Luminescent cadmium(ii) coordination polymers of 1,2,4,5-tetrakis(4-pyridylvinyl)benzene used as efficient multi-responsive sensors for toxic metal ions in water†
Abstract
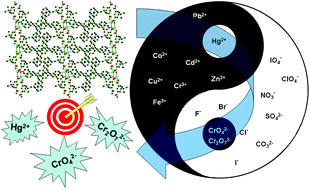
Reactions of Cd(NO3)2·4H2O with 1,2,4,5-tetrakis(4-pyridylvinyl)benzene (4-tkpvb) and 5-tert-butylisophthalic acid (5-tert-H2BIPA), 1,3,5-benzenetricarboxylic acid (1,3,5-H3BTC) or 1,4-naphthalenedicarboxylic acid (1,4-H2NDC) under solvothermal conditions afforded three two-dimensional (2D) Cd(II) coordination polymers [Cd(4-tkpvb)(5-tert-BIPA)]n (1), [{Cd(4-tkpvb)(1,3,5-HBTC)}·0.5DMF]n (2) and [Cd(4-tkpvb)(1,4-NDC)]n (3). Compounds 1–3 were structurally characterized by IR, elemental analysis, powder X-ray diffraction, and single crystal X-ray diffraction. Compounds 1–3 possess unique 2D networks in which 1D double chains [Cd2L2]n (L = 5-tert-BIPA or 1,3,5-HBTC) (1–2) or 1D linear chains [Cd(1,4-NDC)]n (3) are linked by 4-tkpvb ligands. Upon UV light excitation, a 4-tkpvb solution in DMF showed an emission band centered at 446 nm with a shoulder at 475 nm. The addition of Hg2+ ions into the 4-tkpvb solution in DMF remarkably changed its colour from colourless to yellow under natural light, or from blue to grey yellow under UV light, which were clearly visible to the naked eye. Compounds 1–3 suspended in water could emit yellow-green light under UV light irradiation. The representative compound 1 was confirmed to be an uncommon multi-responsive luminescent sensor for Hg2+, CrO42− and Cr2O72− ions in water by the luminescence quenching method. The detection limits for these species were 0.15 μM (Hg2+), 0.08 μM (CrO42−) and 0.12 μM (Cr2O72−), respectively. The luminescence quenching mechanism studies revealed that these quenching processes were involved in either the interaction of Hg2+ with free pyridyl groups in 1 or the overlap between the absorption band of CrO42− or Cr2O72− and the excitation and/or emission bands of 1.
- This article is part of the themed collection: Dalton Transactions Inorganic Symposia


 Please wait while we load your content...
Please wait while we load your content...