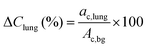Open Access Article
Open Access ArticleClinical evaluation of the radiolanthanide terbium-152: first-in-human PET/CT with 152Tb-DOTATOC
Richard P.
Baum†
 a,
Aviral
Singh†
*a,
Martina
Benešová
bc,
Christiaan
Vermeulen
a,
Aviral
Singh†
*a,
Martina
Benešová
bc,
Christiaan
Vermeulen
 b,
Silvano
Gnesin
b,
Silvano
Gnesin
 d,
Ulli
Köster
d,
Ulli
Köster
 e,
Karl
Johnston
f,
Dirk
Müller
a,
Stefan
Senftleben
a,
Harshad R.
Kulkarni
e,
Karl
Johnston
f,
Dirk
Müller
a,
Stefan
Senftleben
a,
Harshad R.
Kulkarni
 a,
Andreas
Türler
gh,
Roger
Schibli
a,
Andreas
Türler
gh,
Roger
Schibli
 bc,
John O.
Prior
bc,
John O.
Prior
 d,
Nicholas P.
van der Meulen
bg and
Cristina
Müller
d,
Nicholas P.
van der Meulen
bg and
Cristina
Müller
 *bc
*bc
aTHERANOSTICS Centre for Molecular Radiotherapy and Molecular Imaging, ENETS Center of Excellence, Zentralklinik Bad Berka, Germany. E-mail: aviral.singh@zentralklinik.de; Fax: +49 364 585-3515; Tel: +49 364 585-42226
bCenter for Radiopharmaceutical Sciences ETH-PSI-USZ, Paul Scherrer Institut, Villigen-PSI, Switzerland. E-mail: cristina.mueller@psi.ch; Fax: +41 56 310 28 43; Tel: +41 56 310 44 54
cDepartment of Chemistry and Applied Biosciences, ETH Zurich, Zurich, Switzerland
dDepartment of Nuclear Medicine and Molecular Imaging, Lausanne University Hospital, Lausanne, Switzerland
eInstitut Laue-Langevin, Grenoble, France
fISOLDE/CERN, Meyrin, Switzerland
gLaboratory of Radiochemistry, Paul Scherrer Institut, Villigen-PSI, Switzerland
hDepartment of Chemistry and Biochemistry, University of Bern, Bern, Switzerland
First published on 21st August 2017
Abstract
The existence of theragnostic pairs of radionuclides allows the preparation of radiopharmaceuticals for diagnostic and therapeutic purposes. Radiolanthanides, such as 177Lu, are successfully used for therapeutic purposes; however, a perfect diagnostic match is currently not available for clinical use. A unique, multi-disciplinary study was performed using 152Tb (T1/2 = 17.5 h, Eβ+average = 1140 keV, Iβ+ = 20.3%), which resulted in the first-in-human PET/CT images with this promising radionuclide. For this purpose, 152Tb was produced via a spallation process followed by mass separation at ISOLDE, CERN. The chemical separation and quality control, performed at PSI, resulted in a pure product in sufficient yields. Clinical PET phantom studies revealed an increased image noise level, because of the smaller β+ branching ratio of 152Tb as compared to standard PET nuclides at matched activity concentrations; however, the expected recovery would be comparable at matched signal-to-noise ratios in clinical PET. 152Tb was used for labeling DOTATOC, at Zentralklinik Bad Berka, and administered to a patient for a first-in-human clinical study. PET scans were performed over a period of 24 h, allowing the visualization of even small metastases with increased tumor-to-background contrast over time. Based on the results obtained in this work, it can be deduced that PET/CT imaging with 152Tb-labeled targeting agents has promise for clinical application and may be particularly interesting for pre-therapeutic dosimetry.
Introduction
The existence of theragnostic pairs of radionuclides of the same element allows the preparation of chemically identical radiopharmaceuticals for diagnostic and therapeutic purposes. In this regard, radioiodine presents an excellent example, as it has been used successfully to diagnose (123/124I) and treat (131I) differentiated thyroid cancer for several decades.1 Indeed, this was the first theragnostic application of matched radionuclides in nuclear medicine. When utilizing radionuclides in combination with targeting agents, radiometals are commonly preferred over radioiodine due to the increased in vivo stability and an easy preparation of defined radioconjugates.2 There are a number of radiometals potentially useful for radiotheragnostics; among those are matched pairs of yttrium (86Y/90Y), copper (64Cu/67Cu), scandium (43/44Sc/47Sc) and lead (203Pb/212Pb).3–10At Paul Scherrer Institut (PSI), research endeavors in recent years have focused on the investigation of terbium radionuclides.11 Terbium, a lanthanide, is unique in that it presents four medically interesting radionuclides, for SPECT (155Tb) and PET (152Tb) imaging purposes and for α-therapy (149Tb) and β−/Auger-e− therapy (161Tb).11 Interest has been shown in 161Tb, which has similar physical properties to 177Lu and, in addition, emits a substantial number of Auger/conversion electrons.12 De Jong et al. reported on the favorable physical characteristics of 161Tb and presented in vitro and in vivo studies performed with 161Tb-labeled DTPA-octreotide in tumor-bearing rats.13 Preclinical therapy studies performed at PSI demonstrated the superiority of 161Tb over 177Lu, which was attributed to the co-emission of Auger electrons.14,15 Additional side effects to the kidneys were not observed.16 Theoretical calculations of the absorbed radiation dose in spheres of different diameters revealed that 161Tb can effectively irradiate isolated tumor cells and micrometastases, due to its decay spectrum combining β−-particles of medium energy and Auger electrons.17,18 These promising characteristics warrant clinical investigation using 161Tb, when steady supply in sufficient quantities can be guaranteed.
A diagnostic match to therapeutic radiolanthanides is currently not available for clinical use. 68Ga-labeled DOTATOC and DOTATATE are, however, used routinely for the imaging of somatostatin receptor-positive neuroendocrine neoplasms, prior to peptide receptor radionuclide therapy (PRRT) using 177Lu- or 90Y-labeled somatostatin analogues.19–22 While the use of the radiometal 68Ga is considered a success in diagnostic applications, this positron-emitting nuclide is not suitable for pre-therapeutic dosimetry estimations due to its short half-life of only 68 min.
The use of 152Tb may be a solution to address this issue. It decays with a half-life of 17.5 h by the emission of positrons (Eβ+average = 1140 keV, Iβ+ = 20.3%) without the emission of α- or β−-particles, but by the co-emission of several γ-rays (Table 1). It would, thus, be an exact diagnostic match to 161Tb and 149Tb, as well as to other therapeutic radiolanthanides, including 177Lu.23 The current availability of 152Tb is scarce. The nuclide can be produced, however, by proton-induced spallation and on-line mass separation, followed by chemical separation, as previously reported and exemplified at the ISOLDE facility at CERN, Switzerland.11,23,24 An initial proof-of-concept study was performed with 152Tb-labeled folate in a mouse bearing folate receptor (FR)-positive tumors.11 A more extensive in vivo imaging study demonstrated the potential of 152Tb-labeled DOTANOC for the PET/CT imaging of AR42J tumor-bearing mice.23
| Radionuclide | Half-life | Eβ+average (I) | Significant γ-rays (I > 5%), Eγ (I) |
|---|---|---|---|
| Table reproduced from Müller et al., EJNMMI Res., 2016.23 | |||
| 152Tb | 17.5 h | 1140 (20.3%) | 271 (9.53%) |
| 344 (63.5%) | |||
| 586 (9.21%) | |||
| 779 (5.54%) | |||
The aim of the present study was, therefore, to investigate 152Tb in a clinical proof-of-concept PET/CT study. For this purpose, 152Tb was produced at the ISOLDE facility (CERN, Geneva, Switzerland), and was separated from the collection matrix and impurities at PSI. Phantom studies were performed with a clinical PET/CT scanner at Lausanne University Hospital (Centre Hospitalier Universitaire Vaudois or CHUV), Switzerland, and at Zentralklinik Bad Berka (ZBB), Germany, in order to determine the image quality and investigate the possibility of 152Tb-based PET quantification. Finally, DOTATOC was labeled with 152Tb at ZBB's radiopharmacy, and used for a first-in-human clinical PET/CT study.
Experimental
Production of terbium-152 (152Tb)
152Tb was produced through 1.4 GeV proton-induced spallation in a tantalum target (55 g cm−2) at ISOLDE (CERN, Geneva, Switzerland). The spallation products were released online from the hot (∼2000 °C) target and ionized in a hot (∼2000 °C) tungsten ionizer. The cumulative 152Tb yields were significantly boosted by the resonant laser ionization of the radioactive precursor, 152Dy.25 The ions were then accelerated to 30 keV and mass-separated, as previously reported.11,23,24 Ions of mass 152 were implanted into zinc-coated gold foils over a collection period of, typically, 4 hours. The collected samples were allowed to stand, to allow most of the short-lived 152Dy (T1/2 = 2.4 h), as well as the even shorter lived 136Nd and 136Pr oxides collected at the same mass setting (136 + 16 = 152), to decay. The samples were then transported over a distance of 270 km from CERN to PSI.Separation of 152Tb from the collection matrix and quality control of the product
The zinc layer containing 152Tb and impurities of mass 152 (152Dy and 152Gd) and 136 (136Nd/136Pr oxides) was dissolved in 0.1 M HNO3/NH4NO3 at 80 °C. After the addition of H2O, the dissolved zinc layer, along with the radionuclidic implants, was passed through a 50 mm × 5 mm column containing a macroporous strongly acidic cation exchange resin, as previously reported.11 A concentration gradient of α-hydroxy-isobutyric acid (α-HIBA, pH 4.7) ranging from 0.07 to 0.13 M was used for elution. The gradient pump was set to run at 0.6 mL min−1 and reduced to 0.4 mL min−1 after 30 min, when fractions of 1 mL were collected in pre-washed Eppendorf tubes. Small samples (10 μL) were taken from the fractions containing the most activity and measured using an N-type high-purity germanium coaxial detector (EURISYS MESURES, France).For the patient study to be performed at ZBB, the radioactive solutions of the fractions with the highest activity were combined and evaporated to dryness by heating the solution to 80 °C for 1 h, followed by 15 min at 100 °C. The activity was subsequently dissolved in 0.05 M HCl (30% Suprapur, Merck KGaA, Germany). Quality control of the 152Tb product was performed by analyzing the labeling of DOTANOC using analytical high performance liquid chromatography (HPLC), performed on a Merck–Hitachi system, and a radiometric detector. The peptide (DOTANOC acetate, ABX GmbH) was dissolved in Milli-Q water to obtain a stock solution of 1 mM. A mixture of 152Tb in 0.05 M HCl (20 μL), 0.05 M HCl (30 μL), 0.5 M sodium acetate (10 μL, pH 8) and DOTANOC solution (2.4 μL, 1 mM, corresponding to 2.4 nmol) was incubated for 10 min at 95 °C. Afterwards, a 5 μL sample of the labeling solution was diluted with Milli-Q water and injected into an HPLC system equipped with a reversed-phase column (Xterra™, MS, C18, 5 μm, 150 × 4.6 mm; Waters). The mobile phase consisted of Milli-Q water containing 0.1% trifluoroacetic acid (A) and acetonitrile (B). A gradient from 95% A and 5% B to 20% A and 80% B over a period of 15 min was used at a flow rate of 1.0 mL min−1.
PET/CT study of a NEMA NU2 phantom
After chemical separation at PSI, 152Tb (∼150 MBq) was shipped to Lausanne, where it was used for a phantom study at the Nuclear Medicine and Molecular Imaging Department at CHUV. For this purpose, a standard NEMA NU2 phantom composed of a main volume of 9.34 L and six spherical inserts with diameters of 10 mm, 13 mm, 17 mm, 22 mm, 28 mm and 37 mm, respectively, was employed to assess recovery coefficients (RC) in spherical inserts as a function of the insert size. A lung insert was also available for this phantom and was used to estimate the relative lung error (ΔClung). The 152Tb solution was diluted to obtain an activity of 1.8 MBq in 200 mL (activity concentration: 9.0 kBq mL−1) to fill the phantom spheres. An activity of 11.2 MBq in 9.34 L (activity concentration: 1.2 kBq mL−1) was used to fill the main phantom volume, in order to obtain a ratio of 7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 between the activity concentrations in the spheres and background volume, respectively.
1 between the activity concentrations in the spheres and background volume, respectively.
A 10-min list-mode time-of-flight (TOF) PET/CT acquisition was performed with a GE Discovery D690 TOF PET/CT scanner (GE Healthcare, Waukesha, WI).26 Two reconstructions with three iterations and 21 subsets were performed using the vendor's ordered-subset expectation maximization (OSEM) algorithm. The first reconstruction included TOF and resolution recovery corrections (GE-VPFXS) with a post-reconstruction Gaussian filter with a full width at half maximum (FWHM) of 5 mm while the second reconstruction, without TOF and PSP (GE-VPHD), employed a Gaussian filter with a FWHM of 7 mm. The reconstructed field of view had a diameter of 70 cm and a matrix size of 256 × 256, with a slice thickness of 3.27 mm. The CT scan was acquired at 120 kV, 0.8 second gantry rotation time, tube current of 113 mA and a pitch of 1.375. The CT field of view was 70 cm with a matrix size of 512 × 512 and a slice thickness of 3.7 mm.
The PET acquisition was performed with the standard whole-body 18F setup. Direct absolute quantification for 152Tb was not possible, since a specific prompt-gamma correction for this radionuclide was not available. A relative quantification was performed by rescaling the measured average activity concentration in the phantom background to the expected activity concentration, according to the phantom preparation. Average and maximum voxel relative recovery coefficients (RC), as a function of the insert size, were computed by placing a spherical volume of interest (VOI) on each insert and measuring the mean voxel value in the VOI defined by a 3D isocontour at 50%, adapted for background as previously defined27 and the maximum value of the activity concentration divided by the expected activity concentration known from the phantom preparation. According to the NEMA NU2 procedure,28 the relative error in the lung insert was defined by:
Radiolabeling of DOTATOC
A solution of 152Tb in 0.05 M HCl (450 μL, 551 MBq) was prepared at PSI and transported to ZBB. Ten hours after preparation, 152Tb radioactivity was used for the radiolabeling of DOTATOC, as previously reported by Müller et al.23 In brief, DOTATOC (150 μg) was labeled with 152Tb (343 MBq) using a solution of 7.7 mg gentisic acid in 0.4 M sodium acetate buffer (700 μL, pH 4.6). The reaction mixture was incubated at 95 °C for 40 min. Quality control was performed using analytical HPLC (a Jasco PU-1580 system) equipped with a radiometric detector and a reversed-phase column (Jupiter™ Proteo 90 Å, LC, C-18, 4 μm, 250 × 4.6 mm, Phenomenex). The mobile phase consisted of Milli-Q water containing 5% acetonitrile and 0.1% trifluoroacetic acid (A) and acetonitrile containing 0.5% Milli-Q water and 0.1% trifluoroacetic acid (B). The gradient from 100% A to 100% B over a period of 15 min was used, at a flow rate of 1 mL min−1. The reaction solution was diluted with 1 mL sterile 0.9% saline and filtered using a 0.2 μm sterile filter. Samples were taken for sterility and endotoxin testing using an Endosafe®-PTS™ cartridge. The pH value of the final product was then determined.Patient selection and regulatory issues
In accordance with the German Medicinal Act (section 13, subsection 2b), the responsible regulatory authority of the state, Government of Thuringia, and the 1964 Declaration of Helsinki, 152Tb-DOTATOC was administered to a 67-year-old patient with metastatic well-differentiated functional neuroendocrine neoplasm of the ileum, presenting for restaging 8 years after the sixth cycle of PRRT (Table 2). An institutional review board (European Neuroendocrine Tumor Society certified tumor board) approved the study, which was performed in compliance with the regulations of the German Federal Agency for Radiation Protection. Since this was not a formal clinical trial, but a retrospective report on findings obtained within routine clinical care, a formal approval by an ethics committee was not required. The patient signed a substantial written informed consent prior to the investigation and, in addition, he provided signed consent concerning the collection and storage of the clinical data in the institutional database and national registry as well as the anonymized evaluation and publication of these data.| Characteristics | Details |
|---|---|
| Age | 67 years |
| Gender | Male |
| Diagnosis | Metastatic, well-differentiated, functional neuroendocrine neoplasm of the terminal ileum |
| Metastases | Liver, lymph nodes, peritoneum, pancreatic head, intramuscular, pericardium |
| Current disease status | Stable disease |
| Ki-67 of primary tumor | <5% |
| Functionality | Serotonin secretion, carcinoid syndrome |
| Karnofsky Performance Score (at the time of this study) | 100% |
| PRRT schedule: | Cumulative activity administered: |
| May 2005 until October 2007 | |
| 90Y-DOTATATE (n = 5) | 15.2 GBq (90Y-DOTATATE) |
| June 2008 | |
| 177Lu-DOTATATE (n = 1) | 7.5 GBq (177Lu-DOTATATE) |
| Relevant previous surgery | Partial resection of ileum |
| Last restaging with PET/CT | June 2015: |
| 68Ga-DOTATOC PET/CT | |
| Clinical indication | Restaging 8 years after the 6th PRRT cycle |
PET/CT acquisition protocol
PET/CT imaging was performed using a Biograph mCT Flow 64 scanner (Siemens Medical Solutions AG, Erlangen, Germany). Whole-body PET/CT scans were acquired in the supine position from the vertex to the feet at various time intervals. The PET scans were acquired using speeds of 1.1 and 0.8 mm s−1 (for later time points) in flow-motion, which is similar to 2 min per bed or 2.5 min per bed in the stop-and-go acquisition mode. The reconstruction was performed using recon TrueX + ToF with 3 iterations and 21 subsets. The images were reconstructed using a Gaussian filter (2 mm FWHM) and a matrix size of 200 × 200. The CT scans were acquired at 100 kV and at a pitch of 1.5. CareDose4D was applied using a 0.5 second gantry rotation time, 5 mm slice thickness and a matrix size of 512 × 512.PET/CT imaging of a patient using 152Tb-DOTATOC
As part of the routine restaging procedure, blood samples from the patient were sent to the institutional laboratory for analysis. The patient received a bolus intravenous injection of 145 MBq of 152Tb-DOTATOC (2.5 MBq kg−1) in May 2016. No intravenous or oral contrast was given, due to reduced renal function. Four sets of whole-body PET/CT scans of the patient were acquired over two consecutive days. The time points of image acquisition were 25 min, 2 h, 17 h, and 24 h after injection of the radiopharmaceutical, respectively.Analysis of the human PET/CT images
The PET/CT images obtained using 152Tb-DOTATOC were interpreted independently by two physicians (one board-certified nuclear medicine physician and one board-certified radiologist, each with over ten years of experience in reporting PET/CT studies with somatostatin analogues). A qualitative evaluation of the scans was performed by analyzing the bio-distribution of the radiopeptide, along with uptake in the somatostatin receptor (SSTR)-expressing lesions on the whole-body, transverse, coronal and sagittal images. The PET/CT images were compared with those obtained using 68Ga-DOTATOC 11 months previously. Semi-quantitative evaluation using SUV was, however, not performed for this initial study.Results
Production of 152Tb
Due to efficient resonant laser ionization, the ratio between 152Dy and 152Tb was greater than 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 during collection, which means that the mass number 152 isobars consisted of more than 95% 152Dy and less than 5% of 152Tb. Multi reflection time-of-flight mass spectrometer analysis29 showed that the unwanted isobars, namely, 152Gd and 152Eu, occurred to a lesser extent by about 2 orders of magnitude than 152Dy and 152Tb in terms of atomic ratio (i.e. 1 × 10−6 in terms of the activity ratio for 152Eu/152Tb) before chemical separation. Moreover, the atomic ratio of 136Nd/136Pr oxides to all isobar nuclides of mass number 152 was less than 1 × 10−5, and the activity ratio of 136Nd/136Pr oxides to 152Tb was less than 2 × 10−4 at the time of arrival at PSI, decaying to negligible levels before final use. As a result, 152Gd, 152Eu and 136Nd/136Pr could be safely neglected, even if they were not fully separated from 152Dy/152Tb.
1 during collection, which means that the mass number 152 isobars consisted of more than 95% 152Dy and less than 5% of 152Tb. Multi reflection time-of-flight mass spectrometer analysis29 showed that the unwanted isobars, namely, 152Gd and 152Eu, occurred to a lesser extent by about 2 orders of magnitude than 152Dy and 152Tb in terms of atomic ratio (i.e. 1 × 10−6 in terms of the activity ratio for 152Eu/152Tb) before chemical separation. Moreover, the atomic ratio of 136Nd/136Pr oxides to all isobar nuclides of mass number 152 was less than 1 × 10−5, and the activity ratio of 136Nd/136Pr oxides to 152Tb was less than 2 × 10−4 at the time of arrival at PSI, decaying to negligible levels before final use. As a result, 152Gd, 152Eu and 136Nd/136Pr could be safely neglected, even if they were not fully separated from 152Dy/152Tb.
Radiochemical purification of 152Tb
The radiochemical separations were performed typically between 12 and 16 hours after the end of collection. The total measured radioactivity at the beginning of the separation contained about 10% of 152Dy. The gradient elution of the column at low pump speeds ensured the clean elution of 152Tb, together with 152Dy, leaving the remaining contaminants to be eluted with more concentrated solutions afterwards. The eluted fractions containing the highest activities of 152Tb/152Dy were selected and combined to obtain about 675 MBq of 152Tb in ∼1.5 mL of α-HIBA. γ-Spectra taken just after separation confirmed the presence of both 152Tb and 152Dy in the final product. The analysis of the 152Tb solution the day after production revealed a radiochemically pure product as, by this time, all the 152Dy had decayed to 152Tb.As part of the first-in-human study, the 152Tb solution was processed further to ensure high quality radiolabeling. After evaporation of the eluted 152Tb solution and reconstitution in 0.05 M HCl, the 152Tb activity was used for the labeling of DOTANOC at a specific activity of 10 MBq nmol−1. The quality control performed, using HPLC, revealed a radiochemical purity of >98%. Having confirmed the excellent quality of the radionuclide, a solution containing about 500 MBq 152Tb was shipped over a distance of ∼600 km from PSI to ZBB.
As a part of a second experiment, a sample of ∼150 MBq 152Tb in α-HIBA was shipped the morning after separation, from PSI to CHUV, for the performance of human phantom studies.
Phantom studies
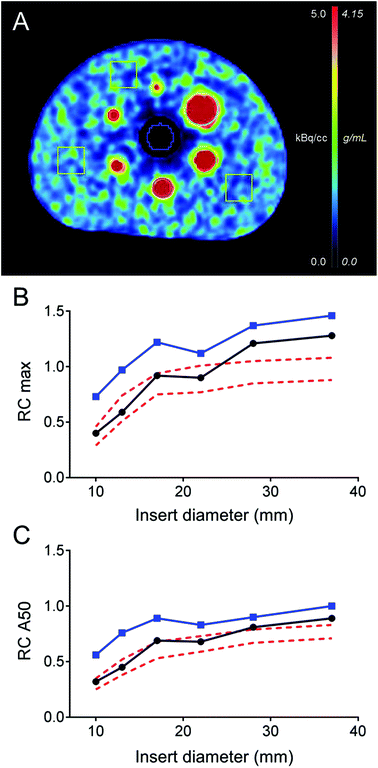
The transaxial view of the NEMA NU2 phantom is shown in Fig. 1A. For both reconstructions, the background variability measured by COV was 25%. The relative maximum and average (50%) recovery coefficients for each spherical insert are shown in Fig. 1B and C. The OSEM3D TOF + PSF reconstruction exhibited maximum and average RC values comparable to the RC reported in the literature for 18F PET reconstructions using TOF and point spread function (PSF) corrections.30 Lower RC values were obtained with the simple OSEM3D reconstruction. Relative lung errors were 12.2% and 40.2% for the OSEM3D TOF + PSF and OSEM3D reconstruction, respectively. These results have been confirmed by an independent phantom experiment performed in the Siemens Biograph mCT Flow 64 PET/CT device at ZBB (data not shown).Preparation of 152Tb-DOTATOC for human use
The radiochemical purity of the final product, performed at ZBB, was >97%, thereby allowing its application without any further purification. The pH value of the final product was 4.6, while the bacterial endotoxin test was negative. Due to the small application volume (<3 mL), it was not necessary to determine and adjust the osmolarity of the final product. The specific activity of the sterile-filtered and applied product (145 MBq) was 1.37 MBq nmol−1, calculated for 150 μg DOTATOC.PET/CT imaging studies of a patient
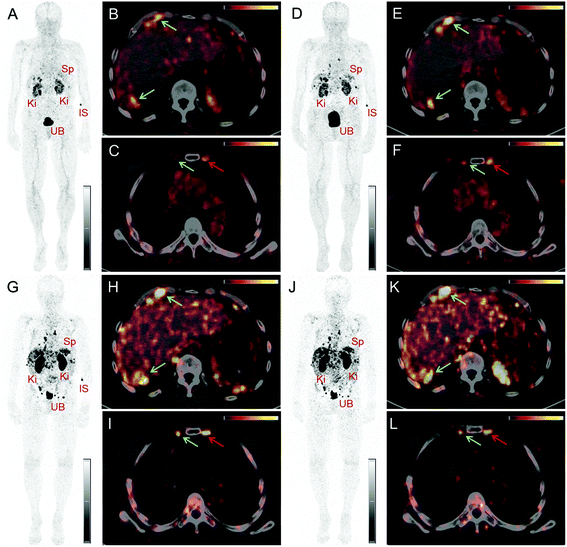
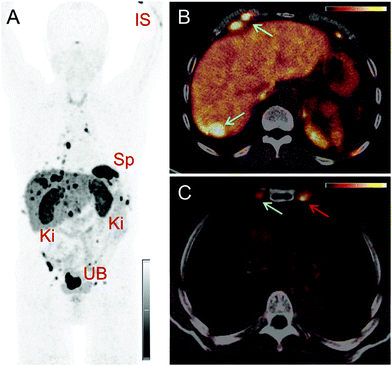
PET/CT images were obtained from a 67-year-old patient with metastatic well-differentiated functional neuroendocrine neoplasm of the ileum, presenting for restaging 8 years after the sixth cycle of PRRT (Fig. 2). The biodistribution of 152Tb-DOTATOC was assessed visually using PET/CT slices acquired at four different time-points on two subsequent days (Fig. 2). The early images performed at 25 min after injection demonstrated uptake in the blood pool, soft tissue, kidneys, and in some lymph node metastases. Moderate-to-low tracer uptake in the liver and spleen was also visualized. Renal excretion of 152Tb-DOTATOC was seen by tracer uptake in the kidneys, the pelvicalyceal system and the urinary bladder. At 2 h after injection, radioactivity was still seen in the blood pool and soft tissue; however, the uptake in the liver, spleen, as well as in lymph node and skeletal metastases was increased. Renal excretion continued, with increasing activity detectable in the urinary bladder. At 17 h after injection of 152Tb-DOTATOC, the tracer was no longer visible in the blood vessels. The uptake was more prominent in SSR-expressing organs, such as the pituitary gland (comparatively moderate-to-low uptake), the spleen, and in multiple SSR-expressing lymph node and skeletal metastases. Very low uptake was present in the shoulders due to inflammatory/degenerative changes. Persistent renal uptake was visible in the kidneys along with urinary bladder activity. Some activity was also detected in the intestines as a consequence of the elimination of 152Tb-DOTATOC. On the delayed images at 24 hours after injection, physiological uptake of the radiopeptide was present in the pituitary gland, spleen, liver, intestines, and kidneys, as well as excreted tracer in the urinary bladder. Even at this delayed time-point, excellent and even increased uptake of the radiopeptide was observed in multiple lymph nodes and skeletal metastases. At all image acquisition time points, the image quality was noisier as compared to 68Ga-based PET scans, which may be attributed to the inability of the software to perform prompt γ-correction for the acquired data.When compared to the previous PET/CT scans performed with 68Ga-DOTATOC in June 2015 (Fig. 3), all known metastases were visualized and no new metastatic lesions were identified in the PET scan obtained with 152Tb-DOTATOC in May 2016. From a clinical point of view, this (stable disease) was the most important finding of the study.
Discussion
In this study, 152Tb, a novel radionuclide for PET imaging, was used for the first time in a human. The 17.5 h half-life of 152Tb allowed its transport over several hundreds of kilometers across Europe, where it was used for the labeling of DOTATOC and PET/CT imaging of a patient with a neuroendocrine neoplasm. The endeavor involved three widely separated research facilities and hospitals, respectively, where the production, separation, radiolabeling and the administration of the final product, 152Tb-DOTATOC, to a patient was carried out.The chemical separation of 152Tb from the target material was performed by ion exchange chromatography at PSI. While it is known that labeling is feasible when using 152Tb directly in the eluted α-HIBA elution solution,11 it was decided to evaporate the liquid and reconstitute the radionuclide in dilute hydrochloric acid to set similar conditions as for commercial radionuclides. That way, efficient radiolabeling of DOTATOC at ZBB for subsequent in-human application was facilitated. By the time the 152Tb product arrived at CHUV and ZBB, respectively, the remaining 152Dy was <0.2% of the 152Tb activity.
The 152Tb PET/CT study with the standard NEMA NU2 phantom showed RC values in agreement with levels typical of clinical 18F- and 68Ga-based PET/CT studies using TOF and PSF corrections.30 RC values obtained with the simple OSEM3D (no-TOF no-PSF) reconstructions were comparable to PET-derived reference levels reported in the EANM guidelines for oncological 18F-FDG PET protocols. As a consequence, expected signal recovery at matched signal-to-noise ratios in clinical 152Tb-based PET/CT procedures should not be different from the established 18F- and 68Ga-based PET/CT. Particularly high values of maximum RC are explained by the important image noise (COV = 25%). A two-fold increased image noise level was expected because of the smaller β+ branching ratio of 152Tb (Iβ+ = 20.3%), as compared to 18F (Iβ+ = 97%), at matched activity concentrations and actual frame durations. The signal recovery in the cold region estimated by the relative lung error in the TOF + PSF 152Tb PET/CT reconstruction (ΔClung = 12.2%) is comparable to the values obtained in TOF + PSF 18F PET/CT.26 The particularly high value of ΔClung = 40.2% obtained for the simple OSEM3D reconstruction can be explained by the important signal pollution in the cold lung region consequent to the lack of TOF information and low acquired statistic. At present, the spatial resolution of 152Tb PET/CT imaging was not assessed. This could be done in future experiments using a specifically designed line resolution phantom. The mean energy and the corresponding mean range in tissues of the β+ emission from 152Tb (1.14 MeV/4.7 mm) are higher than for 18F (0.25 MeV/0.64 mm), but comparable to those for 68Ga (0.836 MeV/3.5 mm).31 Other studies in which 68Ga was compared to 18F in clinically relevant PET/CT setups did not find relevant differences in terms of resolution and signal recovery.31,32 According to these studies, which are supported by the results obtained in the current study, the degradation of 152Tb RC as compared to RC values obtained under clinically relevant conditions for 18F- and 68Ga-based PET is not expected.
This first-in-human application of 152Tb-DOTATOC underlined the potential of using 152Tb for PET imaging. The physiological distribution of 152Tb-DOTATOC was similar, but not identical, to the distribution of 68Ga-DOTATOC. This can be attributed to different and delayed PET acquisition time points, as well as the different amounts of the injected peptide (<50 μg for 68Ga, and 150 μg for 152Tb) used for these studies. Moreover, the different coordination chemistry of 152Tb with DOTA as compared to 68Ga may result in slightly different distribution profiles.
In comparison with the 68Ga-based PET scan, the images obtained with 152Tb-DOTATOC were comparatively noisier, which could possibly result from the lack of prompt γ correction of the scatter fractions from photopeaks with γ-energies >511 keV by the current PET software. Visual assessment of the images revealed lower physiological uptake in the pituitary and thyroid glands in comparison with the previous PET/CT scans obtained with 68Ga-DOTATOC. This may be related to the increased amount of peptide injected in the case of 152Tb-DOTATOC as compared to the amount of peptide used for 68Ga-DOTATOC, resulting in lower specific activity. Nonetheless, the PET/CT images acquired using 152Tb-DOTATOC in this study were adequate for diagnostic purposes. In comparison with the PET/CT scan obtained with 68Ga-DOTATOC, all previously detected lymph node, skeletal and liver lesions were clearly identified and there was no evidence of new or progressive metastases. Information of stable disease, which was obtained in this study, was used for making the clinical decision of further follow-up of the patient; therapeutic intervention was not necessary at this stage.
An accurate semi-quantitative assessment of the acquired images using the standard practice of measuring the standard uptake value (SUV) was not feasible for this study, since the software could not be used for calibration of the PET camera for 152Tb. Should this radionuclide be made available for routine clinical use in future, quantification of accumulated activity will be of particular value. A significant advantage of 152Tb over 68Ga is its considerably longer half-life, thus, rendering 152Tb-based PET imaging a potentially valuable tool for pre-therapeutic dosimetry, allowing optimization of the individualized planning of PRRT with either 177Lu- or 161Tb-labeled DOTA-peptides. Terbium and lutetium belong to the group of lanthanides, with almost identical coordination chemistry and, hence, the tissue distribution profile of the corresponding radiolabeled peptides is likely to be the same. This is not the case for 68Ga-labeled peptides, which have different coordination chemistry. The long half-life of 152Tb provides interesting logistic opportunities, potentially allowing its distribution across Europe and beyond.
Conclusions
This study describes a unique, multi-disciplinary study in which 152Tb was investigated from the production to the first-in-human clinical application. 152Tb was collected via a mass separation process, chemically separated and used for phantom studies as well as for the radiolabeling of DOTATOC. The results of the clinical application demonstrated successful PET/CT imaging using 152Tb-DOTATOC in a patient with neuroendocrine neoplasm, allowing the visualization of even small metastases. Due to the considerably longer half-life of 152Tb, as compared to the currently used 68Ga, this novel radionuclide would be particularly interesting for dosimetry prior to radionuclide therapy. While the current availability of 152Tb is limited, the on-going construction of additional ISOL facilities will increase the possibility of pursuing this concept at a larger scale.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the staff members at the Center for Radiopharmaceutical Sciences at PSI for assistance in the study, as well as the people responsible for radiation safety and radioactive transport for night shifts. The authors are grateful for support by the CERN-ISOLDE teams, in particular the RILIS team and the ISOLTRAP MR-TOF-MS team and thank the radiation safety staff at CERN. The authors would also like to thank Martin Pappon for data acquisition of the phantom study at CHUV, Lausanne, Switzerland. The authors express their gratitude to the physicians, physicists, and nursing staff, as well as the nuclear medicine technologists of the Theranostics Center for Molecular Radiotherapy and Molecular Imaging for patient management at ZBB. This project has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement no. 654002.References
- B. C. Ahn, BioMed Res. Int., 2016, 2016, 1680464 Search PubMed.
- B. M. Zeglis, J. L. Houghton, M. J. Evans, N. Viola-Villegas and J. S. Lewis, Inorg. Chem., 2014, 53, 1880–1899 CrossRef CAS PubMed.
- F. Rösch, H. Herzog, B. Stolz, J. Brockmann, M. Köhle, H. Mühlensiepen, P. Marbach and H. W. Müller-Gartner, Eur. J. Nucl. Med., 1999, 26, 358–366 CrossRef.
- A. Helisch, G. J. Förster, H. Reber, H. G. Buchholz, R. Arnold, B. Goke, M. M. Weber, B. Wiedenmann, S. Pauwels, U. Haus, H. Bouterfa and P. Bartenstein, Eur. J. Nucl. Med. Mol. Imaging, 2004, 31, 1386–1392 CrossRef CAS PubMed.
- C. J. Anderson and R. Ferdani, Cancer Biother. Radiopharm., 2009, 24, 379–393 CrossRef CAS PubMed.
- N. A. Smith, D. L. Bowers and D. A. Ehst, Appl. Radiat. Isot., 2012, 70, 2377–2383 CrossRef CAS PubMed.
- C. Müller, M. Bunka, J. Reber, C. Fischer, K. Zhernosekov, A. Türler and R. Schibli, J. Nucl. Med., 2013, 54, 2168–2174 CrossRef PubMed.
- C. Müller, M. Bunka, S. Haller, U. Köster, V. Groehn, P. Bernhardt, N. van der Meulen, A. Türler and R. Schibli, J. Nucl. Med., 2014, 55, 1658–1664 CrossRef PubMed.
- K. Yong and M. Brechbiel, AIMS Med. Sci., 2015, 2, 228–245 CrossRef PubMed.
- D. Mathe, K. Szigeti, N. Hegedus, I. Horvath, D. S. Veres, B. Kovacs and Z. Szucs, Appl. Radiat. Isot., 2016, 114, 1–6 CrossRef CAS PubMed.
- C. Müller, K. Zhernosekov, U. Köster, K. Johnston, H. Dorrer, A. Hohn, N. T. van der Walt, A. Türler and R. Schibli, J. Nucl. Med., 2012, 53, 1951–1959 CrossRef PubMed.
- S. Lehenberger, C. Barkhausen, S. Cohrs, E. Fischer, J. Grunberg, A. Hohn, U. Koster, R. Schibli, A. Turler and K. Zhernosekov, Nucl. Med. Biol., 2011, 38, 917–924 CrossRef CAS PubMed.
- M. de Jong, W. A. Breeman, B. F. Bernard, E. J. Rolleman, L. J. Hofland, T. J. Visser, B. Setyono-Han, W. H. Bakker, M. E. van der Pluijm and E. P. Krenning, Eur. J. Nucl. Med., 1995, 22, 608–616 CrossRef CAS PubMed.
- C. Müller, J. Reber, S. Haller, H. Dorrer, P. Bernhardt, K. Zhernosekov, A. Türler and R. Schibli, Eur. J. Nucl. Med. Mol. Imaging, 2014, 41, 476–485 CrossRef PubMed.
- J. Grünberg, D. Lindenblatt, H. Dorrer, S. Cohrs, K. Zhernosekov, U. Köster, A. Türler, E. Fischer and R. Schibli, Eur. J. Nucl. Med. Mol. Imaging, 2014, 41, 1907–1915 CrossRef PubMed.
- S. Haller, G. Pellegrini, C. Vermeulen, N. P. van der Meulen, U. Köster, P. Bernhardt, R. Schibli and C. Müller, EJNMMI Res., 2016, 6, 13 CrossRef PubMed.
- C. Champion, M. A. Quinto, C. Morgat, P. Zanotti-Fregonara and E. Hindie, Theranostics, 2016, 6, 1611–1618 CrossRef CAS PubMed.
- E. Hindie, P. Zanotti-Fregonara, M. A. Quinto, C. Morgat and C. Champion, J. Nucl. Med., 2016, 57, 759–764 CrossRef PubMed.
- S. Koukouraki, L. G. Strauss, V. Georgoulias, J. Schuhmacher, U. Haberkorn, N. Karkavitsas and A. Dimitrakopoulou-Strauss, Eur. J. Nucl. Med. Mol. Imaging, 2006, 33, 460–466 CrossRef CAS PubMed.
- A. R. Haug, R. Cindea-Drimus, C. J. Auernhammer, M. Reincke, B. Wangler, C. Uebleis, G. P. Schmidt, B. Goke, P. Bartenstein and M. Hacker, J. Nucl. Med., 2012, 53, 1686–1692 CrossRef CAS PubMed.
- L. Peter, J. Sanger, M. Hommann, R. P. Baum and D. Kaemmerer, Clin. Nucl. Med., 2014, 39, 713–716 CrossRef PubMed.
- P. Sharma, S. Arora, A. Mukherjee, S. Pal, P. Sahni, P. Garg, R. Khadgawat, S. Thulkar, C. Bal and R. Kumar, Clin. Nucl. Med., 2014, 39, 37–43 CrossRef PubMed.
- C. Müller, C. Vermeulen, K. Johnston, U. Köster, R. Schmid, A. Türler and N. P. van der Meulen, EJNMMI Res., 2016, 6, 35 CrossRef PubMed.
- B. J. Allen, G. Goozee, S. Sarkar, G. Beyer, C. Morel and A. P. Byrne, Appl. Radiat. Isot., 2001, 54, 53–58 CrossRef CAS PubMed.
- U. Köster, Nucl. Phys. A, 2002, 701, 441c–451c CrossRef.
- V. Bettinardi, L. Presotto, E. Rapisarda, M. Picchio, L. Gianolli and M. C. Gilardi, Med. Phys., 2011, 38, 5394–5411 CrossRef CAS PubMed.
- R. Boellaard, N. C. Krak, O. S. Hoekstra and A. A. Lammertsma, J. Nucl. Med., 2004, 45, 1519–1527 Search PubMed.
- V. Rosslyn, The Association of Electrical Equipment and Medical Imaging Manufacturers, 2007 Search PubMed.
- R. N. Wolf, D. Beck, K. Blaum, C. Bohm, C. Borgmann, M. Breitenfeldt, F. Herfurth, A. Herlert, M. Kowalska, S. Kreim, D. Lunney, S. Naimi, D. Neidherr, M. Rosenbusch, L. Schweikhard, J. Stanja, F. Wienholtz and K. Zuber, Nucl. Instr. Meth. A, 2012, 686, 82–90 CrossRef CAS.
- E. Quak, P. Y. Le Roux, M. S. Hofman, P. Robin, D. Bourhis, J. Callahan, D. Binns, C. Desmonts, P. Y. Salaun, R. J. Hicks and N. Aide, Eur. J. Nucl. Med. Mol. Imaging, 2015, 42, 2072–2082 CrossRef PubMed.
- A. T. Soderlund, J. Chaal, G. Tjio, J. J. Totman, M. Conti and D. W. Townsend, J. Nucl. Med., 2015, 56, 1285–1291 CrossRef CAS PubMed.
- P. Kench, N. Forwood, K. Willowson and D. Bailey, J. Nucl. Med., 2013, 54, 2121 Search PubMed.
Footnote |
| † These authors contributed equally to this manuscript. |
| This journal is © The Royal Society of Chemistry 2017 |