Open Access Article
Open Access ArticleTwo excellent phase-matchable infrared nonlinear optical materials based on 3D diamond-like frameworks: RbGaSn2Se6 and RbInSn2Se6†
Hua
Lin
ab,
Hong
Chen
ab,
Yu-Jun
Zheng
abc,
Ju-Song
Yu
abc,
Xin-Tao
Wu
a and
Li-Ming
Wu
 *ab
*ab
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, People's Republic of China
bKey Laboratory of Optoelectronic Materials Chemistry and Physics, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, People's Republic of China. E-mail: liming_wu@fjirsm.ac.cn; Tel: +(011)86-591-63173130
cUniversity of Chinese Academy of Sciences, Beijing 100039, People's Republic of China
First published on 10th May 2017
Abstract
Mid- and far-infrared (MFIR) nonlinear optical (NLO) crystals with excellent performances are critical to laser frequency-conversion technology. However, the current commercial MFIR NLO crystals, including AgGaS2 (AGS), AgGaSe2 and ZnGeP2, suffer from certain intrinsic drawbacks and cannot achieve a good balance between large second-harmonic generation (SHG) efficiency and high laser-induced damage thresholds (LIDTs). Herein, we report two new phase-matchable MFIR NLO chalcogenides, specifically RbXSn2Se6 (X = Ga, In), which were successfully synthesized by high-temperature solid-state reactions. The remarkable structural feature of these materials was their 3D diamond-like framework (DLF) stacked by M3Se9 (M = X/Sn) asymmetric building units of vertex-sharing MSe4 tetrahedra along the c axis. Significantly, both of the materials showed the excellent NLO performances with the desired balance between their large SHG efficiencies (4.2 and 4.8 × benchmark AGS) and large LIDTs (8.9 and 8.1 × benchmark AGS), demonstrating that the title compounds meet the crucial conditions as promising MFIR NLO candidates. Furthermore, the crystal structures, synthesis, and theoretical analysis, as well as optical properties are presented herein.
Introduction
In solid-state laser frequency-conversion techniques, second-order nonlinear optical (NLO) crystals are key optoelectronic functional materials based on second-harmonic generation (SHG). Moreover, they also have very important applications in many fields, such as resource exploration, military communications, space anti-missiles and electro-optical countermeasures.1 In general, the state-of-the-art NLO materials can be divided into four classes based on their working wavelength ranges: deep-ultraviolet (DUV, below 200 nm), ultraviolet–visible (UV–vis), near-infrared (IR) and mid-/far-infrared (MFIR) materials. To date, numerous well-known NLO crystals have been discovered that satisfy the practical requirements in the UV–vis and near-IR regions, such as LiB3O5 (LBO),2 β-BaB2O4 (BBO),3 KH2PO4 (KDP)4 and KTiOPO4 (KTP).5 In contrast, materials working in the other two regions are still extremely rare. For example, KBe2BO3F2 (KBBF) is the only practically usable material in the DUV region, but it has a serious layer growth habit and the constituent beryllium is highly toxic, which limits its wide application.6 In addition, only a few commercial NLO crystals, including AgGaS2 (AGS), AgGaSe2 and ZnGeP2, are available in the MFIR region, but unfortunately, they suffer from certain inherent drawbacks, such as a low laser-induced damage threshold (LIDT) and the two-photon absorption of 1 μm laser, which seriously hinders their applications.7 Therefore, the search for new second-order MFIR NLO materials with excellent performances is extremely urgent. Usually, the desirable properties for an MFIR NLO material include the following conditions: (1) large SHG efficiency; (2) high LIDT; (3) moderate optical birefringence (0.04 < Δn < 0.10); (4) broad MFIR transparency region; (5) good physico-chemical properties and thermal stability.8Metal chalcogenides are the most promising materials for second-order NLO applications in the MFIR region due to their fascinating structural features, large SHG efficiency and wide optical transparency, and consequently they have attracted considerable research interest. A large number of non-centrosymmetric (NCS) metal chalcogenides with large SHG efficiencies have been explored and discovered during the last few decades.9–58 Among these, examples that have both a large SHG efficiency (>1 × AGS) and a high LIDT are notably rare, but include SnGa4Se7,31 PbGa2GeSe6,37 Na4MgGe2Se6,42 Na2Hg3M2S8 (M = Si, Ge, Sn),45 [AX3][Ga3PS8] (A = K, Rb; X = Cl, Br),48 Li4HgGe2S7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54 and NaGaIn2Se6
54 and NaGaIn2Se6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55 (a detailed comparison of the properties is displayed in Fig. 1). Moreover, research has shown that macroscopic polarization can be maximized only when the crystal structure belongs to one of the 10 polar point groups.59 As a consequence, the design and synthesis of novel NCS chalcogenides in the field of second-order MFIR NLO materials with a polar structure and good balance between a large SHG efficiency and high LIDT is a hot topic and remains a great challenge.
55 (a detailed comparison of the properties is displayed in Fig. 1). Moreover, research has shown that macroscopic polarization can be maximized only when the crystal structure belongs to one of the 10 polar point groups.59 As a consequence, the design and synthesis of novel NCS chalcogenides in the field of second-order MFIR NLO materials with a polar structure and good balance between a large SHG efficiency and high LIDT is a hot topic and remains a great challenge.
 | ||
| Fig. 1 Comparison of the properties in a series of outstanding MFIR NLO chalcogenides and AgGaS2 (AGS) as the reference. | ||
In an earlier study, we carried out exploratory synthesis on the quaternary alkali metals/transition metals/group 13 metals/chalcogenides (A/XII/XIII/Q) system and discovered a series of novel AM9Q12 (M = XII/XIII) polar compounds with a 3D diamond-like framework (DLF).60–64 Through a comprehensive analysis of the structure–property relationship, we found that 3D DLF structures can provide an adjustable platform for the rational and precise design and development of outstanding MFIR NLO materials. Recently, two new DLF compounds were discovered by our group, namely CsM3Se6 (M = Ga/Sn, In/Sn). Interestingly, compared with the non-phase-matchable (NPM) parent CsM9Se12, they exhibit the desired phase-matchable (PM) behaviour and good NLO properties.65 In this study, we extended the systematic exploratory synthesis to the substitutional flexibility based on the 3D DLF and the other filled alkali metals, resulting in the discovery of two new NCS members in this family, namely RbXSn2Se6 (X = Ga and In). Significantly, they showed excellent NLO performances with a desired balance between their large SHG efficiencies (4.2 and 4.8 × benchmark AGS) and large LIDTs (8.9 and 8.1 × benchmark AGS), demonstrating that the title compounds meet the crucial conditions as promising MFIR NLO candidates. Furthermore, the crystal structures, synthesis and theoretical analysis as well as optical properties are presented herein.
Experimental
Syntheses
The following reactant was used as purchased and stored in a glovebox filled with purified Ar (where the moisture and oxygen level was less than 0.1 ppm), and all the manipulations were performed inside the glovebox. Pure phases of the title compounds were synthesized by a solid-state reaction technique. Based on a large number of explorations on the experimental conditions, including changing the annealing temperature, starting reactant and loading ratio, the optimal synthesis route was determined as following: the mixture of RbCl (3 N), X (X = Ga or In, 5 N), Sn (5 N) and Se (5 N) in the molar ratio of 2/1.5/1.625/6 was placed into fused-silica tubes under vacuum, and annealed at 973 K for 50 h, and then kept at this temperature for 100 h, followed by slow cooling at 3 K h−1 to 473 K, at which point the furnace was turned off. The raw products were washed with distilled water and then dried with ethanol. Deep-red crystals in the millimetre size were obtained (see Fig. S1 in ESI†). Analyses of these compounds with an EDX-equipped JSM6700F FESEM showed the presence of Rb, X, Sn and Se in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, but no other elements (see Fig. S2 in the ESI†). The homogeneous target products were then analyzed using a Rigaku DMAX 2500 diffractometer with Cu-Kα radiation (see Fig. 2). The title compounds were stable in air for more than 5 months.
6, but no other elements (see Fig. S2 in the ESI†). The homogeneous target products were then analyzed using a Rigaku DMAX 2500 diffractometer with Cu-Kα radiation (see Fig. 2). The title compounds were stable in air for more than 5 months.
Property characterizations
The solid-state optical absorption spectra were obtained at room temperature using a PerkinElmer Lambda 950 UV–Vis spectrophotometer. The thermal stability analyses were measured on a NETZSCH STA 449C simultaneous analyser. Powder SHG measurements were performed on a modified Kurtz-NLO system66 using 2.05 μm laser radiation and the particle size of the title compounds and AgGaS2 (as a reference) ranged from 30–210 μm for the measurement, which was carried out as described elsewhere.60–65 The single-pulse measurement method was used to evaluate the powder LIDTs of the polycrystalline title compounds with a AgGaS2 single crystal as the reference (1 × 1 × 2 cm3 single crystals supplied from the Anhui Institute of Optics and Fine Mechanics Chinese Academy of Sciences). Each sample was sieved in the size range of 150–210 μm, and packed into a plastic holder (diameter: 8 mm) with a thickness of about 1 mm. After irradiation by a high-power 1064 nm laser with a pulse width τp of 8 ns, apparent changes of the sample were monitored using an optical microscope. The power of the laser beam was measured by a Nova II sensor with a PE50-DIF-C energy sensor, and the size of the damage spot was measured by a Vernier calliper.Single-crystal X-ray diffraction (XRD)
Single-crystal XRD at room temperature was collected on a Mercury 70 CCD diffractometer with Mo Kα radiation. Absorption correction was carried out67 and the structures were solved by direct methods and refined using the SHELX-97 software.68 The structural refinement was performed with a similar method as that for the BaGa2SnSe6 compound,35 with the difference that the X (X = Ga or In) and Sn atoms were constrained to share the same crystallographic site. During the refinement, the occupancies were restricted by using “EXYZ” and “EADP” commands in SHELX with a fixed X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sn ratio of 1
Sn ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to keep the charge balance. The X
2 to keep the charge balance. The X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sn ratio was also supported by the EDX results. The good matching of the experimental and simulated PXRD patterns together with the observed strong SHG intensity substantiated that the R3 space group was correct. The refinement data are listed in Tables 1 and S1–2.†
Sn ratio was also supported by the EDX results. The good matching of the experimental and simulated PXRD patterns together with the observed strong SHG intensity substantiated that the R3 space group was correct. The refinement data are listed in Tables 1 and S1–2.†
| Formula | RbGaSn2Se6 | RbInSn2Se6 |
|---|---|---|
| a R 1 = ∑||Fo| − |Fc||/∑|Fo|, wR2 = [∑w(Fo2 − Fc2)2/∑w(Fo2)2]1/2. | ||
| fw | 866.33 | 911.43 |
| Crystal system | Trigonal | Trigonal |
| Crystal colour | Deep-red | Deep-red |
| Space group | R3 (no. 146) | R3 (no. 146) |
| a (Å) | 10.4697(2) | 10.6044(8) |
| c (Å) | 9.476(2) | 9.660(2) |
| V (Å3) | 899.5(3) | 940.7(2) |
| Z | 3 | 3 |
| D c (g cm−3) | 4.798 | 4.832 |
| R int | 0.0349 | 0.0326 |
| μ (mm−1) | 28.54 | 26.98 |
| GOOF on F2 | 1.025 | 1.015 |
| R 1, wR2 (I > 2σ(I))a | 0.0228, 0.0400 | 0.0254, 0.0483 |
| R 1, wR2 (all data) | 0.0244, 0.0402 | 0.0274, 0.0487 |
| Largest diff. peak and hole (e Å−3) | 0.807, −0.903 | 1.145, −1.010 |
Computational sections
Theoretical studies were performed by DFT69a with the generalized gradient approximation (GGA)69b as implemented in the VASP.69c The plane-wave basis set with projector augmented wave (PAW),69d,e potentials was used to represent the core electrons. The plane-wave cut-off energy of 600 eV was chosen for all the calculations and denser κ-point grids of 0.02 Å−1 were utilized in the optical property calculations. According to the single-crystal structure refinement results, X and Sn atoms share the 9b Wyckoff sites M with an X occupancy of about 1/3, and therefore, in total, 12 possible models were designed.70 The calculation model was built in the most energetically favourable manner. For calculating the optical properties, scissors operators were applied for the title compounds and AgGaS2 (AGS). The second-order nonlinear susceptibility χabc(−2ω,ω,ω) was calculated through the so-called length-gauge formalism.71 The calculation model and specific parameter settings were as described in our previously reported paper.65Results and discussion
Crystal structure
The single-crystal XRD data revealed that the title compounds are uniaxial crystals and crystallize in the NCS polar trigonal space group R3 (no. 146, Person symbol hR10) with a = 10.4697(2) Å, c = 9.476(2) Å and Z = 3 for RbGaSn2Se6, and a = 10.6044(8) Å, c = 9.660(2) Å and Z = 3 for RbInSn2Se6, which belong to the BaGa2SnSe6-structure type.35 In the asymmetric unit, there is one crystallographic Rb atom (Wyckoff site, 3a) and one M position (Wyckoff site, 9b) randomly occupied by both Ga/In and Sn in the molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and two Se atoms (Wyckoff sites, 9b and 9b), as listed in Table S1.† The remarkable structures of the 3D diamond-like frameworks (DLFs) are formed by the tri-nuclear secondary basic structure unit [M3Se9] (M = X/Sn), which is constructed with three vertex-sharing [MSe4] tetrahedra (see Fig. 3a and b). The normal M–Se distances of 2.4796(9)–2.5471(9) Å (see Table S2 in the ESI†) are consistent with those of BaGa2SnSe6 (M–Se: 2.35570(7)–2.4501(7) Å),35 CsGaSn2Se6 (M–Se: 2.4812(6)–2.4949(8) Å)65 and CsInSn2Se6 (M–Se: 2.5408(7)–2.5493(8) Å).65 Furthermore, the Rb atom fills with 3D DLFs (Fig. 3a) and centres the Se12 cuboctahedron (Fig. 3c). The Rb–Se interatomic distances vary from 3.8290(9) to 3.9462 (8) Å, which are a little longer than those observed in related compounds, such as RbCd4Ga5Se12 (3.804(2)–3.864(9) Å),61 RbCd4In5Se12 (3.872(2)–3.915(8) Å)61 and RbMn4In5Se12 (3.872(8)–3.901(2) Å).61 Besides, it should be emphasized that the identity of the alkali metal on going from Rb+ to Cs+ does not significantly affect the 3D DLF structures, but increases the NLO property of this family of compounds, as discussed below.
2, and two Se atoms (Wyckoff sites, 9b and 9b), as listed in Table S1.† The remarkable structures of the 3D diamond-like frameworks (DLFs) are formed by the tri-nuclear secondary basic structure unit [M3Se9] (M = X/Sn), which is constructed with three vertex-sharing [MSe4] tetrahedra (see Fig. 3a and b). The normal M–Se distances of 2.4796(9)–2.5471(9) Å (see Table S2 in the ESI†) are consistent with those of BaGa2SnSe6 (M–Se: 2.35570(7)–2.4501(7) Å),35 CsGaSn2Se6 (M–Se: 2.4812(6)–2.4949(8) Å)65 and CsInSn2Se6 (M–Se: 2.5408(7)–2.5493(8) Å).65 Furthermore, the Rb atom fills with 3D DLFs (Fig. 3a) and centres the Se12 cuboctahedron (Fig. 3c). The Rb–Se interatomic distances vary from 3.8290(9) to 3.9462 (8) Å, which are a little longer than those observed in related compounds, such as RbCd4Ga5Se12 (3.804(2)–3.864(9) Å),61 RbCd4In5Se12 (3.872(2)–3.915(8) Å)61 and RbMn4In5Se12 (3.872(8)–3.901(2) Å).61 Besides, it should be emphasized that the identity of the alkali metal on going from Rb+ to Cs+ does not significantly affect the 3D DLF structures, but increases the NLO property of this family of compounds, as discussed below.
Optical properties and thermal stabilities
According to the solid-state diffuse-reflectance UV–vis/near-IR spectra at room temperature, the results show that the polycrystalline title compounds possess semiconducting band gaps of 1.80 eV (for RbGaSn2Se6) and 1.92 eV (for RbInSn2Se6), which are consistent with their deep-red colours (Fig. 4). The TG-DTA measurement results of the title compounds in nitrogen are shown in Fig. S3.† As can be seen, the thermal stabilities of the title compounds can be up to 966 K (for RbGaSn2Se6) and 951 K (for RbInSn2Se6), and furthermore, the endothermic peak indicates that these compounds decompose after this temperature. This is in accordance with the PXRD analysis (detailed information is given in Fig. S4†), and such similar examples are also observed in the CsXSn2Se6 (X = Ga, In) compounds.65NLO properties and powder LIDTs
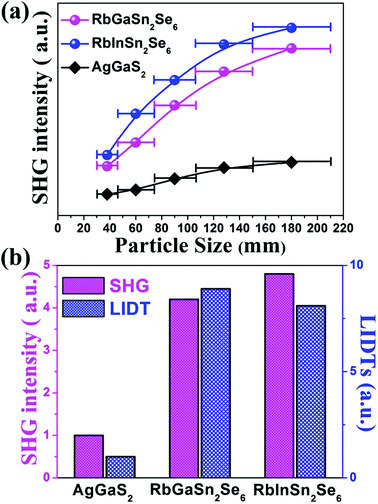
The NLO properties of the title compounds were studied due to the NCS polar space group R3. The powder SHG of RbGaSn2Se6 and RbGaSn2Se6 were investigated with a 2050 nm Q-switch laser and using AgGaS2 as the ref. 66. The particle size versus the SHG intensity curve is illustrated in Fig. 5a. The SHG intensities of the title compounds increased with the increasing particle size, with the peak realized at the particle size of 150–210 μm indicating a PM nature.59 Remarkably, the powder SHG intensities of RbGaSn2Se6 and RbGaSn2Se6 were 4.2 and 4.8 times that of the reference AgGaS2 at the same particle size, respectively (Fig. 5b, pink bar). More importantly, RbInSn2Se6 represents the second strongest powder SHG among the state-of-the-art PM chalcogenides reported to date. Powder LIDT data of the title compounds and benchmark AgGaS2 were collected using the single-pulse powder LIDT method.28 As illustrated in Fig. 5b and Table 2, the title compounds show high LIDTs at an incident laser of 1064 nm, namely 12.82, 11.66 MW cm−2, which are estimated to be 8.9 and 8.1 times greater than that of the benchmark AgGaS2, 1.44 MW cm−2. All of the above results distinguish RbGaSn2Se6 (X = Ga, In) as one of the best MFIR NLO materials known to date.| RbGaSn2Se6 | RbInSn2Se6 | AgGaS2 | |
|---|---|---|---|
| a Calculated at 2050 nm. b Measured on ground polycrystalline samples. c Measured in the particle size range 150–210 μm. | |||
SHG coefficient dij![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (pm V−1) a (pm V−1) |
d 11 = 43.9 | d 11 = 48.8 | d 36 = 18.2 |
| d 15 = 51.5 | d 15 = 60.2 | ||
| d 22 = 23.7 | d 22 = 29.3 | ||
| d 33 = 26.3 | d 33 = 20.4 | ||
| Band-gap (Eg/eV)b | 1.92 | 1.80 | 2.56 |
| Birefringencea | 0.051 | 0.067 | 0.039 |
| Powder SHG intensitiesc | 4.2 × AGS | 4.8 × AGS | AGS |
| Powder LIDTs (MW/cm2)c | 12.82 (8.9 × AGS) | 11.66 (8.1 × AGS) | 1.44 (AGS) |
In the AInSn2Se6 (A = Rb, Cs) compounds, it is noteworthy that the ionic nature of the alkali metal cations does not affect the overall polarity of the structure but significantly affects the SHG and LIDT performances. Taking AInSn2Se6 as an example, the ionic radii only decrease about 8.5% from Cs+ to Rb+ (rCs+ = 1.88 Å and rRb+ = 1.72 Å for CN = 12, respectively), while the powder SHG intensity increase 20% (4.0 × AGS vs. 4.8 × AGS for CsInSn2Se6 and RbInSn2Se6, respectively) and the LIDT decrease 14% (9.2 × AGS vs. 8.1 × AGS for CsInSn2Se6 and RbInSn2Se6, respectively). A similar trend has been seen in some other alkali metal-containing chalcogenides, e.g. A4GeP4Se12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 and AM9Se12.60–64
25 and AM9Se12.60–64
Theoretical studies
In order to understand the structure–property relationship of the title compounds, the linear and nonlinear optical properties were studied with the aid of the ab initio calculations performed by VASP software. The electronic band structures indicated direct band gaps of 1.51 and 1.26 eV for RbGaSn2Se6 and RbGaSn2Se6, respectively (Fig. S5 in ESI†). Such a discrepancy was likely due to a common problem that occurs with GGA calculations.72 As shown in Fig. 6a, the calculated densities of states (DOSs) of the title compounds with the main contributions are similar near the Fermi level. In addition, the origin of the SHG response (Fig. S6 in the ESI†) and the cut-off energy dependence of the largest second-order tensor d15 were also investigated. Fig. 6b shows that in the regions of VB-1 (dominated by the Se-4p and Sn-5p orbitals) and CB-2 (dominated by the Se-4p, Sn-5s and Ga(In)-ns/np orbitals), the d15 values are the most sharply increased, which contributes mainly to the second-order NLO susceptibility. In other words, the NLO activities of the title compounds originate from the condensation of the MSe4 (M = Ga, In) tetrahedral units that built the 3D DLF structures. The calculated d15 values of RbGaSn2Se6 and RbGaSn2Se6 were 51.5 and 60.2 pm V−1 at the wavelength of 2.05 μm (i.e. 0.61 eV), respectively. These values were 2.8 and 3.3 times larger than that of AgGaS2 (d36 = 18.2 pm V−1) at the same wavelength (Fig. 7) and were close to the experimental measurements (4.2 or 4.8 times stronger than AgGaS2 at the range 150–210 μm, see Fig. 5). | ||
| Fig. 7 Calculated frequency-dependent SHG coefficients for RbXSn2Se6 (X = Ga, In) and AgGaS2 (reference). | ||
As shown in Fig. 8, the calculated birefringence (Δn) values of RbGaSn2Se6 and RbInSn2Se6 are 0.051 and 0.067 at the wavelength of 2050 nm (0.61 eV), respectively, both of which are larger than that of AgGaS2 (0.039) and are in an optimal range (0.04 < Δn < 0.10),8 indicating that the title compounds can easily achieve the PM feature in the MFIR range. Such results are similar to those of the Cs-members in this family65 and better than BaGa2XSe6 (X = Si, Ge, Sn) at the same wavelength (Δn > 0.10),26,35 because having too large a Δn will cause issues with two main drawbacks: walk-off effects and self-focus in the conversion process.8 Besides, several key parameters are summarized in Table 2, indicating that the title compounds satisfy the key requirements needed as promising MFIR NLO candidates.
Conclusions
In summary, two new quaternary NLO-active selenides, RbXSn2Se6 (X = Ga, In), were discovered using the reactive flux method. They crystallized in the polar space group R3 and their structures exhibited a 3D diamond-like framework (DLF) composed of MSe4 (M = X/Sn) units that were stacked up by sharing common Se atoms. Remarkably, they displayed excellent NLO performances with concurrently strong SHG efficiencies and large LIDTs, as well as phase-matching features. In particular, RbInSn2Se6 (4.8 × AGS) represents the second strongest powder SHG among the state-of-the-art chalcogenides reported to date in the particle size 150–210 μm. DFT studies were carried out to aid the understanding of the electronic structures and linear and NLO properties. Moreover, the title compounds showed other essential requirements as promising MFIR NLO materials, including a moderate optical birefringence (Δn = 0.051 and 0.067) and good thermal stability (up to 950 K). All these results indicate that the title compounds can be good candidates for MFIR NLO materials. Follow-on efforts to grow large-size crystals are in progress. Furthermore, based upon analyzing their structure–property relationship, this new type of 3D DLF structure represents an unprecedented opportunity for the rational design and development of excellent MFIR NLO materials.Acknowledgements
This work was supported by the NSF of China (21301175, 21233009, 21571020 and 91422303) and the NSF of Fujian Province (2015J01071). We thank Professor Ge Zhang and Doctor Bing-Xuan Li (FJIRSM, CAS) for their help with NLO measurements and Professor Yong-Fan Zhang (Fuzhou University) helping with the DFT calculations.Notes and references
- (a) D. N. Nikogosyan, Nonlinear Optical Crystals: A Complete Survey, Springer-Science, New York, 2005 Search PubMed; (b) Structure–Property Relationships in Nonlinear Optical Crystals II The IR Region, in Structure and Bonding, ed. X.-T. Wu and L. Chen, Series ed. D. M. Mingos, Springer, New York, 2012, vol. 145 Search PubMed; (c) I. Chung and M. G. Kanatzidis, Chem. Mater., 2014, 26, 849–869 CrossRef CAS; (d) C. L. Hu and J. G. Mao, Coord. Chem. Rev., 2015, 288, 1–17 CrossRef CAS; (e) Y. Wang and S. L. Pan, Coord. Chem. Rev., 2016, 323, 15–35 CrossRef CAS; (f) F. Liang, L. Kang, Z. S. Lin, Y. C. Wu and C. T. Chen, Coord. Chem. Rev., 2017, 333, 57–70 CrossRef CAS; (g) S. P. Guo, Y. Chi and G. C. Guo, Coord. Chem. Rev., 2017, 335, 44–57 CrossRef CAS.
- C. T. Chen, Y. C. Wu, A. D. Jiang, B. Wu, G. M. You, R. K. Li and S. J. Lin, J. Opt. Soc. Am. B, 1989, 6, 616–621 CrossRef CAS.
- C. T. Chen, B. C. Wu, A. D. Jiang and G. M. You, Sci. Sin., Ser. B, 1985, 28, 235–243 Search PubMed.
- J. F. Ward and P. A. Franken, Phys. Rev., 1964, 133, A183–A190 CrossRef.
- K. Kato, IEEE J. Quantum Electron., 1991, 27, 1137–1140 CrossRef CAS.
- (a) Y. N. Xia, C. T. Chen, D. Y. Tang and B. C. Wu, Adv. Mater., 1995, 7, 79–81 CrossRef CAS; (b) D. Cyranoski, Nature, 2009, 457, 953–955 CrossRef CAS PubMed.
- (a) A. Harasaki and K. J. Kato, Appl. Phys., 1997, 36, 700–703 CAS; (b) G. C. Catella, L. R. Shiozawa, J. R. Hietanen, R. C. Eckardt, R. K. Route, R. S. Feigelson, D. G. Cooper and C. L. Marquardt, Appl. Opt., 1993, 32, 3948–3951 CrossRef CAS PubMed; (c) G. D. Boyd, E. Buehler and F. G. Storz, Appl. Phys. Lett., 1971, 18, 301–304 CrossRef CAS.
- L. Kang, M. L. Zhou, J. Y. Yao, Z. S. Lin, Y. C. Wu and C. T. Chen, J. Am. Chem. Soc., 2015, 137, 13049–13059 CrossRef CAS PubMed.
- I. Chung, C. D. Malliakas, J. I. Jang, C. G. Canlas, D. P. Weliky and M. G. Kanatzidis, J. Am. Chem. Soc., 2007, 129, 14996–15006 CrossRef CAS PubMed.
- X. S. Lin, G. Zhang and N. Ye, Cryst. Growth Des., 2009, 9, 1186–1189 CAS.
- T. K. Bera, J. I. Jang, J. B. Ketterson and M. G. Kanatzidis, J. Am. Chem. Soc., 2009, 131, 75–77 CrossRef CAS PubMed.
- S. Banerjee, C. D. Malliakas, J. I. Jang, J. B. Ketterson and M. G. Kanatzidis, J. Am. Chem. Soc., 2008, 130, 12270–12272 CrossRef CAS PubMed.
- I. Chung, J. H. Song, J. I. Jang, A. J. Freeman, J. B. Ketterson and M. G. Kanatzidis, J. Am. Chem. Soc., 2009, 131, 2647–2656 CrossRef CAS PubMed.
- Q. C. Zhang, I. Chung, J. I. Jang, J. B. Ketterson and M. G. Kanatzidis, J. Am. Chem. Soc., 2009, 131, 9896–9897 CrossRef CAS PubMed.
- I. Chung, J. I. Jang, C. D. Malliakas, J. B. Ketterson and M. G. Kanatzidis, J. Am. Chem. Soc., 2010, 132, 384–389 CrossRef CAS PubMed.
- T. K. Bera, J. I. Jang, J. H. Song, C. D. Malliakas, A. J. Freeman, J. B. Ketterson and M. G. Kanatzidis, J. Am. Chem. Soc., 2010, 132, 3484–3495 CrossRef CAS PubMed.
- S. Banerjee, J. M. Szarko, B. D. Yuhas, C. D. Malliakas, L. X. Chen and M. G. Kanatzidis, J. Am. Chem. Soc., 2010, 132, 5348–5350 CrossRef CAS PubMed.
- J. Y. Yao, D. J. Mei, L. Bai, Z. S. Lin, W. L. Yin, P. Z. Fu and Y. C. Wu, Inorg. Chem., 2010, 49, 9212–9216 CrossRef CAS PubMed.
- X. M. Jiang, M. J. Zhang, H. Y. Zeng, G. C. Guo and J. S. Huang, J. Am. Chem. Soc., 2011, 133, 3410–3418 CrossRef CAS PubMed.
- M. C. Chen, L. H. Li, Y. B. Chen and L. Chen, J. Am. Chem. Soc., 2011, 133, 4617–4624 CrossRef CAS PubMed.
- H. J. Zhao, Y. F. Zhang and L. Chen, J. Am. Chem. Soc., 2012, 134, 1993–1995 CrossRef CAS PubMed.
- P. Yu, L. J. Zhou and L. Chen, J. Am. Chem. Soc., 2012, 134, 2227–2235 CrossRef CAS PubMed.
- M. C. Chen, L. M. Wu, H. Lin, L. J. Zhou and L. Chen, J. Am. Chem. Soc., 2012, 134, 6058–6060 CrossRef CAS PubMed.
- G. Zhang, Y. J. Li, K. Jiang, H. Y. Zeng, T. Liu, X. G. Chen, J. G. Qin, Z. S. Lin, P. Z. Fu, Y. C. Wu and C. T. Chen, J. Am. Chem. Soc., 2012, 134, 14818–14822 CrossRef CAS PubMed.
- C. D. Morris, I. Chung, S. Park, C. M. Harrison, D. J. Clark, J. I. Jang and M. G. Kanatzidis, J. Am. Chem. Soc., 2012, 134, 20733–20744 CrossRef CAS PubMed.
- W. L. Yin, K. Feng, R. He, D. J. Mei, Z. S. Lin, J. Y. Yao and Y. C. Wu, Dalton Trans., 2012, 41, 5653–5661 RSC.
- L. Kang, D. M. Ramo, Z. S. Lin, P. D. Bristowe, J. G. Qin and C. T. Chen, J. Mater. Chem. C, 2013, 1, 7363–7370 RSC.
- M. J. Zhang, X. M. Jiang, L. J. Zhou and G. C. Guo, J. Mater. Chem. C, 2013, 1, 4754–4760 RSC.
- Q. Wu, X. G. Meng, C. Zhong, X. G. Chen and J. G. Qin, J. Am. Chem. Soc., 2014, 136, 5683–5686 CrossRef CAS PubMed.
- Z. Z. Luo, C. S. Lin, W. L. Zhang, H. Zhang, Z. Z. He and W. D. Cheng, Chem. Mater., 2014, 26, 1093–1099 CrossRef CAS.
- Z. Z. Luo, C. S. Lin, H. H. Cui, W. L. Zhang, H. Zhang, Z. Z. He and W. D. Cheng, Chem. Mater., 2014, 26, 2743–2749 CrossRef CAS.
- J. A. Brant, D. J. Clark, Y. S. Kim, J. I. Jang, J. H. Zhang and J. A. Aitken, Chem. Mater., 2014, 26, 3045–3048 CrossRef CAS.
- K. Feng, L. Kang, Z. S. Lin, J. Y. Yao and Y. C. Wu, J. Mater. Chem. C, 2014, 2, 4590–4596 RSC.
- X. S. Li, L. Kang, C. Li, Z. S. Lin, J. Y. Yao and Y. C. Wu, J. Mater. Chem. C, 2015, 3, 3060–3067 RSC.
- X. S. Li, C. Li, P. F. Gong, Z. S. Lin, J. Y. Yao and Y. C. Wu, J. Mater. Chem. C, 2015, 3, 10998–11004 RSC.
- J. C. Syrigos, D. J. Clark, F. O. Saouma, S. M. Clarke, L. Fang, J. I. Jang and M. G. Kanatzidis, Chem. Mater., 2015, 27, 255–265 CrossRef CAS.
- Z. Z. Luo, C. S. Lin, H. H. Cui, W. L. Zhang, H. Zhang, H. Chen, Z. Z. He and W. D. Cheng, Chem. Mater., 2015, 27, 914–922 CrossRef CAS.
- W. H. Lai, A. S. Haynes, L. Frazer, Y. M. Chang, T. K. Liu, J. F. Lin, I. C. Liang, H. S. Sheu, J. B. Ketterson, M. G. Kanatzidis and K. F. Hsu, Chem. Mater., 2015, 27, 1316–1326 CrossRef CAS.
- A. S. Haynes, F. O. Saouma, C. O. Otieno, D. J. Clark, D. P. Shoemaker, J. I. Jang and M. G. Kanatzidis, Chem. Mater., 2015, 27, 1837–1846 CrossRef CAS.
- Y. F. Shi, Y. K. Chen, M. C. Chen, L. M. Wu, H. Lin, L. J. Zhou and L. Chen, Chem. Mater., 2015, 27, 1876–1884 CrossRef CAS.
- J. A. Brant, D. J. Clark, Y. S. Kim, J. I. Jang, A. Weiland and J. A. Aitken, Inorg. Chem., 2015, 54, 2809–2819 CrossRef CAS PubMed.
- K. Wu, Z. H. Yang and S. L. Pan, Inorg. Chem., 2015, 54, 10108–10110 CrossRef CAS PubMed.
- L. Chao, W. L. Yin, P. F. Gong, X. S. Li, M. L. Zhou, M. Arthur, Z. S. Lin, J. Y. Yao, Y. C. Wu and C. T. Chen, J. Am. Chem. Soc., 2016, 138, 6135–6138 CrossRef PubMed.
- A. S. Haynes, A. Banerjee, F. O. Saouma, C. O. Otieno, J. I. Jang and M. G. Kanatzidis, Chem. Mater., 2016, 28, 2374–2383 CrossRef CAS.
- K. Wu, Z. H. Yang and S. L. Pan, Chem. Mater., 2016, 28, 2795–2801 CrossRef CAS.
- K. Wu, Z. H. Yang and S. L. Pan, Angew. Chem., Int. Ed., 2016, 55, 6713–6715 CrossRef CAS PubMed.
- G. M. Li, K. Wu, Q. Liu, Z. H. Yang and S. L. Pan, J. Am. Chem. Soc., 2016, 138, 7422–7428 CrossRef CAS PubMed.
- B. W. Liu, H. Y. Zeng, X. M. Jiang, G. E. Wang, S. F. Li, L. Xu and G. C. Guo, Chem. Sci., 2016, 7, 6273–6277 RSC.
- Y. Y. Li, P. F. Liu, H. Lin, M. T. Wang and L. Chen, Inorg. Chem. Front., 2016, 3, 952–958 RSC.
- R. H. Duan, J. S. Yu, H. Lin, Y. J. Zheng, H. J. Zhao, S. X. Huang-Fu, M. A. Khan, L. Chen and L. M. Wu, Dalton Trans., 2016, 45, 12288–12291 RSC.
- M. L. Zhou, L. Kang, J. Y. Yao, Z. S. Lin, Y. C. Wu and C. T. Chen, Inorg. Chem., 2016, 55, 3724–3726 CrossRef CAS PubMed.
- W. L. Yin, A. K. Iyer, C. Li, J. Y. Yao and A. Mar, J. Mater. Chem. C, 2017, 5, 1057–1063 RSC.
- Y. Y. Li, J. Q. Wang, P. F. Liu, H. Lin, L. Chen and L. M. Wu, RSC Adv., 2017, 7, 8082–8089 RSC.
- K. Wu, Z. H. Yang and S. L. Pan, Chem. Commun., 2017, 53, 3010–3013 RSC.
- S. F. Li, X. M. Jiang, B. W. Liu, D. Yan, C. S. Lin, H. Y. Zeng and G. C. Guo, Chem. Mater., 2017, 29, 1796–1804 CrossRef CAS.
- G. M. Li, Q. Liu, K. Wu, Z. H. Yang and S. L. Pan, Dalton Trans., 2017, 46, 2778–2784 RSC.
- P. F. Liu, Y. Y. Li, Y. J. Zheng, J. S. Yu, R. H. Duan, H. Chen, H. Lin, L. Chen and L. M. Wu, Dalton Trans., 2017, 46, 2715–2721 RSC.
- Q. C. Zhang, I. Chung, J. I. Jang, J. B. Ketterson and M. G. Kanatzidis, Chem. Mater., 2009, 21, 12–14 CrossRef CAS.
- (a) K. M. Ok, E. O. Chi and P. S. Halasyamani, Chem. Soc. Rev., 2006, 35, 710–717 RSC; (b) E. A. Muller, R. J. Cannon, A. N. Sarjeant, K. M. Ok, P. S. Halasyamani and A. J. Norquist, Cryst. Growth Des., 2005, 5, 1913–1917 CrossRef CAS.
- H. Lin, L. J. Zhou and L. Chen, Chem. Mater., 2012, 24, 3406–3414 CrossRef CAS.
- H. Lin, L. Chen, L. J. Zhou and L. M. Wu, J. Am. Chem. Soc., 2013, 135, 12914–12921 CrossRef CAS PubMed.
- H. Lin, Y. Liu, L. J. Zhou, H. J. Zhao and L. Chen, Inorg. Chem., 2016, 55, 4470–4475 CrossRef CAS PubMed.
- H. Lin, H. Chen, Y. J. Zheng, J. S. Yu and L. M. Wu, Dalton Trans., 2016, 45, 17606–17609 RSC.
- H. Lin, Y. J. Zheng, H. Chen, X. N. Hu, J. S. Yu and L. M. Wu, Chem. – Asian J., 2017, 12, 453–458 CrossRef CAS PubMed.
- H. Lin, L. Chen, J. S. Yu, H. Chen and L. M. Wu, Chem. Mater., 2017, 29, 499–503 CrossRef CAS.
- S. K. Kurtz and T. T. Perry, J. Appl. Phys., 1968, 39, 3798–3813 CrossRef CAS.
- Crystal Clear, version 1.3.5, Rigaku Corp., The Woodlands, TX, 1999 Search PubMed.
- G. M. Sheldrick, SHELXTL, version 5.1, Bruker-AXS, Madison, WI, 1998 Search PubMed.
- (a) G. Kresse and J. Furthmuller, Phys. Rev. B: Condens. Matter, 1996, 54, 11169–11186 CrossRef CAS; (b) J. P. Perdew and Y. Wang, Phys. Rev. B: Condens. Matter, 1992, 45, 13244–13249 CrossRef; (c) G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter, 1999, 59, 1758–1775 CrossRef CAS; (d) P. E. Blöchl, Phys. Rev. B: Condens. Matter, 1994, 50, 17953–17979 CrossRef; (e) P. E. Blöchl, O. Jepsen and O. K. Andersen, Phys. Rev. B: Condens. Matter, 1994, 49, 16223–16234 CrossRef.
- R. Graucrespo, S. Hamad, C. R. A. Catlow and N. H. D. Leeuw, J. Phys.: Condens. Matter, 2007, 19, 219 Search PubMed.
- (a) C. Aversa and J. E. Sipe, Phys. Rev. B: Condens. Matter, 1995, 52, 14636–14645 CrossRef CAS; (b) S. N. Rashkeev, W. R. L. Lambrecht and B. Segall, Phys. Rev. B: Condens. Matter, 1998, 57, 3905–3919 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional crystallographic data, CIF files, TG-DTA, reflection and FT-IR spectra, calculation results, together with additional tables and figures. CCDC 1504918 and 1504919. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7dt01384a |
| This journal is © The Royal Society of Chemistry 2017 |