ScMO(BO3) (M = Ca and Cd): new Sc-based oxyborates featuring interesting edge-sharing sandwich-like chains and UV cut-off edges†
Abstract
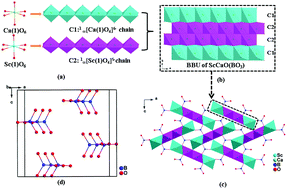
Two new isostructural rare-earth oxyborates ScMO(BO3) (M = Ca and Cd) with a three-dimensional (3D) cationic framework and parallel arranged [BO3] triangles have been synthesized by the flux method. In the 3D cationic framework, an interesting sandwich-like basic building unit (BBU) is constructed by two ∞1[Ca(1)O4]6− chains and two ∞1[Sc(1)O4]5− chains. ScMO(BO3) melt incongruently, which shows that title compounds can be grown by the flux method. The UV cut-off edges for ScCaO(BO3) and ScCdO(BO3) are 230 and 249 nm, respectively. In addition, the first-principles calculations are performed to gain further insights into the relationship between the microscopic electronic structures and associated optical properties.


 Please wait while we load your content...
Please wait while we load your content...