 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A [Ce21] keplerate†
Angelos B.
Canaj
 a,
Milosz
Siczek
b,
Tadeusz
Lis
b,
Mark
Murrie
a,
Milosz
Siczek
b,
Tadeusz
Lis
b,
Mark
Murrie
 *c,
Euan K.
Brechin
*d and
Constantinos J.
Milios
*c,
Euan K.
Brechin
*d and
Constantinos J.
Milios
 *a
*a
aDepartment of Chemistry, The University of Crete, Voutes 71003, Herakleion, Greece. E-mail: komil@uoc.gr
bFaculty of Chemistry, University of Wroclaw, Joliot-Curie 14, Wroclaw 50-383, Poland
cWestCHEM, School of Chemistry, University of Glasgow, University Avenue, Glasgow, G12 8QQ, UK. E-mail: Mark.Murrie@glasgow.ac.uk
dEaStCHEM, School of Chemistry, University of Edinburgh, David Brewster Road, Edinburgh, UK EH9 3FJ, UK. E-mail: E.Brechin@ed.ac.uk
First published on 31st May 2017
Abstract
The solvothermal reaction between Ce(NO3)3·6H2O, 2-amino-isobutyric acid, 2-hydroxy-1-naphthaldehyde and 2-amino-2-methyl-1,3-propanediol in MeOH, in the presence of base, leads to the formation of a unique [CeIV13Ce III8] keplerate cage.
Keplerates are high symmetry polymetallic molecules that contain metal polyhedra that describe both Platonic and Archimedean solids, one inside the other, in a manner akin to Russian dolls.1 They have been described widely in polyoxometalate chemistry, with perhaps the most celebrated example being the spherical {(Mo)Mo5}12M′30 (M′ = FeIII, CrIII, VIV) species whose corner-sharing M′3 triangles (the M′30 unit describes an icosidodecahedron) cause geometric spin frustration, analogous to that seen in planar Kagome lattices.2 Such structures, which are often aesthetically very beautiful, are less well known in homometallic 3d, 4f and heterometallic 3d–4f cages,3–7 and the d-block complexes that have been fully characterised have always shown magnetic behaviour different to that expected from theory, i.e. none have shown frustration effects. A recent study of a family CuII keplerates has suggested this is due to structural disorder, even in systems where the apparent crystallographic symmetry is high.8
The diamagnetic CeIV ion has been used both as an oxidising agent and as a template for the construction of high nuclearity Mn-based Single-Molecule Magnets (SMMs),9 while the CeIII ion, which has no nuclear spin and possesses just one unpaired electron, has been investigated for both its potential Single-Ion Magnet (SIM) behaviour10–13 and for addressing multipolar exchange in f-electron molecules.14 A review of the CSD reveals that only seventeen homometallic [Cen] cages with a nuclearity larger than five have been published. Those with a nuclearity less than ten include two square-pyramidal [Ce5] complexes,15 seven [Ce6] octahedra and trigonal antiprisms,16 and four [Ce8]17 compounds with topologies ranging from bicoronal trigonal prisms to ellipsoids. Species with nuclearities in double figures include a domed, body-centred pentagonal [Ce10] complex,18 a pseudo-spherical [Ce13] lanthaball,19 a spherical [Ce17] cage20 and a [Ce22] species with a condensed metal–oxide core.18 The latter is the largest homometallic cerium cage reported to date.
Solvothermal reaction of Ce(NO3)3·6H2O (1 mmol) with 2-amino-isobutyric acid (Haib; 1 mmol), 2-hydroxy-1-naphthaldehyde (Hnaphth; 1 mmol) and 2-amino-2-methyl-1,3-propanediol (H2amp; 1 mmol) in MeOH, in the presence of base (NEt3) results in the formation of dark brown crystals of the complex [CeIV13CeIII8O8(OH)27(L)12(NO3)9(H2O)4.6(MeOH)3]·9MeOH·4.2H2O (1·9MeOH·4.2H2O) in ∼15% yield (see ESI,† L = the dianion of the Schiff-base between Hnaphth and Haib).‡ Complex 1 crystallises in the trigonal R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) space group with Z = 1 in which Ce1 is at a site with −3 symmetry and Ce5, O1, O4 W are at special positions with 3-fold symmetry. The metallic skeleton of 1 (Fig. 1) describes an outer [CeIII8] cube (BVS: 2.92 and 2.66 for Ce4 and Ce5, respectively) encapsulating an inner [CeIV12] cuboctahedron (BVS: 3.98 and 3.73 for Ce2 and Ce3, respectively), which in turn encapsulates a single CeIV ion (Ce1, BVS: 4.01). Both of the former units show little distortion from ideal polyhedra. The cube, comprising Ce4, Ce5 and symmetry equivalents (s.e.), and of approximate dimensions 7.3 × 7.3 × 7.4 Å, displays Ce⋯Ce⋯Ce angles varying between a minimum of 88.2(6)° and a maximum of 90.6(6)°. The Ce⋯Ce⋯Ce angles of the triangles within the cuboctahedron are in the range of 69.7(6) to 71.4(7)°, close to the expected 60° required for a perfect cuboctahedron.
space group with Z = 1 in which Ce1 is at a site with −3 symmetry and Ce5, O1, O4 W are at special positions with 3-fold symmetry. The metallic skeleton of 1 (Fig. 1) describes an outer [CeIII8] cube (BVS: 2.92 and 2.66 for Ce4 and Ce5, respectively) encapsulating an inner [CeIV12] cuboctahedron (BVS: 3.98 and 3.73 for Ce2 and Ce3, respectively), which in turn encapsulates a single CeIV ion (Ce1, BVS: 4.01). Both of the former units show little distortion from ideal polyhedra. The cube, comprising Ce4, Ce5 and symmetry equivalents (s.e.), and of approximate dimensions 7.3 × 7.3 × 7.4 Å, displays Ce⋯Ce⋯Ce angles varying between a minimum of 88.2(6)° and a maximum of 90.6(6)°. The Ce⋯Ce⋯Ce angles of the triangles within the cuboctahedron are in the range of 69.7(6) to 71.4(7)°, close to the expected 60° required for a perfect cuboctahedron.
 | ||
| Fig. 1 The metallic skeleton of 1. Colour code: CeIII = green, CeIV = brown, central CeIV = yellow. The red line indicates the 3-fold axis crossing Ce5, Ce1 and Ce5′. | ||
By symmetry the central, eight coordinate CeIV ion (Ce1) is in an ideal cube [CeO8] geometry, bonded to eight μ4-O2− ions with Ce–O in the 2.333(5) to 2.338(10) Å range, which also bond to the three CeIV ions that make up the [Ce3] triangles of the inner [Ce12] cuboctahedron. These Ce ions (Ce2, Ce3 and s.e.) are all eight coordinate and in square antiprismatic geometries ([CeO8]). Twenty four μ3-OH− ions (four on each face of the cube) link two Ce ions in the cuboctahedron to one CeIII ion in the cube. Ce4 (and s.e) is eight-coordinate adopting biaugmented trigonal prismatic geometry, while Ce5 (and s.e.) is six-coordinate and in distorted trigonal prismatic geometry. The nitrate anions are of two types: one chelating a Ce ion at the corner of the cube, and one μ-bridging between two Ce ions in the cuboctahedron, protruding out of the centre of the square face of the cube (Fig. 2). The L2− ligands (Fig. 3) are positioned along each edge of the cube, the deprotonated phenol moiety terminally bonded to (cube vertex) Ce4, and the carboxylate moiety bridging between Ce ions (Ce2, Ce3 and s.e.) in the cuboctahedron and Ce4′. Six L2− ligands are disordered over two equivalent positions and coordinate to Ce4 in two alternative ways (through carboxylate or phenolate oxygen atoms). It should also be mentioned that large accessible voids (∼1000 Å3) are observed in the crystal structure.
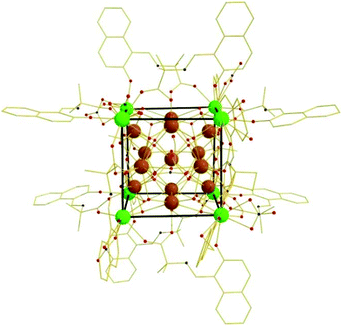 | ||
| Fig. 3 The crystal structure of 1. Colour code: CeIII = green, CeIV = brown, N = blue, O = red, C = gold. H-atoms omitted for clarity. | ||
The structure of 1 is similar to those seen in the [Cu12Mg8] (and analogous) species reported in ref. 8, and somewhat resemble a family of pentadecanuclear [Ln15] clusters whose structures can be described as five vertex-sharing cubane-like [Ln4(μ3-OH)4]8+ units,21 and an octadecanuclear [Cu12Eu6] cluster also possessing a keplerate-like structure.22
Dc magnetic susceptibility measurements were performed on 1 in the 2–300 K temperature range under an applied magnetic field of 0.1 T, and the results are plotted as χMT vs. T in Fig. 4 (top), with the isothermal magnetisation (M vs. H) curves shown in the inset. The room temperature χMT value of 6.46 cm3 mol−1 K is very close to the theoretical value of 6.43 cm3 mol−1 K expected for eight CeIII ions (2F5/2, gJ = 6/7). Upon cooling, the χMT product decreases steadily, reaching 3.34 cm3 mol−1 K at 2 K. Given that the CeIII⋯CeIII separation is over 7 Å, this decrease is consistent with the depopulation of ligand-field sublevels.11,23 In terms of such large “metal–oxide”-like species, magnetic data have been only reported for the [Ce13] ‘lanthaball’ where some Ce⋯Ce distances are much shorter (4–5 Å) and the data are consistent with very weak to zero intramolecular CeIII⋯CeIII interactions.19
 | ||
| Fig. 4 Plot of χMT vs. T for 1 in the 2–300 K temperature range, under an applied field of 0.1 T (top). Inset: M vs. H for 1 in the 0–5 T and 2.0–6.0 K field and temperature ranges. | ||
Even assuming that the CeIII⋯CeIII interactions in 1 are vanishingly small, in order to model the χMT vs. T data, we would need two sets of crystal field parameters (for Ce4 and Ce5, where the local symmetry is approximately C2v and D3h, respectively). The magnetisation isotherms (Fig. 4, inset) do not reach the value expected if each CeIII ion has an mJ = 5/2 ground state (M/NμB = 17.1). Analysis is further complicated by the fact that both ions are in low symmetry coordination environments (SHAPE parameters of 3.58 and 4.62, for Ce4 and Ce5, respectively), which will cause mixing of the different mJ states and hence, it is likely that the ground state of each CeIII ion is not a pure mJ state. Ab initio computational studies could provide insight here.10
We also investigated the dynamic magnetic behaviour of 1 in zero and applied dc fields, as some CeIII single-ion complexes have been shown to show slow magnetic relaxation (Fig. S1†). However, no temperature or frequency dependent out-of-phase susceptibility signals were observed, ruling out the possibility of Single-Molecule Magnet behaviour. CeIII is an oblate ion24 and ideally an axial crystal field is required to stabilise mJ = ±5/2 as the ground state for each ion. We ascribe the lack of slow relaxation of the magnetisation to the non-axial coordination environments at Ce4 and Ce5, which could promote stabilisation of smaller mJ states such as mJ = ±3/2 or mJ = ±1/2 that would lower or destroy the spin-reversal barrier. In addition, the low symmetry coordination environment at each CeIII ion could introduce significant transverse anisotropy, leading to efficient quantum tunnelling.
In summary, we have reported the synthesis of a very rare example of a cerium complex adopting a keplerate “Russian doll” structure, consisting of a cube encapsulating a cuboctahedron encapsulating a single cerium ion. This [Ce21] species is the second largest molecular cerium cluster reported to date, smaller only than the [Ce22] cage reported by Kögerler and co-workers in 2012. Cerium is very much an underused element in molecule-based magnetism despite having a long history in the chemistry and physics of solid-state permanent magnets. The realization that the cores of these molecular species are simple metal oxides conforming to Archimedean and Platonic solids, with building blocks analogous to those seen in POM chemistry, would suggest that extremely large homo- and heterovalent cerium-based “molecular metal oxides” possessing unusual magnetic behaviours are yet to be discovered, which is a tantalizing prospect.
Acknowledgements
EKB thanks the EPSRC for funding.Notes and references
- A. Müller, P. Kögerler and A. W. M. Dass, Coord. Chem. Rev., 2001, 222, 193 CrossRef
.
- J. Schnack, Dalton Trans., 2010, 39, 4677 RSC
.
- L. Zhang, R. Clérac, P. Heijboer and W. Schmitt, Angew. Chem., Int. Ed., 2012, 51, 3007 CrossRef CAS PubMed
.
- L. Zhang and W. Schmitt, J. Am. Chem. Soc., 2011, 133, 11240 CrossRef CAS PubMed
.
- X.-J. Kong, Y.-P. Ren, L.-S. Long, Z. Zheng, R.-B. Huang and L.-S. Zheng, J. Am. Chem. Soc., 2007, 129, 7016 CrossRef CAS PubMed
.
- X.-J. Kong, Y. Wu, L.-S. Long, L.-S. Zheng and Z. Zheng, J. Am. Chem. Soc., 2009, 131, 6918 CrossRef CAS PubMed
.
- J.-B. Peng, X.-J. Kong, Q.-C. Zhang, M. Orendáč, J. Prokleška, Y.-P. Ren, L.-S. Long, Z. Zheng and L.-S. Zheng, J. Am. Chem. Soc., 2014, 136, 17938 CrossRef CAS PubMed
.
- M. A. Palacios, E. Moreno Pineda, S. Sanz, R. Inglis, M. B. Pitak, S. J. Coles, M. Evangelisti, H. Nojiri, C. Heesing, E. K. Brechin, J. Schnack and R. E. P. Winpenny, ChemPhysChem, 2016, 17, 55 CrossRef CAS PubMed
.
- A. J. Tasiopoulos, W. Wernsdorfer, B. Moulton, M. J. Zaworotko and G. Christou, J. Am. Chem. Soc., 2003, 125, 15274 CrossRef CAS PubMed
.
- S. K. Singh, T. Gupta, L. Ungur and G. Rajaraman, Chem. – Eur. J., 2015, 21, 13812 CrossRef CAS PubMed
.
- S. Hino, M. Maeda, K. Yamashita, Y. Kataoka, M. Nakano, T. Yamamura, H. Nojiri, M. Kofu, O. Yamamuro and T. Kajiwara, Dalton Trans., 2013, 42, 2683 RSC
.
- J. J. Le Roy, I. Korobkov, J. E. Kim, E. J. Schelter and M. Murugesu, Dalton Trans., 2014, 43, 2737 RSC
.
- S. Hino, M. Maeda, Y. Ktaoka, M. Nakano, T. Yamamura and T. Kajiwara, Chem. Lett., 2013, 42, 1276 CrossRef CAS
.
- P. Santini, S. Carretta, G. Amoretti, R. Caciuffo, N. Magnani and G. H. Lander, Rev. Mod. Phys., 2009, 81, 807 CrossRef CAS
.
- M. Kritikos, M. Moustiakimov and G. Westin, Inorg. Chim. Acta, 2012, 384, 125 CrossRef CAS
; A. R. Crozier, A. M. Bienfait, C. Maichle-Mösmer, K. W. Törnroosa and R. Anwander, Chem. Commun., 2013, 49, 87 RSC
.
- C. Hennig, A. Ikeda-Ohno, W. Kraus, S. Weiss, P. Pattison, H. Emerich, P. M. Abdala and A. C. Scheinost, Inorg. Chem., 2013, 52, 11734 CrossRef CAS PubMed
; W. Zhu, X. Wu, C. He and C. Duan, Tetrahedron, 2013, 69, 10477 CrossRef
; L. Mathey, M. Paul, C. Copéret, H. Tsurugi and K. Mashima, Chem. – Eur. J., 2015, 21, 13454 CrossRef PubMed
; V. Mereacre, A. M. Ako, M. N. Akhtar, A. Lindemann, C. E. Anson and A. K. Powell, Helv. Chim. Acta, 2009, 92, 2507 CrossRef
; S. M. Taylor, S. Sanz, R. D. McIntosh, C. M. Beavers, S. J. Teat, E. K. Brechin and S. J. Dalgarno, Chem. – Eur. J., 2012, 18, 16014 CrossRef PubMed
; J. Diwu, J. J. Good, V. H. DiStefano and T. E. Albrecht-Schmitt, Eur. J. Inorg. Chem., 2011, 1374 CrossRef
; R. Das, R. Sarma and J. B. Baruah, Inorg. Chem. Commun., 2010, 13, 793 CrossRef
.
- Y. Liu, Z. Lin, C. He, L. Zhao and C. Duan, Dalton Trans., 2010, 39, 11122 RSC
; L. Zhao, S. Qu, C. He, R. Zhang and C. Duan, Chem. Commun., 2011, 47, 9387 RSC
; H. Hou, Y. Wei, Y. Song, Y. Fan and Y. Zhu, Inorg. Chem., 2004, 43, 1323 CrossRef CAS PubMed
; Y. Jiao, J. Zhang, L. Zhang, Z. Lin, C. He and C. Duan, Chem. Commun., 2012, 48, 6022 RSC
.
- I. L. Malaestean, A. Ellern, S. Bacaa and P. Kögerler, Chem. Commun., 2012, 48, 1499 RSC
.
- A. S. R. Chesman, D. R. Turner, B. Moubaraki, K. S. Murray, G. B. Deacon and S. R. Batten, Chem. – Eur. J., 2009, 15, 5203 CrossRef CAS PubMed
.
- B. F. Moore, G. A. Kumar, M.-C. Tan, J. Kohl, R. E. Riman, M. G. Brik, T. J. Emge and J. G. Brennan, J. Am. Chem. Soc., 2011, 133, 373 CrossRef CAS PubMed
.
- R. Wang, H. D. Selby, H. Liu, M. D. Carducci, T. Jin, Z. Zheng, J. W. Anthis and R. J. Staples, Inorg. Chem., 2002, 41, 278 CrossRef CAS PubMed
.
- M. A. Palacios, E. M. Pineda, S. Sanz, R. Inglis, M. B. Pitak, S. J. Coles, M. Evangelisti, H. Nojiri, C. Heesing, E. K. Brechin, J. Schnack and R. E. P. Winpenny, ChemPhysChem, 2016, 17, 55 CrossRef CAS PubMed
.
- S. Hino, M. Maeda, K. Yamashita, Y. Kataoka, M. Nakano, T. Yamamura, H. Nojiri, M. Kofu, O. Yamamuro and T. Kajiwara, Dalton Trans., 2013, 42, 2683 RSC
; A. Ben Khélifa, M. Salah Belkhiria, G. Huang, S. Freslon, O. Guillou and K. Bernot, Dalton Trans., 2015, 44, 16458 RSC
.
- J. D. Rinehart and J. R. Long, Chem. Sci., 2011, 2, 2078 RSC
.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic details, crystallographic information and magnetic data. CCDC 1545734. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7dt01883e |
‡ Crystal data for 1: C192H248.60Ce21N21O118.80, M = 7694.02, trigonal, space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) , a = 20.830(5) Å, α = 106.55(3)°, V = 7617(6) Å3, Z = 1, T = 100 K, R1(I > 2σ) = 0.068 and wR2(all data) = 0.198 for 35 , a = 20.830(5) Å, α = 106.55(3)°, V = 7617(6) Å3, Z = 1, T = 100 K, R1(I > 2σ) = 0.068 and wR2(all data) = 0.198 for 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 039 reflections collected, 8254 observed reflections (I > 2σ(I)) of 14 039 reflections collected, 8254 observed reflections (I > 2σ(I)) of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 429 (Rint = 0.054) unique reflections, GOF = 1.11. CCDC reference number: 1545734. 429 (Rint = 0.054) unique reflections, GOF = 1.11. CCDC reference number: 1545734. |
| This journal is © The Royal Society of Chemistry 2017 |

