaric acid is feasible, and glucaric acid can be used to produce solvents, nylon equivalents, polyesters, fabrics, plastics, and detergents.183 Biological and chemical upgrading pathways will likely have different feedstock quality requirements, and thus either route may become feasible and will depend on the purity of the dilute sugar stream.More unusual hexose-deoxy sugars (fucose and rhamnose) are found in Chlorella, Scenedesmus, and Nannochloropsis.176,184 These sugars can be fermented to 1,2-propanediol, which functions as a feedstock for the formation of polymers, food additives, pharmaceuticals, and textiles.185 For example, rhamnose can be used in a novel conversion pathway for the production of 2,5-dimethylfuran (DMF) with beneficial chemical conversion characteristics of 33% reduction in hydrogen costs and less extreme reaction parameters.186 DMF has been proposed as a potential biofuel due to its higher energy density relative to ethanol.187 Uronic acids are common constituents of algal carbohydrates, especially of the more soluble outer-cell wall polysaccharides.179 These sugar acids may be oxidized to aldaric acids to form FDCA and the salts of aldaric acids and be used in numerous processes such as plastic polymers, fabrics, nylon, carpet fibers, de-rusting, paint stripping of metals, tanning hides, concrete additives, and corrosion inhibitors.188,189
Mannitol is a natural polyol product that can make up a relatively large fraction of the biomass (up to 8% DW) in Nannochloropsis,102 the majority of which would end up in the soluble liquor fraction during the conversion process,3 and thus recovering mannitol as a slipstream might have economic benefits. Sorbitol, the hydrogenation product of glucose can be produced through chemical or biological hydrogenation and, together with mannitol (similar to sorbitol but with a different optical rotation) enter as a feedstock into a range of different applications.175,190 The functionality of mannitol in the coproduct applications listed is thought to be similar and thus parallels can be drawn with sorbitol. All current commercial production of sorbitol is via high-pressure catalytic hydrogenation of D-glucose in a semi-continuous (batch reactor, followed by continuous processing) or continuous process. Sorbitol can be produced as the single product starting from glucose or as a coproduct with mannitol if inverted sugar or high fructose corn syrup is used as the raw material. Reaction temperature and pressure, pH, hydrogen gas flow rate and content of active hydrogen affect sorbitol yield and productivity.191–193 Among the straight-chain polyols that are commercially significant, glycerol, propylene glycol, mannitol and xylitol compete directly with or are used in conjunction with sorbitol in various end uses.
It is probable that other pathways exist for the utilization of microalgal sugars, however, we focused primarily on those that have been recognized as having the most potential to be valorized from aqueous hydrolysis streams from a fractionation process.175 With the advancement of technology and the intensification of research in this area, an increasing number of avenues are likely to become feasible for the use of glucose and other, more unique, microalgal carbohydrates.
6.2 Polymeric carbohydrate structures and routes to polymeric plastics
Polymeric carbohydrates can form the basis of an entire biopolymer industry, based on different pathways for crosslinking polymers. The vast number of algal strains and the complexity of the varying polysaccharides within any one algal cell contribute to far-reaching opportunities for valorization.194 The most common and well-understood polysaccharides found in algae are starch, cellulose, arabinomannan, carrageenan, alginic acid, and chitin.179,180,195,196 Even though in the current configuration of the conversion process described above,3,14 the majority of the polymeric carbohydrates will be hydrolyzed to monomeric saccharides, we include this section on the valorization of polymeric carbohydrates to allow for possible future modifications to the conversion process, e.g. reducing the severity of pretreatment could reduce the completeness of carbohydrate hydrolysis without impacting lipid extractability. This reduced severity would leave a large fraction of the residual biomass as polymeric carbohydrates. Alternatively, harnessing extracellular polymers (algal organic matter) could provide a route to maximizing the polymeric substance utilization from algae.197The conversion of ‘traditional’ polysaccharides, such as starch and cellulose to high-volume, high-value bioplastics has been described extensively before. So far, the polymeric structures of polysaccharides in algal biomass have not been thoroughly described in the literature and it might be difficult to predict the characteristics and the properties of the bioplastics derived from microalgal carbohydrates. Nevertheless, there is a potential abundance of microalgal carbohydrates available, in some strains reaching up to 40% of the dry biomass (e.g. in Chlorella and Scenedesmus20), estimated to amount to 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of polysaccharides generated annually on an algae farm (Fig. 2).
000 tons of polysaccharides generated annually on an algae farm (Fig. 2).
Some of the common forms of carbohydrate-based biopolymers are (i) starch-based plastics (thermoplastic starch TPS and plastarch material PSM) and (ii) cellulose-based plastics (cellulose esters, cellulose acetate, celluloid, and nitrocellulose).198,199 Starch is a relatively simple glucan polymer that is made up of approximately 20–30% amylose (helical polymer of α-1,4 linked glucose) and 70–80% amylopectin (branched polymer of primarily α-1,4 glucose chains linked with α-1,6 branchpoints). Amylose is a straight chain polymer of D-glucose connected units while amylopectin is a branched chain polymer of D-glucose units. Amylose is ideal for the production of thermoplastics because the chains can easily lie close to each other forming weak hydrogen bond interactions between chains; while amylopectin branched chains prohibit forming necessary bonds to transition into a good plastic. In order to turn starch into a bioplastic it must first be chemically treated to eliminate the branching of amylopectin to form amylose, this process is commonly carried out by the addition of acetic acid to cleave any glucose branches. Once the polymer is a homogenous amylose mixture it can be heat treated and cooled into a bioplastic.198–201
Among the carbohydrate-derived bioplastics currently commercialized, PLA is perhaps the most common biodegradable and renewable biopolymer source and has applications in plastic cups, food containers, cutlery, bags, and bottles. PLA is a thermoplastic polyester polymer, which is synthesized via ring-opening polymerization of lactic acid or lactides (cyclic di-ester of lactic acid) with metal catalysts. The characteristics of pliability, flexibility, and durability can be influenced strongly by the addition of plasticizers. PLA has an estimated current consumption at 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tonnes per year and thus a real target market for the high-level production of starch-derived polymers from algae.63,64
000 metric tonnes per year and thus a real target market for the high-level production of starch-derived polymers from algae.63,64
Algae has been shown to have cellulose with higher degrees of crystallinity than a number of other biological sources, including cotton, hemp, flax, and bacterial cellulose.202 Cellulose is a primarily linear glucan polymer of β-1,4 linked glucose units, in a long uniform polymeric chain and can be converted into cellulose acetate, cellulose triacetate, cellulose propionate, nitrocellulose, and cellulose sulfate.203–205 Cellulose-acetate is a long established bioplastic derived from the acetylation of cellulose. The original production process involves the dissolution of cotton (∼90% cellulose) in glacial acetic acid, acetic anhydride, and sulfuric acid (as a catalyst) to disrupt the strong hydrogen bonds between the OH groups that make it rigid.206 Once alcohol groups are replaced with acetate terminal ends, the cotton dissolves into solution, after which water is added to precipitate the cellulose acetate out of solution. After filtering out the fibers, they are dissolved in chloroform, leaving a cellulose acetate plastic after solvent evaporation. This process was later optimized by hydrolyzing cellulose acetate into cellulose diacetate, which is soluble in acetone, a much cheaper and less environmentally toxic solvent.205
Although many of the promising bioplastics stem from the use of proteins, many papers have shown that the addition of polysaccharides to these polymers can yield beneficial intra-molecular property improvements. Polysaccharides from algae can be difficult to harvest given their strong hydrophilic nature and the fact that they are embedded in a complex cell wall matrix architecture of mixtures of protein and carbohydrates.179 Considerable research has been reported on polysaccharide–protein interactions as coacervates, colloid-rich viscous liquids, where the isolated polysaccharides can remain to a certain extent in the complex matrix form. In coacervation, proteins and polysaccharides interact in complex ways, dependent on the ionic and acidic nature of the media, to form gels or precipitates. This approach of emulsification is used extensively in the food industry, e.g. the addition of carrageenan or alginic acid as stabilizers to improve the thermal stability and rheological properties such as milk and yoghurt product firmness, adhesiveness, and gumminess.207,208 Such protein–polysaccharide interactions have been evaluated in the form of edible film polymers and their strength.209 The findings of this research show that protein films were dramatically improved in tensile strength and had greater moisture barriers when combined with polysaccharides alginate, pectin, carrageenan, or konjac flour.209
The addition of polysaccharides to gluten-based bioplastics showed that polysaccharides generally play the role of fillers but can also play the role of a plasticizer. Polysaccharides have also been shown to increase the material elongation characteristics and increase the Young's modulus of the polymers.210 When bioplastics are formed with the addition of chitin, the material showed increase in both tensile strength and Young's modulus, and the material also had noticeably lower water absorption, a desirable trait in the formation of plastics.211
7 Protein as a feedstock for commodity bioproducts
In the conversion and fractionation process of algal biomass (Fig. 2B), the aqueous hydrolyzate fraction along with the residual cell debris is enriched in proteins, peptides and amino acids. Value-added products can be derived from these fractions to improve the process economics, while simultaneously mitigating the environmental impact by allowing extensive nutrient recycling.212–214 In particular, for this review we looked for protein-derived bioproducts that would scale with fuel from algal biomass.7.1 Protein as the basis for food and feed
The use of lipid-extracted algal biomass as a source of human, animal, or microbial nutritional protein and aquaculture feed has been covered in the literature and has the potential to scale with the algae farm scenario described earlier.21,70,81,123,215–221 Related work on producing leaf protein from terrestrial biomass biorefineries for human nutrition may also be applicable to algae biorefineries.7,222,223 The quality (and thus value) of algal protein for human or animal consumption depends on the amino acid composition, in particular the respective concentration of limiting amino acids, palatability and digestibility of the proteins, and the amount of non-protein nitrogen and other potential anti-nutritional components.In general, protein from algae shows good nutritional characteristics,216,224,225 and a typical amino acid composition of the three major genera discussed in this review is shown in Table 8.7,222,223 Integrating food or feed uses of fractionated or extracted algae will need to be tested to ensure that these industrially processed residues remain a good nutritional sources even when produced on a commercial scale.226–228 Furthermore, integrating the use of protein material in an entire process, where all the inputs are accounted for and compatible with a food and feed application is necessary. For example, the utilization of wastewater to supply the nutrients to an algae cultivation will possibly prohibit any nutritional application for the biomass or derived protein. It is likely that the severity of the conditions used to extract lipids and pretreat the biomass may impact any of the listed quality properties. Similarly, any production scenario that involves the use of flue-gas-derived CO2 or wastewater for cultivation, may struggle to demonstrate no negative impact on the quality of the feed derived from the biomass.
| 1 | 2 | 3 | 4 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|
| L-Aspartic acid | 3.64 | 0.7 | 0.65 | 3.85 | 1.15 | 0.93 | 1.39 |
| L-Threonine | 2.13 | 0.5 | 0.45 | 1.88 | 0.59 | 0.49 | 0.74 |
| L-Serine | 1.67 | 0.36 | 0.34 | 1.65 | 0.51 | 0.41 | 0.59 |
| L-Glutamic acid | 4.22 | 0.74 | 0.73 | 4.98 | 1.38 | 1.04 | 1.52 |
| L-Proline | 1.87 | 0.44 | 0.39 | 1.93 | 0.61 | 0.48 | 0.63 |
| L-Glycine | 2.05 | 0.42 | 0.39 | 2.18 | 0.67 | 0.52 | 0.77 |
| L-Alanine | 3.12 | 0.69 | 0.67 | 3.45 | 1.26 | 1.07 | 0.94 |
| L-Cysteine | 0.66 | 0.2 | 0.18 | 0.52 | 0.19 | 0.18 | 0.12 |
| L-Valine | 2.33 | 0.52 | 0.46 | 2.34 | 0.75 | 0.62 | 0.9 |
| L-Methionine | 0.93 | 0.24 | 0.19 | 0.9 | 0.3 | 0.23 | 0.29 |
| L-Isoleucine | 1.63 | 0.36 | 0.32 | 1.64 | 0.5 | 0.4 | 0.66 |
| L-Leucine | 3.43 | 0.75 | 0.65 | 3.73 | 1.17 | 0.92 | 1.24 |
| L-Tyrosine | 1.47 | 0.28 | 0.26 | 1.72 | 0.52 | 0.41 | 0.52 |
| L-Phenylalanine | 2.17 | 0.49 | 0.42 | 2.48 | 0.74 | 0.59 | 0.86 |
| L-Tryptophan | 0.84 | 0.17 | 0.15 | 0.84 | 0.27 | 0.17 | 0.22 |
| L-Lysine | 2.33 | 0.38 | 0.39 | 2.64 | 0.75 | 0.6 | 0.37 |
| L-Histidine | 0.67 | 0.09 | 0.1 | 0.81 | 0.24 | 0.18 | 0.23 |
| L-Arginine | 2.34 | 0.4 | 0.43 | 2.79 | 0.77 | 0.63 | 0.61 |
| Total AA | 37.49 | 7.73 | 7.19 | 40.32 | 12.4 | 9.88 | 12.61 |
| %N | 8.38 | 1.82 | 1.59 | 9.01 | 2.7 | 2.18 | 3.6 |
| Non-protein N (%) | 38.8 | 43.4 | 38.4 | 38.1 | 36.7 | 37.6 | 54.2 |
In addition to human and animal nutrition, partially hydrolyzed algal biomass has been considered as a low-cost microorganism fermentation medium. Hydrolyzed slurries rich in peptides and amino acids have been used to grow E. coli for PHB production,229Lactobacillus lactis and S. cerevisiae for lactic acid and ethanol production, respectively,230 and E. coli and S. cerevisiae for biomass growth.231 However, most of the tests on peptone utilization were run on bench scale fermentations and would need to scale up considerably to absorb the amounts of protein produced in algal fuel production.
That algae can be grown to contain good protein nutritional value is not the only hurdle to overcome for food and feed uses of algal protein. Microalgae are often subjected to nutrient deprivation to induce high lipid production, which can also cause catabolism of proteins and thus potentially change the amino acid profile and perhaps the nutritional value of the algae. Balancing the inverse relationship of algal biomass and thus protein productivity with lipid concentration will be needed to maximize the viability of the entire biorefinery.232,233 The cost of drying or otherwise stabilizing protein needs to be reduced to economical levels for transport to large scale feeding operations. Heavy metals from flue gas, flocculating agents, solvents used to extract algal oil or acid pretreatment may interfere with protein nutrition and such realistic, pilot-scale biorefinery algae samples would need to be tested.223
7.2 Conversion of amino acids
Individual amino acids can be converted to a variety of products, and present viable avenues for large market petrochemical displacement strategies from protein.234 The amino acids lysine and glutamic acid have been proposed for the conversion to platform chemicals.235 Recently, it was demonstrated that an electrodialysis system could separate the positively and negatively charged amino acids, e.g. glutamic and aspartic acid versus lysine and arginine respectively.236 Waste proteins from various sources, including microalgae, can be used to produce bio-based chemicals.237 One way of utilizing amino acid mixtures would be to use fermentative routes to selectively assimilate the mixed amino acids to produce cyanophycin, an insoluble storage polymer of aspartic acid and arginine, often found in cyanobacteria, and thus reduce the number of amino acids for transformation.236 The feasibility of cyanophycin production, from biomass, has been reviewed elsewhere and it is not clear whether such protein-rich polymers are present in Nannochloropsis, Chlorella or Scenedesmus.238Current pathways towards biofuels production tend to not fully recycle all of the reduced nitrogen that is supplied to the cultivation system, a difference that must be made up using energy intensive Haber–Bosch ammonia production.239 An approach to deaminate amino acids and liberate ammonium for nutrient recycling using an E. coli metabolic engineering route has proven to be successful, while also converting the remaining carbon backbones to fuels (e.g. fusel alcohols such as n-butanol and iso-butanol) and chemicals.240,241 This process allows ammonia to be recycled as a fertilizer and, in the case of algae, recycled to the cultivation system. When applied to algal biomass or algal protein-enriched residues, this also allows for the harvesting of fast-growing, protein-rich algae without the need for stress conditions to induce lipid production, along with slower growth. There are however, challenges with this approach, such as channeling the diverse set of amino acids to fewer products and redesigning the cell's nitrogen flux to favor deamination.12 A proof-of-concept Bacillus subtilis system was recently described that excretes proteases, consumes the released amino acids as the sole carbon and nitrogen sources and then converts these to higher alcohols and ammonia, albeit at single digit g L−1 titers.242 Most recently this approach has been demonstrated for the conversion of algal biomass-derived protein, with the successful production of a mixed-alcohol stream, at over 75% efficiency, with composition consistent with the originating amino acid composition.243 Alternative bioproducts are pursued based on a similar protein (and carbohydrate) fermentation pathway, where instead of fusel alcohols, the production of terpenes was targeted.39
7.3 Biomaterials and chemicals from proteins
Various sources of underutilized protein, including algae, have been considered for production of biomaterials and chemicals, such as bioplastics, foams, adhesives, biocomposites and flocculants.244,245 The bioplastic mechanical properties, cost and feedstock quality can be inferior to petroleum plastics requiring suitable plasticizers to modify the biopolymers.89,246 Edible plastic films can be produced from protein feedstocks.247 Most research on protein based plastics uses waste terrestrial feedstocks,248 and little current research has utilized algal proteins as a feedstock for biofilms. Recently, a process to produce polyurethanes using algal proteins was described and initially tested with glycine and then on whole algal protein hydrolyzate.249 Protein was fractionated from algae using flash chromatography then acid hydrolyzed to amino acids and small peptides.250 This peptide mixture was reacted with 1,2-diaminoethane to convert carboxylic acids to amides then reacted with ethylene carbonate to produce urethane polyols. As a proof of concept, up to 5% of the peptide polyol mixture was added to conventional polyols used to produce polyurethane foams. The performance analysis of algal protein infused foams compared favorably to conventional reference polyurethane foams.Similar reaction mechanisms have been described for the production of polyurethane foams from protein-enriched feedstocks, such as polyurethane foams from soybean meal and soy protein isolate plus alkaline-activated (to break disulfide bridges and denature the proteins) versions of the feedstocks.251 Up to 30% soybean meal was used to make foams and the activated feedstocks generally produced better performing foams. A new pathway to produce a novel hyperbranched polyester urethane from D,L-alanine, without the use of isocyanates was recently described.252 Protein extracted from Spirulina platensis and Chlamydomonas reinhardtii mixed with NaOH and various cross-linkers was used to produce adhesives that compared well with similar soy protein adhesives.253 Similarly, gluten and soy protein isolates have been used as binders for formaldehyde-free particleboards or oriented strand boards.254,255
In brief, there are multiple options to catalytically convert the protein fraction to high-value polymers. This is a new area and the dependence of the product properties on the amino acid composition of the feedstock is not yet identified. However, there are sustainability issues with conversion of amino acids, peptides, and proteins from algae into biopolymers, associated with permanent nitrogen nutrient sequestration, which then causes a much reduced level of nitrogen available for recycling back to the cultivation system ponds for growth media. In addition to the nutrient sequestration sustainability penalty, there are also sustainability benefits based on the sequestration of carbon fixed by photosynthesis into the bioplastics.
8 Conclusions
The concept of developing a biorefinery approach to maximize the value derived from algal biomass is placed in the context that is needed to address the pressing technical, economic and sustainability challenges for ultimate commercial realization of a bioeconomy. In this review, we have placed bioproducts in the context of a defined conversion pathway, based on a recently demonstrated fractionation approach, leaving lipids, solubilized carbohydrates and proteins accessible for respective bioproduct routes. This review aims to drive the narrative to a more realistic framework around algae bioenergy with a goal to support a transition in the discussions around algae to an intrinsic biomass value based on biomass composition for upgrading to a suite of fuel and product options, rather than a biomass-to-fuels only pathway which is likely to be challenged in achieving economic viability from algal biomass alone. We strived to place the biorefinery discussion in the context of the large-scale farms that are envisioned for bioenergy production from algae and thus create market opportunities commensurate with the volumes produced in a demonstrated and implemented fractionation pathway. For each of the products derived from algal biomass, a detailed discussion of the market opportunities is given, and placed in the context of the overall value per ton of biomass. In the respective pathway discussions, we focused on the chemistry and the application opportunities where the market size and value of some of the niche products was not available. The technoeconomic impact analysis of the biomass composition on the ultimate cost of the fuel products and with the addition of a thorough market analysis, this work provided a much-needed realistic perspective of algae as feedstocks for fuels and products. The coproduct components described here are discussed as options that are compatible with a demonstrated conversion fractionation process and are scalable to match volumes and market values envisioned to be produced on a farm in a conceptual integrated process. The highly complex nature of the separations and the multiple hypothetical coproduct options presented need to be prioritized as research routes to provide the maximum value for ongoing work. For each of the fractions we highlighted a subset of products and pathways to demonstrate the valorization approaches discussed in this report.Acknowledgements
We are grateful for the valuable input on the material reported here from the following people: Dr Robert McCormick, Dr Mary Biddy, Oliver Palardy, Dr Brenna Black (all NREL), Thomas Garrison, and Michael Kessler (Washington State University). Dr John McGowen, Arizona State University, provided the biomass used for the compositional data presented in Fig. 1 and Table 1. This work was supported by the U.S. Department of Energy under Contract No. DE-AC36-08-GO28308 with the National Renewable Energy Laboratory as part of the BioEnergy Technologies Office (BETO). The U.S. Government retains and the publisher, by accepting the article for publication, acknowledges that the U.S. Government retains a nonexclusive, paid up, irrevocable, worldwide license to publish or reproduce the published form of this work, or allow others to do so, for U.S. Government purposes.References
- R. Davis, J. Markham, C. M. Kinchin, N. Grundl, E. C. D. Tan and D. Humbird, Process Design and Economics for the Production of Algal Biomass: Algal Biomass Production in Open Pond Systems and Processing Through Dewatering for Downstream Conversion, Golden, CO, 2016, http://www.nrel.gov/docs/fy16osti/64772.pdf.
- J. Ruiz Gonzalez, G. Olivieri, J. de Vree, R. Bosma, P. Willems, H. Reith, M. Eppink, D. M. M. Kleinegris, R. H. Wijffels and M. Barbosa, Energy Environ. Sci., 2016, 9, 3036–3043 Search PubMed.
- R. Davis, C. M. Kinchin, J. Markham, E. C. D. Tan, L. M. L. Laurens, D. Sexton, D. Knorr, P. Schoen and J. Lukas, Process Design and Economics for the Conversion of Algal Biomass to Biofuels: Algal Biomass Fractionation to Lipid- and Carbohydrate-Derived Fuel Products, 2014, http://www.nrel.gov/docs/fy14osti/62368.pdf.
- O. Pulz and W. Gross, Appl. Microbiol. Biotechnol., 2004, 65, 635–648 CrossRef CAS PubMed.
- R. H. Wijffels, M. J. Barbosa and M. H. M. Eppink, Biofuels, Bioprod. Biorefin., 2010, 4, 287–295 CrossRef CAS.
- M. J. Biddy, C. J. Scarlata and C. M. Kinchin, Chemicals from biomass: A market assessment of bioproducts with near-term potential (FY14 Q4), Golden, CO, 2014, http://www.nrel.gov/docs/fy16osti/65509.pdf.
- B. E. Dale, M. S. Allen, M. Laser and L. R. Lynd, Biofuels, Bioprod. Biorefin., 2009, 3, 219–230 CrossRef CAS.
- P. M. Foley, E. S. Beach and J. B. Zimmerman, Green Chem., 2011, 13, 1399 RSC.
- IEA Bioenergy, State of Technology Review – Algae Bioenergy, ISBN: 978-1-910154-30-4, http://www.ieabioenergy.com/wp-content/uploads/2017/01/IEA-Bioenergy-Algae-report-update-20170114.pdf, 2017.
- V. H. Smith, Ind. Biotechnol., 2014, 10, 159–161 CrossRef.
- A. A. Koutinas, R. Wang and C. Webb, Biofuels, Bioprod. Biorefin., 2007, 1, 24–38 CrossRef CAS.
- D. G. Wernick and J. C. Liao, Appl. Microbiol. Biotechnol., 2013, 97, 1397–1406 CrossRef CAS PubMed.
- F. K. Davies, R. E. Jinkerson and M. C. Posewitz, Photosynth. Res., 2014, 123, 265–284 CrossRef PubMed.
- L. M. L. Laurens, N. J. Nagle, R. Davis, N. Sweeney, S. Van Wychen, A. Lowell and P. T. Pienkos, Green Chem., 2015, 17, 1145–1158 RSC.
- E. R. Venteris, R. C. McBride, A. M. Coleman, R. L. Skaggs and M. S. Wigmosta, Environ. Sci. Technol., 2014, 48, 3559–3566 CrossRef CAS PubMed.
- M. S. Wigmosta, A. M. Coleman, R. J. Skaggs, M. H. Huesemann and L. J. Lane, Water Resour. Res., 2011, 47, 1–13 CrossRef.
- E. R. Venteris, R. L. Skaggs, A. M. Coleman and M. S. Wigmosta, Environ. Sci. Technol., 2013, 47, 4840–4849 CrossRef CAS PubMed.
- L. M. L. Laurens, P. T. Pienkos, V. L. Harmon and J. McGowen, Algal Res., 2017 DOI:10.1016/j.algal.2017.03.029.
- J. McGowen, E. P. Knoshaug, L. M. L. Laurens, T. A. Dempster, P. T. Pienkos, E. Wolfrum and V. L. Harmon, Algal Res., 2017, 25, 168–177 CrossRef.
- L. M. L. Laurens, S. Van Wychen, J. P. McAllister, S. Arrowsmith, T. A. Dempster, J. McGowen and P. T. Pienkos, Anal. Biochem., 2014, 452, 86–95 CrossRef CAS PubMed.
- C. M. Gatenby, D. M. Orcutt, D. A. Kreeger, B. C. Parker, V. A. Jones and R. J. Neves, J. Appl. Phycol., 2003, 15, 1–11 CrossRef CAS.
- P. Biller and A. B. Ross, Bioresour. Technol., 2011, 102, 215–225 CrossRef CAS PubMed.
- D. Humbird, R. Davis, L. Tao, C. Kinchin, D. Hsu and A. Aden, Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol, Golden, CO, 2011, http://www.nrel.gov/docs/fy11osti/47764.pdf.
- T. Dong, S. Van Wychen, N. Nagle, P. T. Pienkos and L. M. L. Laurens, Algal Res., 2016, 18, 69–77 CrossRef.
- T. Dong, E. P. Knoshaug, R. Davis, L. M. L. Laurens, S. Van Wychen, P. T. Pienkos and N. Nagle, Algal Res., 2015, 19, 316–323 CrossRef.
- D. L. Sills, V. Paramita, M. J. Franke, M. C. Johnson, T. M. Akabas, C. H. Greene and J. W. Tester, Environ. Sci. Technol., 2013, 47, 687–694 CrossRef CAS PubMed.
- J. W. Richardson, M. D. Johnson, X. Zhang, P. Zemke, W. Chen and Q. Hu, Algal Res., 2014, 4, 96–104 CrossRef.
- L. N. Gerber, J. W. Tester, C. M. Beal, M. E. Huntley and D. L. Sills, Environ. Sci. Technol., 2016, 50, 3333–3341 CrossRef CAS PubMed.
- O. K. Lee, A. L. Kim, D. H. Seong, C. G. Lee, Y. T. Jung, J. W. Lee and E. Y. Lee, Bioresour. Technol., 2013, 132, 197–201 CrossRef CAS PubMed.
- G. W. Roberts, B. S. M. Sturm, U. Hamdeh, G. E. Stanton, A. Rocha, T. L. Kinsella, M.-O. P. Fortier, S. Sazdar, M. S. Detamore and S. M. Stagg-Williams, Green Chem., 2015, 17, 2560–2569 RSC.
- U. Jena, N. Vaidyanathan, S. Chinnasamy and K. C. Das, Bioresour. Technol., 2011, 102, 3380–3387 CrossRef CAS PubMed.
- D. López Barreiro, M. Bauer, U. Hornung, C. Posten, A. Kruse and W. Prins, Algal Res., 2015, 9, 99–106 CrossRef.
- Z. Liu and F. S. Zhang, J. Hazard. Mater., 2009, 167, 933–939 CrossRef CAS PubMed.
- D. Mohan, R. Sharma, V. K. Singh, P. Steele and C. U. Pittman, Ind. Eng. Chem. Res., 2012, 51, 900–914 CrossRef CAS.
- N. Borchard, A. Wolf, V. Laabs, R. Aeckersberg, H. W. Scherer, A. Moeller and W. Amelung, Soil Use Manage., 2012, 28, 177–184 CrossRef.
- T. Tsuchida, T. Yoshioka, S. Sakuma, T. Takeguchi and W. Ueda, Ind. Eng. Chem. Res., 2008, 47, 1443–1452 CrossRef CAS.
- B. Yan, L. Z. Tao, Y. Liang and B. Q. Xu, ACS Catal., 2014, 4, 1931–1943 CrossRef CAS.
- R. Davis, A. Aden and P. T. Pienkos, Appl. Energy, 2011, 88, 3524–3531 CrossRef.
- W. Wu and R. W. Davis, Algal Res., 2016, 17, 316–320 CrossRef.
- U. S. Department of Energy, Bioenergy Technologies Office Multi-Year Program Plan, Washington, D.C., 2016, http://energy.gov/sites/prod/files/2016/03/f30/mypp_beto_march2016_2.pdf.
- U. S. Department of Energy, National Algal Biofuels Technology Review, 2016, http://energy.gov/sites/prod/files/2016/06/f33/national_algal_biofuels_technology_review.pdf.
- V. K. Singh, P. Yadav and N. Tadigoppula, Chem. Biol. Interface, 2014, 4, 666–699 Search PubMed.
- X. Luo, P. Su and W. Zhang, Mar. Drugs, 2015, 13, 4231–4254 CrossRef CAS PubMed.
- U. S. Energy Information Administration, US Product Supplied for Crude Oil and Petroleum Products, Petroleum & Other Liquids, 2016.
- International Energy Agency, World Energy Outlook 2014, 2014, http://https://www.iea.org/publications/freepublications/publication/WEO2014.pdf.
- J. A. Glaser, Clean Technol. Environ. Policy, 2007, 9, 249–252 CrossRef.
- H. P. Benecke, B. R. Vijayendran, D. B. Garbark and K. P. Mitchell, Clean: Soil, Air, Water, 2008, 36, 694–699 CrossRef CAS.
- United Soybean Board (2010), http://soynewuses.org, A survey of Recent Chemical Price Trends: The potential impact of rising petrochemical prices on soy use for industrial applications, http://www.compositesworld.com/columns/harvest-season-ahead-for-soy-resins, accessed March 2017.
- United Soybean Board (2012), http://soynewuses.org, Market Opportunity Summary: Soy-based Thermoset Plastics, http://soynewuses.org/wp-content/uploads/44422_MOS_Plastics.pdf, accessed March 2017.
- M. Szycher, Szycher's Handbook of Polyurethanes, 2nd edn, 2013 Search PubMed.
- M. F. Sonnenschein, Polyurethanes: science, technology, markets, and trends, Wiley, 2014 Search PubMed.
- Y. Li, Biofuels, 2011, 2, 357–359 CrossRef CAS.
- American Chemistry Council, The Economic Benefits of the U.S. Polyurethanes Industry, 2015.
- L. Shen, J. Haufe and M. K. Patel, Product Overview and Market Projection of Emerging Bio-based Plastics, 2009, http://plasticker.de/news/docs/PROBIP2009_Final_June_2009.pdf.
- M. A. Borowitzka, J. Appl. Phycol., 2013, 25, 743–756 CrossRef CAS.
- Algae Industry Magazine, Omega-3 Ingredients Market Est. $3.79 billion by 2022.
- M. V. Hegde, A. A. Zanwar and S. P. Adekar, Fatty Acids: Keys to Nutritional Health, Springer International Publishing, 2016 Search PubMed.
- D. Rust and S. Wildes, Surfactants; a market opportunity study update, 2008 Search PubMed.
- United Soybean Board, http://soynewuses.org, Market Opportunity Summary: Soy-based Surfactants, 2012.
- P. York, U. B. Kompella and B. Y. Sherkunov, Supercritical Fluid Technology for Drug Product Development, CRC Press, Taylor & Francis LLC, 2004 Search PubMed.
- C. A. G. Quispe, C. J. R. Coronado and J. A. Carvalho, Renewable Sustainable Energy Rev., 2013, 27, 475–493 CrossRef CAS.
- M. J. Biddy, R. Davis, D. Humbird, L. Tao, N. Dowe, M. T. Guarnieri, J. G. Linger, E. M. Karp, D. Salvachúa, D. R. Vardon and G. T. Beckham, ACS Sustainable Chem. Eng., 2016, 4, 3196–3211 CrossRef CAS.
- A. Fradet and M. Tessier, in Synthetic Methods in Step-Growth Polymers, ed. T. E. Rogers and M. E. Long, John Wiley & Sons, Inc., Hoboken, NJ, 2003 Search PubMed.
- Smithers Pira, The Future of Bioplastics for Packaging to 2020, 2015.
- R. Tudor and M. Ashley, Platinum Met. Rev., 2007, 51, 116 CrossRef CAS.
- B. G. Harvey, H. A. Meylemans and R. L. Quintana, Green Chem., 2012, 14, 2450–2456 RSC.
- ICIS Chemical Business, US chemical Profile: 2-EH, 2013.
- K. H. M. Cardozo, T. Guaratini, M. P. Barros, V. R. Falcão, A. P. Tonon, N. P. Lopes, S. Campos, M. A. Torres, A. O. Souza, P. Colepicolo and E. Pinto, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2007, 146, 60–78 CrossRef PubMed.
- K. Samarakoon and Y.-J. Jeon, Food Res. Int., 2012, 48, 948–960 CrossRef CAS.
- P. Spolaore, C. Joannis-Cassan, E. Duran and A. Isambert, J. Biosci. Bioeng., 2006, 101, 87–96 CrossRef CAS PubMed.
- E. M. Torres, D. Hess, B. T. McNeil, T. Guy and J. C. Quinn, Ecotoxicol. Environ. Saf., 2017, 139, 367–376 CrossRef CAS PubMed.
- K. Napan, L. Teng, J. C. Quinn and B. D. Wood, Algal Res., 2015, 8, 83–88 CrossRef.
- K. Napan, D. Hess, B. McNeil and J. C. Quinn, J. Visualized Exp., 2015, e52936 Search PubMed.
- Alltech, 2015 Global Feed Survey, 2015, http://www.alltech.com/sites/default/files/global-feed-survey-2015.pdf.
- M. Sprague, J. R. Dick and D. R. Tocher, Sci. Rep., 2016, 6, 21892 CrossRef CAS PubMed.
- A. Muller-Feuga, Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2013, pp. 613–627 Search PubMed.
- K. K. Lum, J. Kim and X. G. Lei, J. Anim. Sci. Biotechnol., 2013, 4, 53 CrossRef PubMed.
- S. L. Lodge-Ivey, L. N. Tracey and A. Salazar, J. Anim. Sci., 2014, 92, 1331–1342 CrossRef CAS PubMed.
- R. S. Stokes, M. L. Van Emon, D. D. Loy and S. L. Hansen, J. Anim. Sci., 2015, 93, 5386 CrossRef CAS PubMed.
- C. Greene, M. E. Huntley, I. Archibald, L. N. Gerber, D. L. Sills, J. Granados, J. W. Tester, C. M. Beal, M. J. Walsh, R. R. Bidigare, S. L. Brown, W. P. Cochlan, Z. I. Johnson, X. G. Lei, S. C. Machesky, D. G. Redalje, R. E. Richardon, V. Kiron and V. Corless, Oceanography, 2016, 29, 10–15 Search PubMed.
- E. W. Becker, Biotechnol. Adv., 2007, 25, 207–210 CrossRef CAS PubMed.
- R. S. Stokes, M. L. Van Emon, D. D. Loy and S. L. Hansen, J. Anim. Sci., 2014, 93, 5386 CrossRef PubMed.
- P. G. Peiretti and G. Meineri, Livest. Sci., 2008, 118, 173–177 CrossRef.
- D. Patterson and D. M. Gatlin III, Aquaculture, 2013, 416, 92–98 CrossRef.
- R. A. Efroymson and V. H. Dale, Ecol. Indic., 2015, 49, 1–13 CrossRef.
- R. A. Efroymson, V. H. Dale and M. H. Langholtz, GCB Bioenergy, 2016, 1673, 1–19 Search PubMed.
- M. J. Walsh, L. Gerber Van Doren, D. L. Sills, I. Archibald, C. M. Beal, X. G. Lei, M. E. Huntley, Z. Johnson and C. H. Greene, Environ. Res. Lett., 2016, 11, 114006 CrossRef.
- A. I. Orr, W. J. Burkholder and S. A. Benz, Algae Biomass Summit, 2010.
- T. Mekonnen, P. Mussone, H. Khalil and D. Bressler, J. Mater. Chem. A, 2013, 1, 13379 CAS.
- M. M. Reddy, S. Vivekanandhan, M. Misra, S. K. Bhatia and A. K. Mohanty, Prog. Polym. Sci., 2013, 38, 1653–1689 CrossRef CAS.
- F. Zhang, T. Endo, R. Kitagawa, H. Kabeya and T. Hirotsu, J. Mater. Chem., 2000, 10, 2666–2672 RSC.
- F. Zhang, H. Kabeya and R. Kitagawa, J. Mater. Sci., 2000, 5, 2603–2609 CrossRef.
- M. A. Zeller, R. Hunt, A. Jones and S. Sharma, J. Appl. Polym. Sci., 2013, 130, 3263–3275 CrossRef CAS.
- T. Otsuki and F. Zhang, J. Appl. Polym. Sci., 2004, 92, 812–816 CrossRef CAS.
- T. E. Kipngetich and M. Hillary, Int. J. Green Herb. Chem., 2013, 2, 15–19 Search PubMed.
- B. Shi, G. Wideman and J. H. Wang, J. Polym. Environ., 2011, 20, 124–131 CrossRef.
- S. Balaji, K. Gopi and B. Muthuvelan, Algal Res., 2013, 2, 278–285 CrossRef.
- S. M. Haase, B. Huchzermeyer and T. Rath, J. Appl. Phycol., 2011, 24, 157–162 CrossRef.
- F. Hempel, A. S. Bozarth, N. Lindenkamp, A. Klingl, S. Zauner, U. Linne, A. Steinbüchel and U. G. Maier, Microb. Cell Fact., 2011, 10, 81 CrossRef CAS PubMed.
- W. Chaogang, H. Zhangli, L. Anping and J. Baohui, J. Phycol., 2010, 46, 396–402 CrossRef.
- D. Simionato, M. A. Block, N. La Rocca, J. Jouhet, E. Maréchal, G. Finazzi and T. Morosinotto, Eukaryotic Cell, 2013, 12, 665–676 CrossRef CAS PubMed.
- J. Jia, D. Han, H. G. Gerken, Y. Li, M. Sommerfeld, Q. Hu and J. Xu, Algal Res., 2015, 7, 66–77 CrossRef.
- F. O. Holguin and T. Schaub, Algal Res., 2013, 2, 43–50 CrossRef.
- A. A. Koutinas, A. Chatzifragkou, N. Kopsahelis, S. Papanikolaou and I. K. Kookos, Fuel, 2014, 116, 566–577 CrossRef CAS.
- S. M. Mudge, Fatty Alcohols – a review of their natural synthesis and environmental distribution, Bangor, UK, 2005, http://www.aciscience.org/docs/Fatty_Alcohols_Mudge_2005.pdf, accessed March 2017.
- X. Yu, T. Dong, Y. Zheng, C. Miao and S. Chen, Eur. J. Lipid Sci. Technol., 2014, 117, 730–737 CrossRef.
- T. Dong, E. P. Knoshaug, P. T. Pienkos and L. M. L. Laurens, Appl. Energy, 2016, 177, 879–895 CrossRef CAS.
- B. P. Nobre, F. Villalobos, B. E. Barragán, A. C. Oliveira, A. P. Batista, P. A. S. S. Marques, R. L. Mendes, H. Sovová, A. F. Palavra and L. Gouveia, Bioresour. Technol., 2013, 135, 128–136 CrossRef CAS PubMed.
- G. A. Thompson, Biochim. Biophys. Acta, 1996, 1302, 17–45 CrossRef.
- J. Liu, J. Huang, Z. Sun, Y. Zhong, Y. Jiang and F. Chen, Bioresour. Technol., 2011, 102, 106–110 CrossRef CAS PubMed.
- H. He, R. P. Rodgers, A. G. Marshall and C. S. Hsu, Energy Fuels, 2011, 25, 4770–4775 CrossRef CAS.
- G. W. Patterson, in Physiology and Biochemistry of Sterols, ed. G. W. Patterson and W. D. Nes, American Oil Chemists' Society, Champaign, IL, 1991, pp. 118–157 Search PubMed.
- Y. Lu, W. Zhou, L. Wei, J. Li, J. Jia, F. Li, S. Smith and J. Xu, Biotechnol. Biofuels, 2014, 7, 81 CrossRef PubMed.
- T. Rezanka and K. Sigler, Prog. Lipid Res., 2009, 48, 206–238 CrossRef CAS PubMed.
- D. J. Pyle, R. A. Garcia and Z. Wen, J. Agric. Food Chem., 2008, 56, 3933–3939 CrossRef CAS PubMed.
- M. Hoffmann, K. Marxen, R. Schulz and K. H. Vanselow, Mar. Drugs, 2010, 8, 2526–2545 CrossRef CAS PubMed.
- E. Belarbi, E. Molina and Y. Chisti, Enzyme Microb. Technol., 2000, 26, 516–529 CrossRef CAS PubMed.
- Z. Cohen, C. Bigogno and I. Khozin-Goldberg, Phytochemistry, 2002, 60, 135–143 CrossRef.
- G. Su, K. Jiao, Z. Li, X. Guo, J. Chang, T. Ndikubwimana, Y. Sun, X. Zeng, Y. Lu and L. Lin, Bioprocess Biosyst. Eng., 2016, 1–8 CAS.
- H. G. Tababa, S. Hirabayashi and K. Inubushi, J. Appl. Phycol., 2012, 24, 887–895 CrossRef CAS PubMed.
- G. Mahajan and M. Kamat, Appl. Microbiol. Biotechnol., 1995, 43, 466–469 CrossRef CAS.
- T. Dong, J. Wang, C. Miao, Y. Zheng and S. Chen, Bioresour. Technol., 2013, 136, 8–15 CrossRef CAS PubMed.
- A. B. Walker and D. L. Berlinsky, N. Am. J. Aquac., 2011, 73, 76–83 Search PubMed.
- Q. Hu, M. Sommerfeld, E. Jarvis, M. Ghirardi, M. Posewitz, M. Seibert and A. Darzins, Plant J., 2008, 54, 621–639 CrossRef CAS PubMed.
- E. M. Grima, A. G. Giménez and A. R. Medina, Chemicals from Microalgae, CRC Press, 1999 Search PubMed.
- R. Davis, D. Fishman, E. D. E. Frank and M. S. M. Wigmosta, Renewable Diesel from Algal Lipids: An Integrated Baseline for Cost, Emissions, and Resource Potential from a Harmonized Model, Golden, CO, 2012, http://www.nrel.gov/docs/fy12osti/55431.pdf.
- T. G. Smagala, E. Christensen, K. M. Christison, R. E. Mohler, E. Gjersing and R. L. McCormick, Energy Fuels, 2013, 27, 237–246 CrossRef CAS.
- A. Lewinska, J. Zebrowski, M. Duda, A. Gorka and M. Wnuk, Molecules, 2015, 20, 22872–22880 CrossRef CAS PubMed.
- E. G. Hammond, L. A. Johnson, C. Su, T. Wang and P. White, in Bailey's Industrial Oil and Fat Products, ed. F. Shahidi, John Wiley & Sons, Inc., 6th edn, 2005 Search PubMed.
- U. Schörken and P. Kempers, Eur. J. Lipid Sci. Technol., 2009, 111, 627–645 CrossRef.
- A. Behr and J. P. Gomes, Eur. J. Lipid Sci. Technol., 2010, 112, 31–50 CrossRef CAS.
- N. Burris, J. Am. Oil Chem. Soc., 1983, 60, 806–811 CrossRef CAS.
- C. N. Mulligan, Environ. Pollut., 2005, 133, 183–198 CrossRef CAS PubMed.
- I. Johansson and M. Svensson, Curr. Opin. Colloid Interface Sci., 2001, 6, 178–188 CrossRef CAS.
- K. Holmberg, Curr. Opin. Colloid Interface Sci., 2001, 6, 148–159 CrossRef CAS.
- B. M. Folmer, Adv. Colloid Interface Sci., 2003, 103, 99–119 CrossRef CAS PubMed.
- P. Weschayanwiwat, J. F. Scamehorn and P. J. Reilly, J. Surfactants Deterg., 2005, 8, 65–72 CrossRef CAS.
- Z. C. Zhang, M. Dery, S. Zhang and D. Steichen, J. Surfactants Deterg., 2004, 7, 211–215 CrossRef CAS.
- M. Hato, H. Minamikawa and T. Kato, in Sugar-based Surfactants: Fundamentals and Applications, ed. C. Carnero Ruiz, CRC Press, Boca Raton, FL, 2008, p. 664 Search PubMed.
- R. Hashim, Liq. Cryst., 2012, 39, 1–17 CrossRef CAS.
- S. A. Urbin, J. J. Scheibel, P. K. Vinson, P. K. Depa, R. T. Reilman and M. P. Steffy USPTO 20140148375 A1, 2014.
- Z. S. Petrovic, X. Wan, O. Bilic, A. Zlataníc, J. Hong, I. Javni, M. Ionescu, J. Milic and D. Degruson, J. Am. Oil Chem. Soc., 2013, 90, 1073–1078 CrossRef CAS.
- S. Dinda, A. V. Patwardhan, V. V. Goud and N. C. Pradhan, Bioresour. Technol., 2008, 99, 3737–3744 CrossRef CAS PubMed.
- V. V. Goud, N. C. Pradhan and A. V. Patwardhan, J. Am. Oil Chem. Soc., 2006, 83, 635–640 CrossRef CAS.
- A. E. Gerbase, J. R. Gregório, M. Martinelli, M. C. Brasil and A. N. F. Mendes, J. Am. Oil Chem. Soc., 2002, 79, 179–181 CrossRef CAS.
- P. Lathi and B. Mattiasson, Appl. Catal., B, 2007, 69, 207–212 CrossRef CAS.
- R. A. Holser, Ind. Crops Prod., 2008, 27, 130–132 CrossRef CAS.
- K. D. Carlson and S. P. Chang, J. Am. Oil Chem. Soc., 1985, 62, 934–939 CrossRef CAS.
- X. Hang and H. Yang, J. Am. Oil Chem. Soc., 1999, 76, 89–92 CrossRef CAS.
- S. G. Tan and W. S. Chow, Polym.-Plast. Technol. Eng., 2010, 49, 1581–1590 CrossRef CAS.
- T. F. Garrison, M. R. Kessler and R. C. Larock, Polymer, 2014, 55, 1004–1011 CrossRef CAS.
- Y. Xia, Z. Zhang, M. R. Kessler, B. Brehm-Stecher and R. C. Larock, ChemSusChem, 2012, 5, 2221–2227 CrossRef CAS PubMed.
- T. F. Garrison, Z. Zhang, H.-J. Kim, D. Mitra, Y. Xia, D. P. Pfister, B. Brehm-Stecher, R. C. Larock and M. R. Kessler, Macromol. Mater. Eng., 2014, 299, 1042 CAS.
- Oil and Gas Product News, World market for fuel additives, 2013.
- Chevron Company, Motor Gasolines Technical Review, 1996, FTR-1, pp. 1–69.
- Chevron Company, Diesel Fuels Technical Review, 1998, FTR-2, pp. 1–70.
- P. Richards, Automotive Fuels Reference Book, Society of Automotive Engineers, Warrendale, PA, 3rd edn, 2014 Search PubMed.
- S. Q. A. Rizvi, ASTM Fuels and Lubricants Handbook, ASTM International, West Conshohocken, PA, 2003 Search PubMed.
- CRC Report No. 667 Diesel Fuel Storage and Handling, September 2014.
- B. Bai, J. Zhou, M. Yang, Y. Liu, X. Xu and J. Xing, Bioresour. Technol., 2015, 185, 56–61 CrossRef CAS PubMed.
- B. H. Garth and F. H. Schmidt, USPTO 4214876A, 1980.
- 40 CFR Parts 79 and 80 Regulation of Fuels and Fuel Additives, Federal Register: November 18, 1996, vol. 61(223).
- ASTM, D975-14a Standard Specification for Diesel Fuel Oils, West Conshohocken, PA, 2014.
- C. P. Henry Jr, USPTO 3917466 A, 1975.
- G. J. Suppes, M. Goff, M. L. Burkhart, K. Bockwinkel, M. H. Mason, J. B. Botts and J. A. Heppert, Energy Fuels, 2001, 15, 151–157 CrossRef CAS.
- M. Morales, P. Y. Dapsens, I. Giovinazzo, J. Witte, C. Mondelli, S. Papadokonstantakis, K. Hungerb and J. Pérez-ramírez, Energy Environ. Sci., 2015, 8, 558–567 CAS.
- The Ohio State University, Turning Crude Glycerin into Polyurethane Foam and Biopolyols, http://ohioline.osu.edu/factsheet/AEX-654-11, Ohioline, 2011.
- L. Balduyck, S. Bijttebier, C. Bruneel, G. Jacobs, S. Voorspoels, J. Van Durme, K. Muylaert and I. Foubert, Algal Res., 2016, 18, 281–287 CrossRef.
- A. E. F. Abomohra, W. Jin and M. El-Sheekh, Energy Convers. Manage., 2016, 108, 23–29 CrossRef CAS.
- R. D. Cortright, R. R. Davda and J. A. Dumesic, Nature, 2002, 418, 964 CrossRef CAS PubMed.
- G. Suppes, W. Sutterlin and M. Dasari, USPTO 7943805 B2, 2011.
- L. Craciun, G. P. Benn, J. Dewing, G. W. Schriver, W. J. Peer, B. Siebenhaar and U. Siegrist, USPTO 0113822 A1, 2010.
- Y. Lu and R. C. Larock, ACS Symposium Series, 2010, vol. 1043, pp. 87–102 Search PubMed.
- L. Ott, M. Bicker and H. Vogel, Green Chem., 2006, 8, 214–220 RSC.
- U.S. Department of Energy, Top Value Added Chemical from Biomass: Volume I – Results of Screening for Potential Candidates from Sugars and Synthesis Gas, Washington D.C, 2004.
- D. Templeton, M. Quinn, S. Van Wychen, D. Hyman and L. M. L. Laurens, J. Chromatogr. A, 2012, 1270, 225–234 CrossRef CAS PubMed.
- E. G. V. Percival and A. G. Ross, J. Chem. Soc., 1951, 720–726 RSC.
- S. M. Read, G. Currie and A. Bacic, Carbohydr. Res., 1996, 281, 187–201 CrossRef CAS PubMed.
- Z. A. Popper, G. Michel, C. Hervé, D. S. Domozych, W. G. T. Willats, M. G. Tuohy, B. Kloareg and D. B. Stengel, Annu. Rev. Plant Biol., 2011, 62, 567–590 CrossRef CAS PubMed.
- S. Pieper, I. Unterieser, F. Mann and P. Mischnick, Carbohydr. Res., 2012, 352, 166–176 CrossRef CAS PubMed.
- H. Lechat, M. Amat, J. Mazoyer, A. Buléon and M. Lahaye, J. Appl. Phycol., 2000, 902, 891–902 CrossRef.
- M. Bomgardner, Chem. Eng. News, 2014, 92, 10–14 Search PubMed.
- Y. Wu, Y. Enomoto-Rogers, H. Masaki and T. Iwata, ACS Sustainable Chem. Eng., 2016, 4, 3812–3819 CrossRef CAS.
- A. Abo-Shady, Y. Mohamed and T. Lasheen, Biol. Plant., 1993, 35, 629–632 CrossRef CAS.
- R. K. Saxena, P. Anand and S. Saran, Indian J. Microbiol., 2010, 50, 2–11 CrossRef CAS PubMed.
- H. Rasmussen, K. H. Mogensen, M. D. Jeppesen, H. R. Sørensen and A. S. Meyer, Biomass Bioenergy, 2016, 93, 209–216 CrossRef CAS.
- Y. Román-Leshkov, C. J. Barrett, Z. Y. Liu and J. A. Dumesic, Nature, 2007, 447, 982–985 CrossRef PubMed.
- A. Abbadi, K. F. Gotlieb and J. B. M. Meiberg, Green Chem., 1999, 1, 231–235 RSC.
- F. van der Klis, A. E. Frissen and J. van Haveren, ChemSusChem, 2013, 6, 1640–1645 CrossRef CAS PubMed.
- M. Malveda, A. Bland, Y. Kazuteru and X. Ma, Chemical Economics Handbook. Sorbitol (693.1000), Englewood, CO, 2014 Search PubMed.
- D. Cao, B. Yu, S. Zhang, L. Cui, J. Zhang and W. Cai, Appl. Catal., A, 2016, 528, 59–66 CrossRef CAS.
- J. Zhang, L. Wang, F. Liu, X. Meng, J. Mao and F. S. Xiao, Catal. Today, 2015, 242, 249–254 CrossRef CAS.
- J. C. Bersot, N. Jacquel, R. Saint-Loup, P. Fuertes, A. Rousseau, J. P. Pascault, R. Spitz, F. Fenouillot and V. Monteil, Macromol. Chem. Phys., 2011, 212, 2114–2120 CrossRef CAS.
- B. Ray, Carbohydr. Polym., 2006, 66, 408–416 CrossRef CAS.
- D. S. Domozych, Int. J. Plant Sci., 2007, 168, 763–774 CrossRef CAS.
- D. S. Domozych, K. D. Stewart and K. R. Mattox, J. Mol. Evol., 1980, 15, 1–12 CrossRef CAS PubMed.
- M. Pivokonsky, J. Naceradska, I. Kopecka, M. Baresova, B. Jefferson, X. Li and R. K. Henderson, Crit. Rev. Environ. Sci. Technol., 2016, 46, 291–335 CrossRef CAS.
- L. Avérous, J. Macromol. Sci., Polym. Rev., 2004, 44, 231–274 CrossRef.
- Y. Zhang, X. Yuan, Q. Liu and A. Hrymak, J. Polym. Environ., 2012, 20, 315–325 CrossRef CAS.
- L. T. Sin, A. R. Rahmat and W. A. W. A. Rahman, Polylactic Acid, 2007, vol. 52 Search PubMed.
- Y. F. Zuo, J. Gu, Z. Qiao, H. Tan, J. Cao and Y. Zhang, Int. J. Biol. Macromol., 2015, 72, 391–402 CrossRef CAS PubMed.
- D. Klemm, B. Heublein, H. P. Fink and A. Bohn, Angew. Chem., Int. Ed., 2005, 44, 3358–3393 CrossRef CAS PubMed.
- S. Fischer, K. Thümmler, B. Volkert, K. Hettrich, I. Schmidt and K. Fischer, Macromol. Symp., 2008, vol. 262, pp. 89–96 Search PubMed.
- A. Biswas, B. C. Saha, J. W. Lawton, R. L. Shogren and J. L. Willett, Carbohydr. Polym., 2006, 64, 134–137 CrossRef CAS.
- K. J. Edgar, C. M. Buchanan, J. S. Debenham, P. A. Rundquist, B. D. Seiler, M. C. Shelton and D. Tindall, Prog. Polym. Sci., 2001, 26, 1605–1688 CrossRef CAS.
- H. N. Cheng, M. K. Dowd, G. W. Selling and A. Biswas, Carbohydr. Polym., 2010, 80, 450–453 CrossRef.
- K. A. Lunardello, F. Yamashita, M. De Toledo Benassi and C. M. V. B. De Rensis, Int. J. Dairy Technol., 2012, 65, 260–267 CrossRef CAS.
- Y. Doleyres, L. Schaub and C. Lacroix, J. Dairy Sci., 2005, 88, 4146–4156 CrossRef CAS PubMed.
- K. Coughlan, N. B. Shaw, J. F. Kerry and J. P. Kerry, J. Food Sci., 2004, 69, 271–276 CrossRef.
- L. S. Zárate-Ramírez, A. Romero, C. Bengoechea, P. Partal and A. Guerrero, Carbohydr. Polym., 2014, 112, 24–31 CrossRef PubMed.
- H. Zheng, Z. Tan, Y. R. Zhan and J. Huang, J. Appl. Polym. Sci., 2003, 90, 3676–3682 CrossRef CAS.
- B. G. Subhadra and M. Edwards, Appl. Energy, 2011, 88, 3515–3523 CrossRef.
- K. Soratana, V. Khanna and A. E. Landis, Appl. Energy, 2013, 112, 194–204 CrossRef CAS.
- K. Soratana, W. J. Barr and A. E. Lan dis, Bioresour. Technol., 2014, 159, 157–166 CrossRef CAS PubMed.
- J. M. Romero García, F. G. Acién Fernández and J. M. Fernández Sevilla, Bioresour. Technol., 2012, 112, 164–170 CrossRef PubMed.
- L. Mišurcová, F. Buňka, J. Vávra Ambrožová, L. Machů, D. Samek and S. Kráčmar, Food Chem., 2014, 151, 120–125 CrossRef PubMed.
- I. S. Chronakis, Novel Macromolecules in Food Systems, Elsevier, 2000, vol. 41 Search PubMed.
- B. J. Kelly and M. T. Brown, J. Appl. Phycol., 2000, 12, 317–324 CrossRef.
- B. Subhadra, J. Sci. Food Agric., 2011, 91, 2–13 CrossRef CAS PubMed.
- C. Shi, S. Dong, F. Wang, Q. Gao and X. Tian, Aquaculture, 2013, 416, 296–301 CrossRef.
- A. J. Vizcaino, G. Lopez, M. I. Saez, J. A. Jimenez, A. Barros, L. Hidalgo, J. Camacho-Rodriguez, T. F. Martinez, M. C. Ceron-Garcia and F. J. Alarcon, Aquaculture, 2014, 431, 34–43 CrossRef CAS.
- S. S. Fernandez, A. P. Padilla and S. Mucciarelli, Plant Foods Hum. Nutr., 1999, 54, 251–259 CrossRef CAS PubMed.
- S. Chiesa and E. Gnansounou, Bioresour. Technol., 2011, 102, 427–436 CrossRef CAS PubMed.
- D. Samek, L. Mišurcova, L. Machu, F. Bunka and M. Fišera, Turk. J. Biochem., 2013, 38, 360–368 CrossRef.
- D. W. Templeton and L. M. L. Laurens, Algal Res., 2015, 11, 359–367 CrossRef.
- H. L. Bryant, I. Gogichaishvili, D. Anderson, J. W. Richardson, J. Sawyer, T. Wickersham and M. L. Drewery, Algal Res., 2012, 1, 185–193 CrossRef.
- M. L. Drewery, J. E. Sawyer, W. E. Pinchak and T. A. Wickersham, J. Anim. Sci., 2014, 92, 4642–4649 CrossRef CAS PubMed.
- J. C. McCann, M. L. Drewery, J. E. Sawyer, W. E. Pinchak and T. A. Wickersham, J. Anim. Sci., 2014, 92, 5063–5075 CrossRef CAS PubMed.
- A. Sathish, K. Glaittli, R. C. Sims and C. D. Miller, J. Polym. Environ., 2014, 22, 272–277 CrossRef CAS.
- M.-T. Gao, T. Shimamura, N. Ishida and H. Takahashi, J. Biosci. Bioeng., 2012, 114, 330–333 CrossRef CAS PubMed.
- W. Kightlinger, K. Chen, A. Pourmir, D. W. Crunkleton, G. L. Price and T. W. Johannes, Electron. J. Biotechnol., 2014, 17, 14–18 CrossRef.
- N. Hempel, I. Petrick and F. Behrendt, J. Appl. Phycol., 2012, 24, 1407–1418 CrossRef CAS PubMed.
- C. Adams and B. Bugbee, J. Phycol., 2014, 50, 356–365 CrossRef CAS PubMed.
- E. Scott, F. Peter and J. Sanders, Appl. Microbiol. Biotechnol., 2007, 75, 751–762 CrossRef CAS PubMed.
- R. A. Sheldon, Green Chem., 2014, 16, 950 RSC.
- J. P. M. Sanders, J. H. Clark, G. J. Harmsen, H. J. Heeres, J. J. Heijnen, S. R. A. Kersten, W. P. M. van Swaaij and J. A. Moulijn, Chem. Eng. Process. Process Intensif., 2012, 51, 117–136 CrossRef CAS.
- T. M. Lammens, M. C. R. Franssen, E. L. Scott and J. P. M. Sanders, Biomass Bioenergy, 2012, 44, 168–181 CrossRef CAS.
- H. Mooibroek, N. Oosterhuis, M. Giuseppin, M. Toonen, H. Franssen, E. Scott, J. Sanders and A. Steinbüchel, Appl. Microbiol. Biotechnol., 2007, 77, 257–267 CrossRef CAS PubMed.
- Y.-X. Huo, D. G. Wernick and J. C. Liao, Curr. Opin. Biotechnol., 2012, 23, 406–413 CrossRef CAS PubMed.
- E. I. Lan and J. C. Liao, Bioresour. Technol., 2013, 135, 339–349 CrossRef CAS PubMed.
- Y.-X. Huo, K. M. Cho, J. G. L. Rivera, E. Monte, C. R. Shen, Y. Yan and J. C. Liao, Nat. Biotechnol., 2011, 29, 346–351 CrossRef CAS PubMed.
- K.-Y. Choi, D. G. Wernick, C. A. Tat and J. C. Liao, Metab. Eng., 2014, 23, 53–61 CrossRef CAS PubMed.
- W. Wu, M. B. Tran-Gyamfi, J. D. Jaryenneh and R. W. Davis, Algal Res., 2016, 19, 162–167 CrossRef.
- T. Mekonnen, P. Mussone and D. Bressler, Crit. Rev. Biotechnol., 2014, 1–12 Search PubMed.
- R. Kumar, D. Liu and L. Zhang, J. Biobased Mater. Bioenergy, 2008, 2, 1–24 CrossRef.
- C. J. R. Verbeek and L. E. van den Berg, Macromol. Mater. Eng., 2010, 295, 10–21 CrossRef CAS.
- V. M. Hernandez-Izquierdo and J. M. Krochta, J. Food Sci., 2008, 73, R30–R39 CrossRef CAS PubMed.
- W. Shi and M.-J. Dumont, J. Mater. Sci., 2013, 49, 1915–1930 CrossRef.
- S. Kumar, E. Hablot, J. L. G. Moscoso, W. Obeid, P. G. Hatcher, B. M. DuQuette, D. Graiver, R. Narayan and V. Balan, J. Mater. Sci., 2014, 49, 7824–7833 CrossRef CAS.
- J. L. Garcia-Moscoso, W. Obeid, S. Kumar and P. G. Hatcher, J. Supercrit. Fluids, 2013, 82, 183–190 CrossRef CAS.
- Y. Mu, X. Wan, Z. Han, Y. Peng and S. Zhong, J. Appl. Polym. Sci., 2012, 124, 4331–4338 CrossRef CAS.
- Y.-M. Bao, G.-R. Shen, J. He and Y.-S. Li, Green Chem., 2012, 14, 2243 RSC.
- J. J. Roy, L. Sun and L. Ji, J. Appl. Phycol., 2014, 26, 1415–1422 CrossRef CAS.
- S. Khosravi, F. Khabbaz, P. Nordqvist and M. Johansson, Ind. Crops Prod., 2010, 32, 275–283 CrossRef CAS.
- T. H. Mekonnen, P. G. Mussone, P. Choi and D. C. Bressler, Macromol. Mater. Eng., 2014, 299, 1003–1012 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |
