Open Access Article
Open Access ArticleMutual interference of Cu and Zn ions in Alzheimer's disease: perspectives at the molecular level
Elena
Atrián-Blasco†
 ab,
Amandine
Conte-Daban†
ab and
Christelle
Hureau
ab,
Amandine
Conte-Daban†
ab and
Christelle
Hureau
 *ab
*ab
aCNRS, LCC (Laboratoire de Chimie de Coordination), 205 route de Narbonne, BP 44099 31077 Toulouse Cedex 4, France. E-mail: christelle.hureau@lcc-toulouse.fr
bUniversity of Toulouse, UPS, INPT, 31077 Toulouse Cedex 4, France
First published on 22nd September 2017
Abstract
While metal ions such as copper and zinc are essential in biology, they are also linked to several amyloid-related diseases, including Alzheimer's disease (AD). Zinc and copper can indeed modify the aggregation pathways of the amyloid-β (Aβ) peptide, the key component encountered in AD. In addition, the redox active copper ions do produce Reactive Oxygen Species (ROS) when bound to the Aβ peptide. While Cu(I) or Cu(II) or Zn(II) coordination to the Aβ has been extensively studied in the last ten years, characterization of hetero-bimetallic Aβ complexes is still scarce. This is also true for the metal induced Aβ aggregation and ROS production, for which studies on the mutual influence of the copper and zinc ions are currently appearing. Last but not least, zinc can strongly interfere in therapeutic approaches relying on copper detoxification. This will be exemplified with a biological lead, namely metallothioneins, and with synthetic ligands.
Introduction
Alzheimer's disease (AD) is the most common neurodegenerative disease, with a prevalence of around 35 million patients worldwide.1 This number is expected to triple within the next 35 years, making AD a current major global public health problem. In the brain, one of the hallmarks of the disease is the extracellular accumulation of Amyloid-β (Aβ) peptides into senile plaques. The amyloid cascade hypothesis describes this process: Aβ is present in healthy brains in soluble and monomeric forms. In contrast, in AD brains, these peptides aggregate into oligomers and then fibrils which assemble themselves into so-called senile plaques.2,3 In the last few years, therapies based on inhibiting the aggregation of the Aβ peptides have failed clinical trials and, as a consequence, attention has been paid to other factors.4 Indeed, AD has been linked to the dyshomeostasis of both Cu(I/II) and Zn(II) ions.5 Regarding Zn(II) ions, both increased and decreased concentrations have been measured in AD brains compared to healthy brains.6 Similarly, Cu ions are present in a 2-fold higher concentration in the CNS6 and in higher concentrations in the hippocampus while intra-neuronal Cu(I) deficiency has been reported to be a key factor in AD.7,8 Important levels of these metal ions have also been found in the senile plaques: 0.4 mM of Cu and 1 mM of Zn(II).9,10 Consequently, these metal ions are supposed to play a key role in the aggregation of the Aβ peptides in vivo and thus in the associated amyloid cascade process.11,12 In addition, the literature reveals the impact of metal ions on the Aβ aggregation in vitro, although contradictory effects have been reported.11,12 They can modify either the kinetics of aggregation or the morphology of the aggregates. While there are many divergent reports on the impact of metal ions, two main consensual features are described: (i) the importance of the ratio between metal ions and peptides,11–13 and (ii) the different effects produced by Cu and Zn ions.11 Metal-induced aggregation is also described for other neurodegenerative diseases such as Parkinson's and Prion diseases, all characterized by misfolding of amyloidogenic proteins and metal ion dyshomeostasis.6,14In AD, another important consequence of this dyshomeostasis is the production of Reactive Oxygen Species (ROS) by the Cu–Aβ complex, which catalyses the reduction of O2, in the presence of a reductant such as ascorbate.9,15 Indeed, it has been proposed that superoxide, hydrogen peroxide and hydroxyl radicals, which are the resulting species of the incomplete reduction of O2, attack the surrounding biomolecules, thus participating in the global oxidative stress observed in AD.16
Within this context, metal chelation (using chelators)‡ or metal redistribution (using metallophores)‡ therapeutic approaches against AD have been under focus during the past few years.8,17 In general, Cu is considered as the most pertinent target due to the ROS related toxicity of this redox competent ion.8 The first generations of ligands‡ were capable of removing Cu(II) from Aβ peptides, stopping ROS production and favouring the disaggregation of Aβ senile plaques.8 Clioquinol and PBT2 have shown to be the best candidates and have gone under clinical trials, however, both have failed in phase II.18 To explain such unsuccessful results, it has first been proposed that these ligands cannot differentiate between the toxic Cu bound to Aβ and the Cu bound to essential metalloproteins. A new hypothesis relies on a too weak discrimination between the Cu(II) target and the Zn ions.19,20 In fact, the significance of the selectivity in the development of ligands in the wide context of the chelation therapy has already been reviewed.21,22 Particularly, the need for selectivity between the targeted toxic metal ion and other biological metal ions has been discussed for actinide decorporation,23 and the need for Cu/Zn selectivity in the case of Wilson's disease.24
Therefore, different approaches are under focus to find new generations of ligands:17,25 (i) prochelators,26 which can be activated by H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 or β-secretase28 directly in the brain; (ii) multi-target compounds, e.g. those with an Aβ recognition moiety and a chelating moiety;29,30 (iii) Cu(II)-chelators with a glucose moiety31 or nanoparticles32 as transporters through the Blood Brain Barrier.
27 or β-secretase28 directly in the brain; (ii) multi-target compounds, e.g. those with an Aβ recognition moiety and a chelating moiety;29,30 (iii) Cu(II)-chelators with a glucose moiety31 or nanoparticles32 as transporters through the Blood Brain Barrier.
It seems clear that the brain is a highly complex system, which also makes AD an intricate illness. There are many factors that could be implicated in the development of the disease, such as the different metal ions present in the synaptic cleft altogether with a wide range of biomolecules. The interaction of all of them could be relevant for the etiology, but trying to understand the basis requires starting by the simplest systems. For example, from a bio-inorganic chemistry approach of the disease, the effort was put on monometallic systems i.e. Cu–Aβ,10,33 or Zn–Aβ.34–36 Nevertheless, this is partially representative of the reality. Hence, a first step toward a more realistic picture would be to study the mutual influence of both metal ions in their interaction with the peptide at the molecular level, in order to understand the fundamental aspects of AD and to improve the development of ligands.
In this review, we report first the coordination chemistry of Aβ peptides with Cu(II), Cu(I) or Zn(II) and also of hetero-bimetallic species. The impact of the co-presence of Cu and Zn ions on the metal-induced Aβ aggregation and/or on the ROS production by the Cu–Aβ complex is then described. Finally, the importance of considering Zn in Cu-based chelation therapy is discussed. Personal points of view are also given about future research lines with respect to these three aspects.
Coordination chemistry of Cu and Zn to Aβ
Monometallic complexes
The amyloid-β (Aβ) peptide is a 40–42 amino-acid residue long peptide, of which the more hydrophobic region, the C-terminal, is prone to aggregation. The high affinity metal binding site of Aβ peptides is found at residues 1–16. For this reason, Aβ1–16 is proposed as a model for the coordination and redox properties of the full-length peptides. Coordination of Cu(II), Cu(I) and Zn(II) to the amyloid-β peptides has been extensively studied and probed by many different techniques. The most accepted coordination spheres for these metal ions, as well as their corresponding affinities, are summarized in Fig. 1. In the case of Cu(II), two different binding modes can be found at physiological pH, known as components I and II. They both show a distorted square-planar geometry and share the terminal amine as a ligand. Component I, which is predominant at lower pH, binds to Cu(II) through the N-terminal amine, the carbonyl from the amide bond of Asp1–Ala2, and the imidazole nitrogen (Nim) from two His residues, His6 and His13 or His14 in equilibrium. In component II, predominant at higher pH values, the nitrogen atom from the Asp1–Ala2 amide bond is deprotonated and binds to the Cu(II) ion, together with the N-terminal amine, the CO from the Ala2–Glu3 peptide bond and one imidazole group from a His residue.10,37 A second site has been described for the Aβ1–28 peptide but its low affinity would make it a biologically irrelevant coordination site.38 Cu(I) is bound in a linear fashion by two among the three possible Nim of His6, His13 and His14 in an equilibrium, in which the His13–His14 pair seems to be the preferred ligands.39–41 The first 16 amino acids are also involved in the coordination of Zn(II). Previous studies stated the involvement of the three histidine residues in the coordination site.42–48 The N-terminal amine and the Glu11 residue (among others) would be also involved in the coordination.34–36 A more recent study proposes a different coordination sphere deduced from the study by 1H-NMR and X-ray absorption spectroscopy of Aβ1–16 and a range of modified peptides.34 The Zn(II) ion has a tetrahedral binding to two histidine residues (His6 and His13 or His14), and two carboxylate residues (Glu11 and Asp1 or Glu3 or Asp7, with a preference for Asp1). In this case, the N-terminal amine does not coordinate to Zn(II) at physiological pH. This proposition is in line with previously reported affinity studies on the very same series of modified peptides.49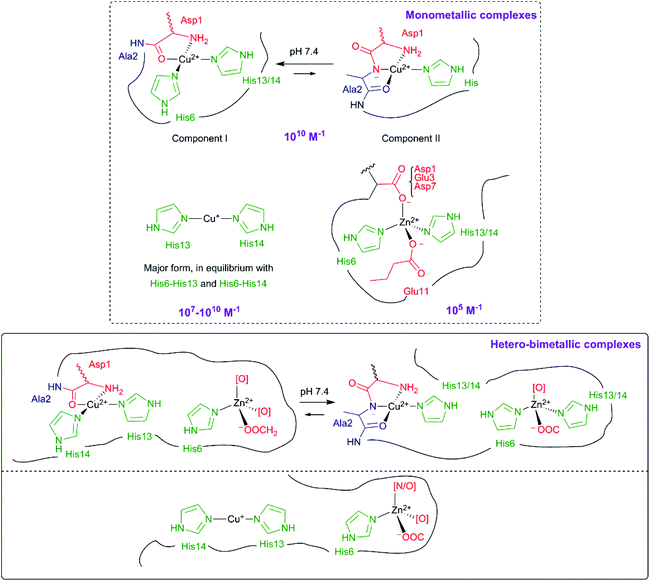 | ||
| Fig. 1 Proposed coordination modes of Cu(II),10 Cu(I),10 Zn(II)34 and the mixed metal Cu(II),Zn(II) or Cu(I),Zn(II) complexes52,53 based on experimental results and bibliographic data. Affinity constant values (Ka) at pH 7.4 for Cu(II),57 Cu(I)58,59 and Zn(II)49 are given in M−1 (bold, purple). | ||
Hetero-bimetallic complexes
A seminal competition study observed that Cu(II) displaced more than 95% of Zn(II) bound to Aβ.50 The first research regarding the simultaneous binding of Cu(II) and Zn(II) to Aβ peptides was performed by Damante et al.51 where they used the polyethylene glycol (PEG) conjugated peptide, which has enhanced water solubility and could aid in the spectroscopic studies. By using potentiometric measurements altogether with UV-Vis, CD and EPR spectroscopy studies, they shed light on the mutual interference of the metal ions. At pH >7, a hetero-bimetallic complex of Cu(II), Zn(II) and Aβ at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is formed. Addition of up to 4-fold equivalents of Zn(II) to a Cu(II)–Aβ solution shifts the copper binding mode from component I to component II (Fig. 1), while His13 and His14 would be involved in Zn(II) binding. The addition of Zn(II) would not release Cu(II), probably due to the high affinity of the peptide for Cu(II), but would modify its distribution in the available binding sites. Spectroscopic data obtained by Alies et al.52 support this hypothesis. Furthermore, Silva et al.53 have also corroborated the shift of components for Cu(II) bound to Aβ upon addition of increasing equivalents of Zn(II) (see Table 1). Contrary to the study of Damante et al.,51 they also probed a more important contribution of His13 and His14 as ligands for Cu(II) in the presence of Zn(II).53 In a further study, they proposed that Zn(II) and Cu(II) do compete for one binding site.54 However, this is in disagreement with the respective affinity values of Cu(II) and Zn(II) for the Aβ peptide that differ by at least three orders of magnitude (Fig. 1). The most likely explanation for such unexpected observation is linked to the experimental procedure that may force precipitation of either Zn(II)–Aβ or Cu(II)–Aβ and modify the associated thermodynamic equilibria.54 Indeed, with other sample preparation, only the formation of the hetero-bimetallic complex was observed.52 Alies et al.52 have investigated as well the Cu(I)/Zn(II) system which could be highly relevant in vivo. They used NMR spectroscopy and XANES to study the co-presence of Cu(I) and Zn(II). Indeed, XANES allows to distinguish the different oxidation states of the Cu ion and to observe the environments of Cu and Zn simultaneously since Cu and Zn K-edges are close enough to be recorded on the very same sample. While Zn(II) only slightly impacts the Cu(II) binding site (inducing a weak shift from component I to component II), both Cu(I) and Cu(II) influence the coordination sphere of Zn(II). Notably, in the case of the mixed Cu(I)/Zn(II) complex, Cu(I) remains coordinated to two histidine residues, leaving only one available for Zn(II) binding (Fig. 1).
1 is formed. Addition of up to 4-fold equivalents of Zn(II) to a Cu(II)–Aβ solution shifts the copper binding mode from component I to component II (Fig. 1), while His13 and His14 would be involved in Zn(II) binding. The addition of Zn(II) would not release Cu(II), probably due to the high affinity of the peptide for Cu(II), but would modify its distribution in the available binding sites. Spectroscopic data obtained by Alies et al.52 support this hypothesis. Furthermore, Silva et al.53 have also corroborated the shift of components for Cu(II) bound to Aβ upon addition of increasing equivalents of Zn(II) (see Table 1). Contrary to the study of Damante et al.,51 they also probed a more important contribution of His13 and His14 as ligands for Cu(II) in the presence of Zn(II).53 In a further study, they proposed that Zn(II) and Cu(II) do compete for one binding site.54 However, this is in disagreement with the respective affinity values of Cu(II) and Zn(II) for the Aβ peptide that differ by at least three orders of magnitude (Fig. 1). The most likely explanation for such unexpected observation is linked to the experimental procedure that may force precipitation of either Zn(II)–Aβ or Cu(II)–Aβ and modify the associated thermodynamic equilibria.54 Indeed, with other sample preparation, only the formation of the hetero-bimetallic complex was observed.52 Alies et al.52 have investigated as well the Cu(I)/Zn(II) system which could be highly relevant in vivo. They used NMR spectroscopy and XANES to study the co-presence of Cu(I) and Zn(II). Indeed, XANES allows to distinguish the different oxidation states of the Cu ion and to observe the environments of Cu and Zn simultaneously since Cu and Zn K-edges are close enough to be recorded on the very same sample. While Zn(II) only slightly impacts the Cu(II) binding site (inducing a weak shift from component I to component II), both Cu(I) and Cu(II) influence the coordination sphere of Zn(II). Notably, in the case of the mixed Cu(I)/Zn(II) complex, Cu(I) remains coordinated to two histidine residues, leaving only one available for Zn(II) binding (Fig. 1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn
Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aβ ratios, at pH 7.4
Aβ ratios, at pH 7.4
| Ref. | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ≥8 ≥8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
|---|---|---|---|---|---|
| 52 | ∼80/20 | 55/45 | 40/60 | — | — |
| 53 | 65/35 | 53/47 | 40/60 | 35/65 | 31/61 |
Kinetics of metal binding by Aβ peptides
Another important parameter to take into account would be the kinetics of the interaction between metal ions and Aβ peptides. A pioneering study by Pedersen et al.55 investigated the rate of the binding of Cu(II) to Aβ, and its impact on the aggregation. In the same context, a recent study by Branch et al.56 has measured the rate of Cu(II)–Aβ complex formation. The component I binding mode is very rapidly formed, while component II forms through component I as an intermediate. The association rates of Cu(II) and Aβ reported vary between 1 and 100 ms. As a consequence, the association between Aβ and Cu(II) is fast and is thus of biological relevance.Concluding remarks and prospective
All the studies (summarized in Table 2) agree on the mutual influence of Zn(II) and Cu(II) on their coordination. Zn(II) impacts the binding mode of Cu(II) at physiological pH as observed by the shift from component I to component II, whereas Cu(I) maintains its bis-His coordination sphere in the presence of Zn(II). Moreover, Zn(II) coordination is affected by the presence of both Cu(I) and Cu(II).Further studies would be needed to evaluate how the affinities of Cu(I/II) and Zn(II) are influenced by the co-presence of the metal ions and to determine the exact nature of the coordination spheres of Zn(II) in the Cu(I/II),Zn(II) hetero-bimetallic complexes. In addition, probing hetero-bimetallic species of other biologically relevant Aβ peptides (truncated peptides, murine Aβ and Familial Alzheimer's Disease mutants) is also of interest as well as the kinetics of interactions of Aβ with Cu(I), Aβ with Zn(II), and the mutual influence of Cu and Zn.
Aggregation and ROS production
Aggregation
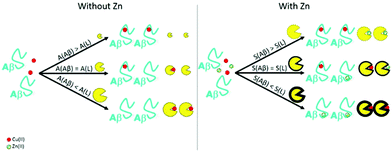
Aβ aggregation is the catalytic auto-assembly of the monomeric peptide (mathematically described by a sigmoid-shape curve).11 Metal-induced aggregation has been widely studied: different pH conditions, temperature, concentration, metal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) peptide ratio, etc. seem to have an influence on the aggregation rate and species formed. The influence of Cu(II) on the Aβ aggregation seems to be mainly dependent on its ratio (see Fig. 2, left),61 and may lead to the production of highly cytotoxic oligomers.55,62,63 In the case of aggregation in the presence of Zn(II), even small sub-stoichiometric quantities of the metal ion have an important influence on the aggregation of the Aβ peptides (see Fig. 2, right).60,63–68
peptide ratio, etc. seem to have an influence on the aggregation rate and species formed. The influence of Cu(II) on the Aβ aggregation seems to be mainly dependent on its ratio (see Fig. 2, left),61 and may lead to the production of highly cytotoxic oligomers.55,62,63 In the case of aggregation in the presence of Zn(II), even small sub-stoichiometric quantities of the metal ion have an important influence on the aggregation of the Aβ peptides (see Fig. 2, right).60,63–68
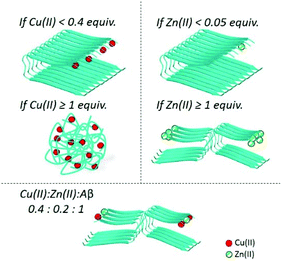 | ||
Fig. 2 This figure resumes the aggregation with different Cu(II)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn(II) Zn(II)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aβ ratios.60 Zn is the ion that dominates the Aβ metal-induced assembly even in the presence of Cu. For all of these aggregation experiments, metal ions have been added at the beginning, i.e. these are metal-induced aggregations. Aβ ratios.60 Zn is the ion that dominates the Aβ metal-induced assembly even in the presence of Cu. For all of these aggregation experiments, metal ions have been added at the beginning, i.e. these are metal-induced aggregations. | ||
Intra and inter-molecular Zn(II) binding promotes a fast aggregation of species different from apo-fibrils. In the pioneering work by Mayes et al.69, incubation of Aβ1–42 with equimolar quantities of Cu(II) and Zn(II) showed the coexistence of both small amorphous (non-fibrillar) aggregates and fibrils. Later, Matheou et al.60 showed that Zn(II) has a greater influence on the aggregation as, at even a quarter less of Zn(II) than Cu(II), the ThT aggregation curve was strongly affected and typical Zn(II)-induced aggregates could be observed by TEM (see Fig. 2, bottom). At the suprastoichiometric ratio of Cu(II) and/or Zn(II), Attanasio et al.70 reported the formation of non-fibrillar aggregates.
ROS production by monomeric species
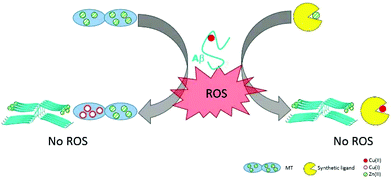
Since Zn(II) and Cu(I/II) have a clear mutual influence on their coordination to the Aβ peptides and on the metal-induced aggregation, Zn(II) could also have an impact on the ROS production by the Cu–Aβ complex. In this sense, a few studies have taken into account the co-presence of these metal ions and some of them have even indicated a protective role of Zn(II).71 The first studies on the protective role of Zn(II) in Cu(II)–Aβ-induced ROS production were carried out by Bush and co-workers.71 They found that the co-presence of Zn(II) improved the survival of cells incubated with Cu(II) and Aβ1–42 altogether with a decrease of the level of H2O2 production. They proposed a shift of the Cu ion in the peptide apart from its redox-active binding site as the origin of the redox-silencing properties associated with Zn(II). As it has been reported later, Zn(II) effectively displaces Cu(II) to its component II binding mode (see the previous paragraph); however, Alies et al.52 showed that this has no impact on the ROS production as monitored by ascorbate consumption and H2O2 production (two of the most used methods to probe the production of ROS by Cu–peptide systems, see Fig. 3). In other words, while Zn(II) impacts the coordination of Cu(II) to Aβ, its addition at the stoichiometric ratio has no effect on the Cu–Aβ-induced ROS production and associated cellular toxicity (Fig. 4A). This is in line with a recent study proposing that, in contrast to what can be expected based on the redox potential of Cu(II) in component I or II,75 the coordination mode of Cu(II) is not the predominant factor in ROS production.76 Other factors such as the metal ion ratio or aggregation states might influence the ROS production by mixed metal complexes and it may be of interest to further investigate them as well.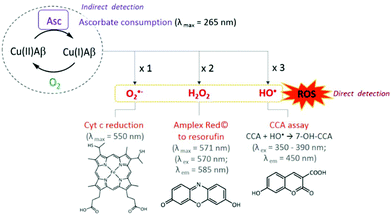 | ||
| Fig. 3 ROS production from Cu–Aβ complexes and the classical assays for the detection of ROS produced15 (O2˙−,72 H2O2,52 HO˙,73) and indirect monitoring of Asc consumption.74 | ||
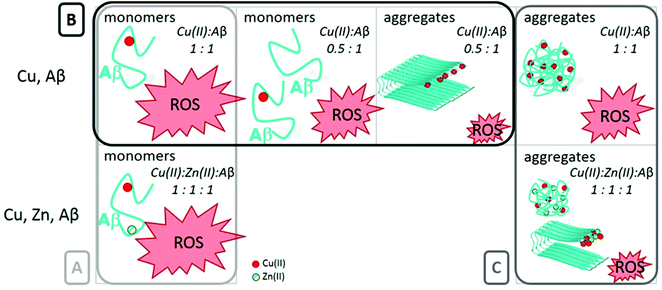 | ||
Fig. 4 Panel A. This part represents the identical ROS production of a monomeric monometallic Cu–Aβ complex and of a monomeric hetero-bimetallic Cu,Zn–Aβ complex.52 Panel B. This part represents the ROS production by different Cu(II)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aβ ratios and by different states of aggregation of the peptide: fibrils produce less ROS than monomers, for the 0.5 Aβ ratios and by different states of aggregation of the peptide: fibrils produce less ROS than monomers, for the 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio.52,78 Panel C. This last part compares the ROS production by amorphous aggregates of Cu(II)–Aβ to the ROS production of aggregates in the presence of Zn(II).69 Note that for all of the aggregation experiments, metal ions have been added at the beginning, i.e. these are metal-induced aggregations. 1 ratio.52,78 Panel C. This last part compares the ROS production by amorphous aggregates of Cu(II)–Aβ to the ROS production of aggregates in the presence of Zn(II).69 Note that for all of the aggregation experiments, metal ions have been added at the beginning, i.e. these are metal-induced aggregations. | ||
ROS-production by aggregated species
The relationship between the aggregation states of the Cu–Aβ complexes and their ROS production in vitro has been described in the literature. Cu–Aβ aggregated species are able to produce ROS,69 but at a lower level than the Cu–Aβ monomeric complex (see Fig. 4B).77,78 Mayes et al.69 have also incorporated into their research the ROS production of mixed metal Cu,Zn–Aβ aggregates (see Fig. 4C). They studied the degradation of H2O2 and the consequent HO˙ production by different Cu–Aβ and Cu,Zn–Aβ aggregated species. They observed that Aβ aggregates are capable of degrading H2O2 both when they have been formed in the presence of Cu(II) and when the metal has been added after fibril formation. In addition, increasing the ratio of Zn(II) over Cu(II)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aβ diminished the quantity and the rate at which H2O2 was degraded. They also corroborate the “antioxidant” effect associated with Zn(II) with less intense oxidation of the peptides, followed by carbonyl group formation indicative of peptide oxidation.
Aβ diminished the quantity and the rate at which H2O2 was degraded. They also corroborate the “antioxidant” effect associated with Zn(II) with less intense oxidation of the peptides, followed by carbonyl group formation indicative of peptide oxidation.
Concluding remarks
In this part, studies have shown that metal-induced aggregation and/or ROS production are already complex in monometallic systems for which there are many studies reported (see reviews:11,12,79). There are still not enough reported data on hetero-bimetallic systems to reach any kind of general conclusion about the mutual influence of Cu and Zn ions on aggregation and/or ROS production. Future studies should address this issue and include more detailed investigations on the impact of different factors such as Cu to Zn ratios, metal to peptide ratios, pH, mixture of Aβ peptides (Aβ1–40, Aβ4–40, Aβ11–40, etc.)… In this context, pioneering aggregation studies performed in CSF mimicking media70 or in CSF from AD patients are worth noting.80Besides, kinetic aspects may also be extremely important for aggregation. This includes determining how the metal (Zn(II) or Cu(II)) addition during the aggregation matters both for the kinetics of the aggregation process and for the morphology of the aggregates. Knowing the rate of Zn and/or Cu-induced structural rearrangement to aggregation prone species would also be of significance.
Metal ion-based therapy
Due to the important toxicity shown by Cu ions within the context of AD, many Cu(II) ligands have been developed in the past few years.17,25 They all can remove Cu(II) from Aβ peptides, and reduce its associated toxicity. One of the requirements for the design of ligands is their affinity towards Cu(II) compared to Aβ: it should be higher than that of the peptide (see Fig. 5, left). Nevertheless, their affinity should be lower than that of important metalloproteins, in order to avoid deleterious side effects. Furthermore, as previously mentioned, Zn(II) is present in high concentrations in the synaptic cleft and in the senile plaques and has an important impact on the Cu(II) coordination and the associated ROS production and Aβ aggregation. For this reason, some researchers have studied the impact of Zn(II) on the Cu(II) chelation therapy.Metallothioneins: a biological model
Vašák and co-workers have studied the impact of metallothionein 3 (Zn7-MT-3) on the toxicity of the Cu(II)–Aβ complex.81,82 There is a swap of metallic ions between the Aβ peptides and Zn7-MT-3 (see Fig. 6, left). The mechanism proposed includes the dissociation of the Cu(II)–Aβ complex and the chelation of the free metal ion by the metallothionein.83 Also, the formation of two disulphide bonds triggers the reduction of Cu(II) into Cu(I), forming an air-stable Cu(I)4-thiolate cluster inside the Cu(I)4Zn4MT-3 protein. The removal of Cu ions from the Aβ peptides and its consequent binding as an air-stable Cu(I)-complex silence the redox capability of Cu. Therefore, this swap has a protective effect against Cu(II)–Aβ toxicity.82 Another study by West's group84 focused on the metallothionein 2A (MT-2A) which is the major human-expressed subtype of metallothionein and, under stressful situations, it can be secreted near the synaptic cleft where aggregation of Aβ peptides occurs. West and his colleagues have demonstrated that under physiological conditions, Zn7-MT-2A can also remove Cu ions from Aβ, stop the ROS production and prevent changes in the metallic balance within neurons. When the same experiments were performed with the apo metallothionein, no impact on the Cu removal or on the diminution of the neurotoxicity was detected. Recently, Bal and co-workers have also studied the metal swap between Zn7-MT-3 and the truncated peptide Aβ4–16. In this case, the high affinity of the peptide prevents the sequestration of Cu(II) by the metallothionein.85 Besides, the impact of the Zn7-MT-3 has also been studied for the α-synuclein and the Prion proteins and the swap of metallic ions is observed. Cu(II) is sequestered by the metallothionein, silencing its redox activity and preventing the metal-induced aggregation of the protein.86,87Synthetic ligands and selectivity issue
Later, our group has highlighted the impact of the presence of Zn(II) within the Cu(II)-chelation context.19 Two synthetic chelators were tested, and while both are able to retrieve Cu(II) from Aβ, only one removes Cu(II) from Aβ in the presence of Zn(II). For the latter one and as in the case of Zn7-MT-3, there is a swap of metallic ions between the peptide and the chelator, and the resulting species cannot produce ROS (see Fig. 6, right). We propose the following explanation (based on thermodynamics only): a good chelator needs not only a high affinity constant for Cu(II) (see Fig. 5, left) but also high Cu(II) over Zn(II) selectivity, that corresponds to the ratio between its affinity constant for Cu(II) and for Zn(II) (see Fig. 5, right). Actually, the Cu(II) over Zn(II) selectivity of the chelator should overcome that of the Aβ peptides themselves, which is high (about 4 orders of magnitude, see Fig. 1).There are several studies reported in the literature that address the removal of Cu(II) or Zn(II) from Aβ,88–91 and some introduce the importance of having a high Cu(II) over Zn(II) selectivity for the ligand.92–94 However, to the best of our knowledge, the importance of overcoming the Cu(II) over Zn(II) selectivity of Aβ was not taken into account. In other words, the removal of Cu(II) from Aβ by a chelator in the presence of Zn(II) was not thoroughly described except in ref. 19. Nevertheless, Zn(II) interference in Cu(II) removal from Aβ should be taken into account as it can either promote protective effects when the metal swap occurs19,82,84 or be detrimental if not.19
Prospective
Conclusions
In this review, we have focused on the mutual influence of Cu and Zn regarding their coordination to Aβ peptides, as well as the resulting impact on the aggregation and ROS production. We have also highlighted the importance of the co-presence of both Cu(II) and Zn(II) for the metal ion chelation/redistribution therapeutic approaches.As detailed through the present manuscript, future chemical work will undoubtedly include studies of the interaction of both metal ions with the peptide in the presence of other biologically relevant components, such as glutamate, acetylcholine, other metal ions, metallothioneins… This will bridge the gap between in vitro and in vivo studies, probing metal ion–peptide interactions under more complex conditions but still at the molecular level.
Abbreviations
| Aβn–m | Amyloid-β starting from the amino acid residue number n to the number m. |
| AD | Alzheimer's disease |
| Ala | Alanine |
| Asc | Ascorbate |
| Asp | Aspartic acid |
| ATCUN | Amino-terminal copper and nickel |
| CCA | Coumarin-3-carboxylic acid |
| CD | Circular dichroism |
| CNS | Central nervous system |
| CW-ESR | Continuous-wave electron spin resonance |
| Cyt c | Cytochrome c |
| EPR | Electronic paramagnetic resonance |
| ESEEM | Electron spin echo envelope modulation |
| EXAFS | Extended X-ray absorption fine structure |
| Glu | Glutamic acid |
| His | Histidine |
| LC-ESI-MS | Liquid chromatography–electro spray ionization-mass spectrometry |
| MT-2A | Metallothionein-2A |
| MT-3 | Metallothionein-3 |
| Nim | Nitrogen from the imidazole ring |
| NMR | Nuclear magnetic resonance |
| PBT2 | 5,7-Dichloro-2-[(dimethylamino)methyl]quinolin-8-ol |
| PEG | Polyethylene glycol |
| ROS | Reactive oxygen species |
| TEM | Transmission electron microscopy |
| ThT | Thioflavin T |
| UV-Vis | UV-Visible |
| XANES | X-ray absorption near edge structure |
Acknowledgements
The ERC aLzINK grant (ERC-StG-638712) is acknowledged for financial support. We thank warmly Prof. Peter Faller for inspiring discussions.Notes and references
- C. Ising, M. Stanley and D. M. Holtzman, Clin. Pharmacol. Ther., 2015, 98, 469–471 CrossRef CAS PubMed.
- R. Riek and D. S. Eisenberg, Nature, 2016, 539, 227–235 CrossRef PubMed.
- R. Roychaudhuri, M. Yang, M. M. Hoshi and D. B. Teplow, J. Biol. Chem., 2009, 284, 4749–4753 CrossRef CAS PubMed.
- D. A. Drachman, Alzheimers Dement., 2014, 10, 372–380 CrossRef PubMed.
- K. J. Barnham and A. I. Bush, Curr. Opin. Chem. Biol., 2008, 12, 222–228 CrossRef CAS PubMed.
- H. Kozłowski, M. Luczkowski, M. Remelli and D. Valensin, Coord. Chem. Rev., 2012, 256, 2129–2141 CrossRef.
- P. S. Donnelly, Z. Xiao and A. G. Wedd, Curr. Opin. Chem. Biol., 2007, 11, 128–133 CrossRef CAS PubMed.
- K. J. Barnham and A. I. Bush, Chem. Soc. Rev., 2014, 43, 6727–6749 RSC.
- A. S. Pithadia and M. H. Lim, Curr. Opin. Chem. Biol., 2012, 16, 67–73 CrossRef CAS PubMed.
- C. Hureau, Coord. Chem. Rev., 2012, 256, 2164–2174 CrossRef CAS.
- P. Faller, C. Hureau and O. Berthoumieu, Inorg. Chem., 2013, 52, 12193–12206 CrossRef CAS PubMed.
- J. H. Viles, Coord. Chem. Rev., 2012, 256, 2271–2284 CrossRef CAS.
- V. Togu, A. Tiiman and P. Palumaa, Metallomics, 2011, 3, 250–261 RSC.
- M. Rowińska-Żyrek, M. Salerno and H. Kozłowski, Coord. Chem. Rev., 2015, 284, 298–312 CrossRef.
- S. Chassaing, F. Collin, P. Dorlet, J. Gout, C. Hureau and P. Faller, Curr. Top. Med. Chem., 2012, 12, 2573–2595 CrossRef CAS PubMed.
- H. W. Querfurth and F. M. LaFerla, N. Engl. J. Med., 2010, 362, 329–344 CrossRef CAS PubMed.
- M. A. Santos, K. Chand and S. Chaves, Coord. Chem. Rev., 2016, 327–328, 287–303 CrossRef CAS.
- A. Robert, Y. Liu, M. Nguyen and B. Meunier, Acc. Chem. Res., 2015, 48, 1332–1339 CrossRef CAS PubMed.
- A. Conte-Daban, A. Day, P. Faller and C. Hureau, Dalton Trans., 2016, 45, 15671–15678 RSC.
- M. Nguyen, L. Vendier, J.-L. Stigliani, B. Meunier and A. Robert, Eur. J. Inorg. Chem., 2017, 600–608 CrossRef CAS.
- O. Andersen, Chem. Rev., 1999, 99, 2683–2710 CrossRef CAS PubMed.
- L. E. Scott and C. Orvig, Chem. Rev., 2009, 109, 4885–4910 CrossRef CAS PubMed.
- A. E. V. Gorden, J. Xu, K. N. Raymond and P. Durbin, Chem. Rev., 2003, 103, 4207–4282 CrossRef CAS PubMed.
- P. Delangle and E. Mintz, Dalton Trans., 2012, 41, 6335–6370 RSC.
- N. Xia and L. Liu, Mini-Rev. Med. Chem., 2014, 14, 271–281 CrossRef CAS PubMed.
- Q. Wang and K. J. Franz, Acc. Chem. Res., 2016, 49, 2468–2477 CrossRef CAS PubMed.
- M. G. Dickens and K. J. Franz, ChemBioChem, 2010, 11, 59–62 CrossRef CAS PubMed.
- D. S. Folk and K. J. Franz, J. Am. Chem. Soc., 2010, 132, 4994–4995 CrossRef CAS PubMed.
- C. Rodríguez-Rodríguez, M. Telpoukhovskaia and C. Orvig, Coord. Chem. Rev., 2012, 256, 2308–2332 CrossRef.
- S. Noël, S. Cadet, E. Gras and C. Hureau, Chem. Soc. Rev., 2013, 42, 7747–7762 RSC.
- T. Storr, L. E. Scott, M. L. Bowen, D. E. Green, K. H. Thompson, H. J. Schugar and C. Orvig, Dalton Trans., 2009, 3034–3043 RSC.
- Z. Cui, P. R. Lockman, C. S. Atwood, C. H. Hsu, A. Gupte, D. D. Allen and R. J. Mumper, Eur. J. Pharm. Biopharm., 2005, 59, 263–272 CrossRef CAS PubMed.
- S. C. Drew and K. J. Barnham, Acc. Chem. Res., 2011, 44, 1146–1155 CrossRef CAS PubMed.
- B. Alies, A. Conte-Daban, S. Sayen, F. Collin, I. Kieffer, E. Guillon, P. Faller and C. Hureau, Inorg. Chem., 2016, 55, 10499–10509 CrossRef CAS PubMed.
- V. Tõugu and P. Palumaa, Coord. Chem. Rev., 2012, 256, 2219–2224 CrossRef.
- C. Migliorini, E. Porciatti, M. Luczkowski and D. Valensin, Coord. Chem. Rev., 2012, 256, 352–368 CrossRef CAS.
- C. Hureau and P. Dorlet, Coord. Chem. Rev., 2012, 256, 2175–2187 CrossRef CAS.
- L. Guilloreau, L. Damian, Y. Coppel, H. Mazarguil, M. Winterhalter and P. Faller, J. Biol. Inorg. Chem., 2006, 11, 1024–1038 CrossRef CAS PubMed.
- J. Shearer and V. A. Szalai, J. Am. Chem. Soc., 2008, 130, 17826–17835 CrossRef CAS PubMed.
- C. Hureau, V. Balland, Y. Coppel, P.-L. Solari, E. Fonda and P. Faller, J. Biol. Inorg. Chem., 2009, 14, 995–1000 CrossRef CAS PubMed.
- R. A. Himes, G. Y. Park, G. S. Siluvai, N. J. Blackburn and K. D. Karlin, Angew. Chem., Int. Ed., 2008, 47, 9084–9087 CrossRef CAS PubMed.
- Y. Mekmouche, Y. Coppel, K. Hochgräfe, L. Guilloreau, C. Talmard, H. Mazarguil and P. Faller, ChemBioChem, 2005, 6, 1663–1671 CrossRef CAS PubMed.
- C. D. Syme and J. H. Viles, Biochim. Biophys. Acta, Proteins Proteomics, 2006, 1764, 246–256 CrossRef CAS PubMed.
- S. Zirah, S. A. Kozin, A. K. Mazur, A. Blond, M. Cheminant, I. Segalas-Milazzo, P. Debey and S. Rebuffat, J. Biol. Chem., 2006, 281, 2151–2161 CrossRef CAS PubMed.
- J. Danielsson, R. Pierattelli, L. Banci and A. Gräslund, FEBS J., 2007, 274, 46–59 CrossRef CAS PubMed.
- E. Gaggelli, A. Janicka-Klos, E. Jankowska, H. Kozłowski, C. Migliorini, E. Molteni, D. Valensin, G. Valensin and E. Wieczerzak, J. Phys. Chem. B, 2008, 112, 100–109 CrossRef CAS PubMed.
- C. A. Damante, K. Ősz, Z. Nagy, G. Pappalardo, G. Grasso, G. Impellizzeri, E. Rizzarelli and I. Sóvágó, Inorg. Chem., 2009, 48, 10405–10415 CrossRef CAS PubMed.
- P. O. Tsvetkov, A. A. Kulikova, A. V. Golovin, Y. V. Tkachev, A. I. Archakov, S. A. Kozin and A. A. Makarov, Biophys. J., 2010, 99, L84–L86 CrossRef CAS PubMed.
- S. Noël, S. Bustos Rodriguez, S. Sayen, E. Guillon, P. Faller and C. Hureau, Metallomics, 2014, 6, 1220–1222 RSC.
- A. Clements, D. Allsop, D. M. Walsh and C. H. Williams, J. Neurochem., 1996, 66, 740–747 CrossRef CAS PubMed.
- C. A. Damante, K. Ösz, Z. Nagy, G. Grasso, G. Pappalardo, E. Rizzarelli and I. Sóvágó, Inorg. Chem., 2011, 50, 5342–5350 CrossRef CAS PubMed.
- B. Alies, I. Sasaki, O. Proux, S. Sayen, E. Guillon, P. Faller and C. Hureau, Chem. Commun., 2013, 49, 1214 RSC.
- K. I. Silva and S. Saxena, J. Phys. Chem. B, 2013, 117, 9386–9394 CrossRef CAS PubMed.
- E. De Santis, V. Minicozzi, O. Proux, G. C. Rossi, K. I. Silva, M. J. Lawless, F. Stellato, S. Saxena and S. Morante, J. Phys. Chem. B, 2015, 119, 15813–15820 CrossRef CAS PubMed.
- J. T. Pedersen, K. Teilum, N. H. H. Heegaard, J. Østergaard, H.-W. W. Adolph and L. Hemmingsen, Angew. Chem., Int. Ed., 2011, 50, 2532–2535 CrossRef CAS PubMed.
- T. Branch, P. Girvan, M. Barahona and L. Ying, Angew. Chem., Int. Ed., 2015, 54, 1227–1230 CrossRef CAS PubMed.
- B. Alies, E. Renaglia, M. Rózga, W. Bal, P. Faller and C. Hureau, Anal. Chem., 2013, 85, 1501–1508 CrossRef CAS PubMed.
- B. Alies, B. Badei, P. Faller and C. Hureau, Chem. – Eur. J., 2012, 18, 1161–1167 CrossRef CAS PubMed.
- T. R. Young, A. Kirchner, A. G. Wedd and Z. Xiao, Metallomics, 2014, 6, 505–517 RSC.
- C. J. Matheou, N. D. Younan and J. H. Viles, J. Mol. Biol., 2016, 428, 2832–2846 CrossRef CAS PubMed.
- S. Jun and S. Saxena, Angew. Chem., Int. Ed., 2007, 46, 3959–3961 CrossRef CAS PubMed.
- C. J. Matheou, N. D. Younan and J. H. Viles, Biochem. J., 2015, 466, 233–242 CrossRef CAS PubMed.
- A. K. Sharma, S. T. Pavlova, J. Kim, J. Kim and L. M. Mirica, Metallomics, 2013, 5, 1529–1536 RSC.
- L. Pan and J. C. Patterson, PLoS One, 2013, 8, e70681 CAS.
- P. Giannozzi, K. Jansen, G. La Penna, V. Minicozzi, S. Morante, G. C. Rossi and F. Stellato, Metallomics, 2012, 4, 156–165 RSC.
- B. Alies, P.-L. Solari, C. Hureau and P. Faller, Inorg. Chem., 2012, 51, 701–708 CrossRef CAS PubMed.
- Y. Miller, B. Ma and R. Nussinov, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 9490–9495 CrossRef CAS PubMed.
- A. I. Bush, W. H. Pettingell, G. Multhaup, M. D. Paradis, J.-P. Vonsattel, J. F. Gusella, K. Beyreuther, C. L. Masters and R. E. Tanzi, Science, 1994, 265, 1464–1467 CAS.
- J. Mayes, C. Tinker-Mill, O. Kolosov, H. Zhang, B. J. Tabner and D. Allsop, J. Biol. Chem., 2014, 289, 12052–12062 CrossRef CAS PubMed.
- F. Attanasio, P. De Bona, S. Cataldo, M. F. M. Sciacca, D. Milardi, B. Pignataro and G. Pappalardo, New J. Chem., 2013, 37, 1206–1215 RSC.
- M. P. Cuajungco, L. E. Goldstein, A. Nunomura, M. A. Smith, J. T. Lim, C. S. Atwood, X. Huang, Y. W. Farrag, G. Perry and A. I. Bush, J. Biol. Chem., 2000, 275, 19439–19442 CrossRef CAS PubMed.
- K. Reybier, S. Ayala, B. Alies, J. V. Rodrigues, S. Bustos Rodriguez, G. La Penna, F. Collin, C. M. Gomes, C. Hureau and P. Faller, Angew. Chem., Int. Ed., 2016, 55, 1085–1089 CrossRef CAS PubMed.
- C. Cheignon, F. Collin, P. Faller and C. Hureau, Dalton Trans., 2016, 45, 12627–12631 RSC.
- C. Cheignon, P. Faller, D. Testemale, C. Hureau and F. Collin, Metallomics, 2016, 8, 1081–1089 RSC.
- L. G. Trujano-Ortiz, F. J. González and L. Quintanar, Inorg. Chem., 2015, 54, 4–6 CrossRef CAS PubMed.
- C. Cheignon, M. Jones, E. Atrián-Blasco, I. Kieffer, P. Faller, F. Collin and C. Hureau, Chem. Sci., 2017, 8, 5107–5118 RSC.
- R. C. Nadal, S. E. J. Rigby and J. H. Viles, Biochemistry, 2008, 47, 11653–11664 CrossRef CAS PubMed.
- J. T. Pedersen, S. W. Chen, C. B. Borg, S. Ness, J. M. Bahl, N. H. H. Heegaard, C. M. Dobson, L. Hemmingsen, N. Cremades and K. Teilum, J. Am. Chem. Soc., 2016, 138, 3966–3969 CrossRef CAS PubMed.
- P. Faller and C. Hureau, Chem. – Eur. J., 2012, 18, 15910–15920 CrossRef CAS PubMed.
- E. R. Padayachee, H. Zetterberg, E. Portelius, J. Borén, J. L. Molinuevo, N. Andreasen, R. Cukalevski, S. Linse, K. Blennow and U. Andreasson, Brain Res., 2016, 1651, 11–16 CrossRef CAS PubMed.
- G. Meloni, P. Faller and M. Vašák, J. Biol. Chem., 2007, 282, 16068–16078 CrossRef CAS PubMed.
- G. Meloni, V. Sonois, T. Delaine, L. Guilloreau, A. Gillet, J. Teissie, P. Faller and M. Vašák, Nat. Chem. Biol., 2008, 4, 366–372 CrossRef CAS PubMed.
- J. T. Pedersen, C. Hureau, L. Hemmingsen, N. H. H. Heegaard, J. Østergaard, M. Vašák and P. Faller, Biochemistry, 2012, 51, 1697–1706 CrossRef CAS PubMed.
- R. S. Chung, C. Howells, E. D. Eaton, L. Shabala, K. Zovo, P. Palumaa, R. Sillard, A. Woodhouse, W. R. Bennett, S. Ray, J. C. Vickers and A. K. West, PLoS One, 2010, 5, e12030 Search PubMed.
- N. E. Wezynfeld, E. Stefaniak, K. Stachucy, A. Drozd, D. Płonka, S. C. Drew, A. Krężel and W. Bal, Angew. Chem., Int. Ed., 2016, 55, 8235–8238 CrossRef CAS PubMed.
- G. Meloni and M. Vašák, Free Radical Biol. Med., 2011, 50, 1471–1479 CrossRef CAS PubMed.
- G. Meloni, A. Crameri, G. Fritz, P. Davies, D. R. Brown, P. M. H. Kroneck and M. Vašák, ChemBioChem, 2012, 13, 1261–1265 CrossRef CAS PubMed.
- A. K. Sharma, S. T. Pavlova, J. Kim, D. Finkelstein, N. J. Hawco, N. P. Rath, J. Kim and L. M. Mirica, J. Am. Chem. Soc., 2012, 134, 6625–6636 CrossRef CAS PubMed.
- A. Lakatos, É. Zsigó, D. Hollender, N. V. Nagy, L. Fülöp, D. Simon, Z. Bozsó and T. Kiss, Dalton Trans., 2010, 39, 1302–1315 RSC.
- C. Deraeve, C. Boldron, A. Maraval, H. Mazarguil, H. Gornitzka, L. Vendier, M. Pitié and B. Meunier, Chem. – Eur. J., 2008, 14, 682–696 CrossRef CAS PubMed.
- T. Chen, X. Wang, Y. He, C. Zhang, Z. Wu, K. Liao, J. Wang and Z. Guo, Inorg. Chem., 2009, 48, 5801–5809 CrossRef CAS PubMed.
- T. Storr, M. Merkel, G. X. Song-Zhao, L. E. Scott, D. E. Green, M. L. Bowen, K. H. Thompson, B. O. Patrick, H. J. Schugar and C. Orvig, J. Am. Chem. Soc., 2007, 129, 7453–7463 CrossRef CAS PubMed.
- S. Lee, X. Zheng, J. Krishnamoorthy, M. G. Savelieff, H. M. Park, J. R. Brender, J. H. Kim, J. S. Derrick, A. Kochi, H. J. Lee, C. Kim, A. Ramamoorthy, M. T. Bowers and M. H. Lim, J. Am. Chem. Soc., 2014, 136, 299–310 CrossRef CAS PubMed.
- J.-S. Choi, J. J. Braymer, R. P. R. Nanga, A. Ramamoorthy and M. H. Lim, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 21990–21995 CrossRef CAS PubMed.
- E. Atrián-Blasco, E. Cerrada, A. Conte-Daban, D. Testemale, P. Faller, M. Laguna and C. Hureau, Metallomics, 2015, 7, 1229–1232 RSC.
- G. R. Walke, D. S. Ranade, S. N. Ramteke, S. Rapole, C. Satriano, E. Rizzarelli, G. A. Tomaselli, G. Trusso Sfrazzetto and P. P. Kulkarni, Inorg. Chem., 2017, 56, 3729–3732 CrossRef CAS PubMed.
- P. J. Crouch, L. W. Hung, P. A. Adlard, M. Cortes, V. Lal, G. Filiz, K. A. Perez, M. Nurjono, A. Caragounis, T. Du, K. Laughton, I. Volitakis, A. I. Bush, Q.-X. Li, C. L. Masters, R. Cappai, R. A. Cherny, P. S. Donnelly, A. R. White and K. J. Barnham, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 381–386 CrossRef CAS PubMed.
- W. Bal, M. Sokołowska, E. Kurowska and P. Faller, Biochim. Biophys. Acta, Gen. Subj., 2013, 1830, 5444–5455 CrossRef CAS PubMed.
- H. Eury, C. Bijani, P. Faller and C. Hureau, Angew. Chem., Int. Ed., 2011, 50, 901–905 CrossRef CAS PubMed.
- B. Alies, V. Borghesani, S. Sayen, I. Kieffer, E. Guillon, P. Faller and C. Hureau, manuscript in preparation.
- A. A. Belaidi and A. I. Bush, J. Neurochem., 2016, 139, 179–197 CrossRef CAS PubMed.
- N. Singh, S. Haldar, A. K. Tripathi, K. Horback, J. Wong, D. Sharma, A. Beserra, S. Suda, C. Anbalagan, S. Dev, C. K. Mukhopadhyay and A. Singh, Antioxid. Redox Signaling, 2014, 20, 1324–1363 CrossRef CAS PubMed.
- M. Salkovic-Petrisic, A. Knezovic, J. Osmanovic-Barilar, U. Smailovic, V. Trkulja, P. Riederer, T. Amit, S. Mandel and M. B. H. Youdim, Life Sci., 2015, 136, 108–119 CrossRef CAS PubMed.
- F. Bousejra-ElGarah, C. Bijani, Y. Coppel, P. Faller and C. Hureau, Inorg. Chem., 2011, 50, 9024–9030 CrossRef CAS PubMed.
- P. Girvan, T. Miyake, X. Teng, T. Branch and L. Ying, ChemBioChem, 2016, 17, 1732–1737 CrossRef CAS PubMed.
Footnotes |
| † These authors contributed equally. |
| ‡ Chelators: molecules capable of binding Cu ions with a high affinity constant, which then cannot release them rapidly. Metallophores: molecules capable of binding Cu ions and releasing them in another place. Ligands: molecules capable of binding Cu ions, with no precision on their releasing ability (a ligand can keep the metal ions or can release them, i.e. a ligand can either be a chelator or a metallophore). |
| This journal is © The Royal Society of Chemistry 2017 |