Open Access Article
Open Access ArticleSensitization of NIR luminescence of Yb3+ by Zn2+ chromophores in heterometallic complexes with a bridging Schiff-base ligand†
Anatoly P.
Pushkarev
 *ab,
Tatyana V.
Balashova
a,
Andrey A.
Kukinov
c,
Maxim V.
Arsenyev
*ab,
Tatyana V.
Balashova
a,
Andrey A.
Kukinov
c,
Maxim V.
Arsenyev
 ac,
Artem N.
Yablonskiy
cd,
Denis I.
Kryzhkov
cd,
Boris A.
Andreev
ac,
Artem N.
Yablonskiy
cd,
Denis I.
Kryzhkov
cd,
Boris A.
Andreev
 cd,
Roman V.
Rumyantcev
a,
Georgy K.
Fukin
a and
Mikhail N.
Bochkarev
*ac
cd,
Roman V.
Rumyantcev
a,
Georgy K.
Fukin
a and
Mikhail N.
Bochkarev
*ac
aG. A. Razuvaev Institute of Organometallic Chemistry of Russian Academy of Sciences, Tropinina 49, 603950 Nizhny Novgorod, Russian Federation. E-mail: anatoly.pushkarev@gmail.com; mboch@iomc.ras.ru; Fax: +7 (831) 4627497; Tel: +7 (831) 4627709
bDepartment of Nanophotonics and Metamaterials, ITMO University, 197101 St. Petersburg, Russian Federation
cNizhny Novgorod State University, Gagarina avenue 23/2, 603950 Nizhny Novgorod, Russian Federation
dInstitute for Physics of Microstructures of Russian Academy of Sciences, 7 ul. Akademicheskaya, 603950 Nizhny Novgorod, Russian Federation
First published on 26th July 2017
Abstract
Herein, complexes [ZnL]2 (1), {(H2O)Zn(μ-L)Yb[OCH(CF3)2]3} (2), {[(CF3)2HCO]Zn(μ-L)Yb[OCH(CF3)2](μ-OH)}2 (3), and [(H2O)Ln2(L)3] (Ln = Yb (4) and Gd (5)) containing a bridging Schiff-base ligand (H2L = N,N′-bis(3-methoxy salicylidene)phenylene-1,2-diamine) were synthesized. The compounds 1–4 were structurally characterized. The ytterbium derivatives 2–4 exhibited bright NIR metal-centred photoluminescence (PL) of Yb3+ ion under one- (λex = 380 nm) and two-photon (λex = 750 nm) excitation. The superior luminescence properties of complex 2, which was suggested as a marker for NIR bioimaging, were explained via the strong absorption of the 375 nm LMCT state of the ZnL chromophore, efficient energy transfer from ZnL towards Yb3+ through a reversible ligand-to-lanthanide electron transfer process, and absence of luminescence quenchers (C–H and O–H groups) in the first coordination sphere of the rare-earth atom.
Introduction
Discovered more than half a century ago, nowadays, lanthanides have undoubtedly become a part of sustainable living owing to their broad practical applications such as in strong magnets, active (lasers and fiber amplifiers) and passive (LEDs, OLEDs, PVs, and OPVs) optoelectronic devices, phosphors for white lighting, and biomedical imaging.1–7 Except lanthanum and lutetium, Ln3+ ions are able to generate long-lived line-like f–f emission in response to direct photoexcitation at metal absorption bands or energy transfer from excited states of sensitizing ligands. These features, along with reduced scattering of near-infrared (NIR) light in the biological media, make lanthanide-based materials, emitting in the first biological transparency window (λ = 700–1100 nm), very attractive for in-depth, time-gated imaging of thick tissues. For this purpose, Nd, Sm, and Yb complexes are potentially well-suited in combination with a two-photon (2P) excitation technique and signal registration at a wavelength longer than the incident laser pulse wavelength.8–11 Since signal-to-noise ratio versus laser power has quadratic dependence in this case and a common source of two-photon sensitization is a femtosecond Ti:sapphire laser, which operates most efficiently in the 740–800 nm range, it is of great importance to design lanthanide complexes that exhibit both two-photon absorption bands in the 370–400 nm spectral region and intensive NIR radiation.A promising strategy for the synthesis of rare-earth derivatives extending their absorption in the visible region is binding of Ln3+ ions to d-block metal chromophores (e.g. Cr3+,12 Zn2+,13 Ru2+,14 Pt2+,15 and Ir3+ (ref. 16)). These chromophores successfully harvest visible light due to severe absorbing singlet and triplet ligand-to-metal (LMCT) or metal-to-ligand (MLCT) charge transfer states and efficiently transmit energy to emissive levels of f-block metals. A ZnL complex with a salen-type Schiff-base ligand (H2L = N,N′-bis(3-methoxy salicylidene)phenylene-1,2-diamine) has been reported as one of the possible d-metal units suitable for the design of d–f heteronuclear compounds and sensitization of metal-centred luminescence.17–22 Because of compartmental nature of the ligand, Zn2+ and Ln3+ centres could be allocated to its inner N2O2 and outer O4 cavities, respectively; this yielded various single- and double-decker structures or molecules with ZnL fragments arranged in a crosswise fashion depending on the reaction stoichiometry and presence of other anionic or neutral ligands during the synthesis. Thereby, a number of Nd,17–20 Eu,21 Tb,21,22 and Yb20 complexes were established to reveal the f–f luminescence, whereas Dy23 complexes were found to be high performance single-ion magnets. In addition to the existing NIR-emissive Nd and Yb compounds, there is a study reported by Pasatoiu et al. that describes two-photon (λex = 775)-induced photoluminescence (PL) of Zn–Tb and Zn–Sm heterobimetallic compounds comprising a salen-type ligand with a 2,2-dimethyl-1,3-propanediyl linker.24 All the abovementioned findings provide a research background for a study on the application of Zn–Ln salen-derived probes in bioimaging.
Herein, we present synthesis, structures, and photophysical properties of the complexes [ZnL]2 (1), {(H2O)Zn(μ-L)Yb[OCH(CF3)2]3} (2), {[(CF3)2HCO]Zn(μ-L)Yb[OCH(CF3)2](μ-OH)}2 (3), and [(H2O)Ln2(L)3] (Ln = Yb (4) and Gd (5)). Their optical characteristics in the solid state and MeCN solution were determined via steady-state and time-resolved spectroscopic techniques. Moreover, the role of Zn-containing chromophore in sensitization of Yb3+ emission in response to one- and two-photon excitation has been discussed.
Experimental
General procedures
All manipulations were conducted under vacuum using a Schlenk line technique. Solvents were purified by distillation over sodium/benzophenone ketyl (DME) and calcium hydride. (MeCN). HOCH(CF3)2 was purchased from a commercial supplier. Ligand H2L was synthesized via a condensation reaction of o-vanillin and o-phenylenediamine in MeOH. The complexes Ln[N(SiMe3)2]3 (Ln = Gd and Yb) and {(DME)Yb[OCH(CF3)2]3}2 were synthesized according to the procedures reported in literature.25,26 The C, H, and N elemental analyses were performed using a Vario EL cube analyzer. Metal content in the compounds [(H2O)Ln2(L)3] (Ln = Gd and Yb) was analyzed via complexometric titration. IR spectra were obtained using a PerkinElmer 577 spectrometer in the 4000–450 cm−1 region as a Nujol mull on KBr plates. UV-vis absorption spectroscopy was performed in a 1 cm quartz cuvette using a PerkinElmer Lambda-25 from 200 to 700 nm; excitation and emission spectra were obtained via PerkinElmer LS-55 in the 200–700 nm range. Emission spectra in the NIR range were obtained using an Ocean Optics NIR512 spectrometer.Synthesis
X-ray crystallography
The X-ray diffraction data for 1–4 were obtained using Bruker AXS SMART APEX (1 and 2) and Oxford Xcalibur Eos (3 and 4) diffractometers (Mo-Kα radiation, ω-scan technique, and λ = 0.71073 Å). The intensity data were integrated by SAINT (1 and 2) and CrysAlisPro (3 and 4) programs.27,28 All structures were solved by the dual method29 and refined on Fhkl2 using the SHELXTL package.30 All non-hydrogen atoms were anisotropically refined. Hydrogen atoms were placed at the calculated positions and refined in the riding-model (Uiso(H) = 1.5Ueq(C) in CH3-groups and Uiso(H) = 1.2Ueq(C) in other ligands). SADABS (1 and 2) and SCALE3 ABSPACK (3 and 4) were used to perform absorption corrections.31,32 The crystal of 3 contains solvate molecules of acetonitrile. The main crystallographic data and structure refinement details for 1–4 are presented in Table S1.† CCDC 1537232 (1 and 2), 1537231 (3), and 1537230 (4)† contain the supplementary crystallographic data for this study.Time-resolved and two-photon spectroscopy
The PL decay times were measured under pulsed excitation with a third harmonic of a Spectra-Physics Nd:YAG laser at 355 nm (pulse duration – 10 ns). The PL signal was dispersed using a grating spectrometer Acton-2300 and detected using a cooled InP/InGaAs-based PMT Hamamatsu H10330A-75 in the 900–1500 nm range. Moreover, two-photon excitation (λex = 750 nm) of NIR emission of 2–4 was performed using a Tsunami Spectra-Physics Ti:Sa laser (pulse duration – 120 fs and repetition frequency – 80 MHz). The laser beam spot size at the focal plane was approximately 5 μm. To filter out the laser radiation from the PL signal, an interference filter cutting off wavelengths below 850 nm was exploited. The PL signal was dispersed using a TriVista Princeton Instruments spectrometer and detected by silicon CCD from Princeton Instruments.Results and discussion
Synthesis
The complex [ZnL]2 (1) was obtained as an orange crystalline precipitate via the reaction of H2L with two equivalents of Zn(Et)2 in MeCN. According to the single crystal X-ray analysis data, 1 is a double-decker molecule having unoccupied outer O4 cavities of the ligands. To produce heterometallic d–f complexes with Yb3+ located in the outer O4 compartment of L2−, a simple strategy of mixing the products 1 and {(DME)Yb[OCH(CF3)2]3}2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 in MeCN was adopted. The latter precursor has been selected for the following reasons: (i) hexafluoro-isopropoxide groups do not sensitize the luminescence of Yb3+; hence, the sensitization effect can be clearly referred to the ZnL → Yb energy transfer; (ii) OCH(CF3)2− ligands contain minimum C–H oscillators, which are known NIR emission quenchers; (iii) {(DME)Yb[OCH(CF3)2]3}2 is most likely capable of dissociating when it reacts with a zinc dimer because its Yb atoms have unfilled coordination sphere (coordination number is 6) and bear sterically non-hindering ligands that are unable to shield the metal centers from the capturing polydentate ZnL chromophore. It has been reported that in the related d–f derivatives, the fifth axial coordination site of Zn2+ can be occupied by various neutral ligands (such as aqua,18,24 acetonitrile,17 and pyridine20). Since during bioimaging, a luminescent marker inevitably interacts with water molecules, it is reasonable to introduce H2O as a neutral ligand to saturate the coordination spheres of metal atoms in the complexes. In this way, the mixture of two equivalents of aqua, 1, and {(DME)Yb[OCH(CF3)2]3}2 in MeCN was stirred at room temperature for 1 h to afford a pale yellow crystalline precipitate, {(H2O)Zn(μ-L)Yb[OCH(CF3)2]3} (2).
26 in MeCN was adopted. The latter precursor has been selected for the following reasons: (i) hexafluoro-isopropoxide groups do not sensitize the luminescence of Yb3+; hence, the sensitization effect can be clearly referred to the ZnL → Yb energy transfer; (ii) OCH(CF3)2− ligands contain minimum C–H oscillators, which are known NIR emission quenchers; (iii) {(DME)Yb[OCH(CF3)2]3}2 is most likely capable of dissociating when it reacts with a zinc dimer because its Yb atoms have unfilled coordination sphere (coordination number is 6) and bear sterically non-hindering ligands that are unable to shield the metal centers from the capturing polydentate ZnL chromophore. It has been reported that in the related d–f derivatives, the fifth axial coordination site of Zn2+ can be occupied by various neutral ligands (such as aqua,18,24 acetonitrile,17 and pyridine20). Since during bioimaging, a luminescent marker inevitably interacts with water molecules, it is reasonable to introduce H2O as a neutral ligand to saturate the coordination spheres of metal atoms in the complexes. In this way, the mixture of two equivalents of aqua, 1, and {(DME)Yb[OCH(CF3)2]3}2 in MeCN was stirred at room temperature for 1 h to afford a pale yellow crystalline precipitate, {(H2O)Zn(μ-L)Yb[OCH(CF3)2]3} (2).
Suitable for X-ray analysis, the crystals containing molecules of both 1 and 2 within one unit cell were obtained from the similar reaction in which double excess of 1 was exploited. Heating of the former mixture at 80 °C for 2 h resulted in the formation of yellow crystals of {[(CF3)2HCO]Zn(μ-L)Yb[OCH(CF3)2](μ-OH)}2 (3). To compare the contribution of the ZnL chromophore antenna towards the sensitization of Yb3+ NIR emission with that of the L2− ligand, a homometallic dinuclear product, [(H2O)Yb2(L)3] (4), was prepared via the reaction of Yb[N(SiMe3)2]3 with H2L in DME in the presence of water. Since the first triplet excited state is commonly recognized to be a key mediator in the energy transfer between an organic ligand and a rare-earth ion, a gadolinium analogue, (5), was synthesized in a similar fashion for the determination of the 3T1 energy level of the ligand. The compounds 1–5 are air- and moisture-stable and have poor (1, 4, and 5) or moderate (2 and 3) solubility in MeCN and DMSO solvents. Schematic of the reactions is depicted in Scheme 1.
Description of the structures
The structural unit of the crystals containing complexes 1 and 2 consists of two neutral heterometallic Yb–Zn molecules (Fig. 1a) between which a dimer molecule [ZnL]2 (Fig. 1b) is disposed. The relatively soft Zn2+ ion is located in the inner N2O2 cavity and the hard Yb3+ ion in the outer O2O2 cavity of the L2− ligand. Ytterbium cation is coordinated by four oxygen atoms of the Schiff-base and three oxygen atoms of the isopropoxide groups. As a result, the coordination number of the Yb atom is equal to seven, and its coordination environment is distorted pentagonal bipyramid. The zinc atom in molecule 2 has a distorted tetragonal pyramidal coordination. It deviates from the basal plane (O(2)O(3)N(1)N(2)) of a tetragonal pyramid by 0.694 Å. The Yb–O(OCH(CF3)2) distances lie in the narrow range of 2.131(2)–2.135(2) Å and are in agreement with the analogous distances in other ytterbium complexes.26,33 As expected, the bridging Yb–μ2-O(2 and 3) distances (2.293(2)–2.310(2) Å) notably exceed the terminal Yb–O(CH(CF3)2) distances. In turn, the Yb(1)–O(methoxy) distances are maximal (2.547(2) and 2.566(2) Å) as compared to other Yb–O bond lengths in this molecule. The Zn(1)–O(2 and 3) and Zn(1)–N(1 and 2) distances are equal to 2.028(2) and 2.036(2) and 2.065(2) and 2.065(2) Å, respectively. The Zn(1)⋯O(8) (H2O) distance (2.002(2) Å) is slightly shorter than the Zn(1)–O(2 and 3) bond lengths. The structure of the heterometallic fragment is typical for related dinuclear Zn(μ-Schiff-base)Ln complexes [(C5H5N)Zn(μ-Schiff-base)Nd(NO3)3], [(MeCN)Zn(μ-Schiff-base)Nd(NO3)3], and [(H2O)Zn(μ-Schiff-base)Sm(NO3)3].17,34,35 All Zn–O, Zn–N, and Yb⋯Zn distances are in the normal range and comparable to those of other dinuclear Zn(μ-Schiff-base)Ln complexes.17,34–36 The shortest Yb⋯F distance in the bimetallic Yb–Zn molecule is 3.713 Å that exceeds the sum of R(Yb3+) = 0.925 Å (ref. 37) and R(F)vdW = 1.4 Å.38 The metal centres of the [ZnL]2 (1) structural unit have a distorted tetragonal pyramidal coordination, as with the molecule 2. The Zn atoms deviate from the basal plane of the tetragonal pyramid by 0.273 Å. The Zn(2)O(12)Zn(2A)O(12A) fragment is centrosymmetric. The Zn–O and Zn⋯Zn distances inside this fragment are 2.030(2) and 2.118(2) and 2.9913(7) Å, respectively. These distances agree with the analogous distances in related dimeric complexes.39,40 The main geometrical parameters of the dimer molecule are close to the analogous parameters in the ZnL fragment of the Yb–Zn molecule. It is worth noting that the Zn–μ2-O distance in 1 (2.1180(19) Å) is slightly longer than the Zn–O(H2O) (2.002(2) Å) distance in the Zn(μ-L)Yb fragment. As distinct from the Zn(2)O(12)Zn(2A)O(12A) fragment of 1, the Yb(1)O(2)Zn(1)O(3) metallocycle in 2 is not planar. The dihedral angles between the YbOO and ZnOO planes are equal to 17.43°. It should be noted that the ligands in the zinc dimer are parallel to each other. The rings A–B and C–D are offset to each other, and the distance between the centres of these rings is 3.733 Å, which satisfies the criterion of the presence of π–π interaction between them.41 | ||
| Fig. 1 Molecular structures of 1 (a) and 2 (b). Thermal ellipsoids are drawn at the 30% probability level. H atoms are omitted for clarity. | ||
Moreover, two molecules of 2 and one molecule of 1 are combined via hydrogen bonds between H2O molecules and oxygen atoms of the salen-type ligand (Fig. 2). The intermolecular O⋯H distances are in the 1.94–2.14 Å range and reflect a specific interaction between 1 and 2.42
According to the X-ray data, complex 3 is a heterometallic tetranuclear compound. Herein, two Zn(μ-L)Yb fragments are connected via two μ2-OH− anions (Fig. 3a and b). As distinct from the Yb(1)O(13)Yb(2)O(14) fragment (the dihedral angles between YbOO planes is 9.01°), the Yb(1)O(1)Zn(1)O(3) and Yb(2)O(7)Zn(2)O(9) metallocycles in 3 are not planar. The dihedral angles between the YbOO and ZnOO planes are equal to 31.64 and 25.21°. Similar to the case of complex 2, the Yb and Zn atoms in 3 have distorted pentagonal bipyramidal and tetragonal pyramidal coordinations, respectively. The Yb–O, Zn–O, and Zn–N distances vary in the ranges of 2.0895(15)–2.5502(14), 1.9291(15)–2.0270(14), and 2.0449(17)–2.0575(17) Å, respectively. The Yb–O(methoxy) distances are maximal (2.4796(14)–2.5502(14) Å) as compared to other Yb–O bond lengths in 3 as well as in complex 2. Overall, the geometric parameters in 3 are close to the analogous parameters in the complex 2. It should be noted that one of the Schiff-bases at the Zn(1) atom has a planar conformation, whereas the other has a butterfly conformation. The dihedral angles between benzene rings connected with oxygen atoms are equal to 4.43° for planar conformation and 23.14° for butterfly conformation. The Yb–OH distances (2.1590(14)–2.2356(14) Å) and Yb(1)⋯Yb(2) (3.53628(12) Å) in 3 perfectly agree with the corresponding distances in the related Ni–Yb tetrameric complex.43
 | ||
| Fig. 3 Molecular structure of 3 (a) and the central core (b). Thermal ellipsoids are drawn at the 30% probability level. H atoms are omitted for clarity. | ||
Complex 4 has a dimeric structure containing two Yb3+ ions, two terminal and one bridging Schiff-bases, as well as an additionally coordinated H2O molecule (Fig. 4). The molecular structure of 4 is similar to that of the related compound [(CH3OH)Yb2L3].44 Contrary to those in the complexes 2 and 3, the ytterbium atoms in 4 are located in the N2O2 cavity of the terminal L2− ligands. The Yb(1) cation is coordinated by four heteroatoms of the terminal ligand and four heteroatoms of the bridging Schiff-base. Thereby, the coordination environment of the Yb(1) cation is a distorted square antiprism, and the coordination number is equal to eight. In addition, another Yb3+ ion is coordinated by four heteroatoms of one terminal ligand and only two oxygen atoms of the bridging ligand. Moreover, the Yb(2) atom is bound to the H2O molecule. It is important to note that the distance between the Yb(2) atom and oxygen of one methoxy group (O(8)) of the bridging ligand (3.056(2) Å) is significantly shorter than others (Yb(2)–O(6,10,12) lying between 3.855 and 4.866 Å). As a consequence, the coordination number of the Yb(2) cations increases up to eight, and the coordination environment is a distorted square antiprism, as with the Yb(1) atom. It should be noted that the Topos program45 confirms the Yb(2)⋯O(8) interaction. Moreover, two terminal ligands are twisted in a butterfly style. The dihedral angles between benzene rings connected with oxygen atoms are equal to 55.17 and 47.21°.
 | ||
| Fig. 4 Molecular structure of 4 (a) and the central core (b). Thermal ellipsoids are drawn at the 30% probability level. H atoms are omitted for clarity. | ||
The bridging ligand is not planar as well. The Yb–O and Yb–N distances vary in the ranges of 2.1724(18)–2.3466(17) and 2.455(2)–2.515(2) Å, respectively. The Yb⋯Yb distance is 3.78559(18) Å. These geometrical parameters are in accordance with those reported for the related [(CH3OH)Yb2L3] compound.44 Selected bond lengths and angles are summarized in Table 1.
| 1 | |||
| Zn(2)–O(10) | 1.958(2) | O(10)–Zn(2)–O(12) | 95.07(8) |
| Zn(2)–O(12) | 2.030(2) | O(10)–Zn(2)–N(3) | 91.88(9) |
| Zn(2)–N(3) | 2.046(3) | O(12)–Zn(2)–N(3) | 167.18(9) |
| Zn(2)–N(4) | 2.073(2) | O(10)–Zn(2)–N(4) | 157.39(9) |
| Zn(2)–O(12A) | 2.1180(19) | O(12)–Zn(2)–N(4) | 88.97(9) |
| Zn(2)–Zn(2A) | 2.9913(7) | N(3)–Zn(2)–N(4) | 80.57(10) |
| 2 | |||
| Zn(1)–O(8) | 2.002(2) | O(2)–Zn(1)–O(3) | 77.73(8) |
| Zn(1)–O(2) | 2.028(2) | O(2)–Zn(1)–N(2) | 142.27(9) |
| Zn(1)–O(3) | 2.036(2) | O(3)–Zn(1)–N(2) | 88.10(9) |
| Zn(1)–N(2) | 2.065(2) | O(2)–Zn(1)–N(1) | 88.07(9) |
| Zn(1)–N(1) | 2.065(2) | O(3)–Zn(1)–N(1) | 138.45(9) |
| Yb(1)–O(7) | 2.131(2) | N(2)–Zn(1)–N(1) | 79.68(10) |
| Yb(1)–O(5) | 2.132(2) | O(3)–Yb(1)–O(2) | 67.29(7) |
| Yb(1)–O(6) | 2.135(2) | O(3)–Yb(1)–O(4) | 63.23(7) |
| Yb(1)–O(3) | 2.293(2) | O(2)–Yb(1)–O(4) | 130.41(7) |
| Yb(1)–O(2) | 2.310(2) | O(3)–Yb(1)–O(1) | 129.87(7) |
| Yb(1)–O(4) | 2.547(2) | O(2)–Yb(1)–O(1) | 62.59(7) |
| Yb(1)–O(1) | 2.566(2) | O(4)–Yb(1)–O(1) | 166.41(7) |
| Yb(1)–Zn(1) | 3.4581(3) | ||
| 3 | |||
| Zn(1)–O(5) | 1.9291(15) | O(1)–Zn(1)–O(3) | 77.00(6) |
| Zn(1)–O(1) | 2.0111(14) | O(1)–Zn(1)–N(1) | 89.33(6) |
| Zn(1)–O(3) | 2.0270(14) | O(3)–Zn(1)–N(1) | 141.05(6) |
| Zn(1)–N(1) | 2.0449(17) | O(1)–Zn(1)–N(2) | 143.98(6) |
| Zn(1)–N(2) | 2.0474(17) | O(3)–Zn(1)–N(2) | 88.41(6) |
| Zn(2)–O(11) | 1.9336(15) | N(1)–Zn(1)–N(2) | 81.62(7) |
| Zn(2)–O(9) | 2.0207(14) | O(9)–Zn(2)–O(7) | 76.41(6) |
| Zn(2)–O(7) | 2.0263(14) | O(9)–Zn(2)–N(4) | 88.63(6) |
| Zn(2)–N(4) | 2.0550(17) | O(7)–Zn(2)–N(4) | 135.94(6) |
| Zn(2)–N(3) | 2.0575(17) | O(9)–Zn(2)–N(3) | 143.57(7) |
| Yb(1)–O(6) | 2.1053(15) | O(7)–Zn(2)–N(3) | 87.66(6) |
| Yb(1)–O(13) | 2.1590(14) | N(4)–Zn(2)–N(3) | 80.35(7) |
| Yb(1)–O(14) | 2.2150(14) | O(1)–Yb(1)–O(3) | 67.20(5) |
| Yb(1)–O(1) | 2.2684(14) | O(1)–Yb(1)–O(4) | 131.37(5) |
| Yb(1)–O(3) | 2.2744(14) | O(3)–Yb(1)–O(4) | 64.19(5) |
| Yb(1)–O(4) | 2.4912(14) | O(1)–Yb(1)–O(2) | 63.29(5) |
| Yb(1)–O(2) | 2.5502(14) | O(3)–Yb(1)–O(2) | 130.40(5) |
| Yb(2)–O(12) | 2.0895(15) | O(4)–Yb(1)–O(2) | 165.25(5) |
| Yb(2)–O(14) | 2.1692(14) | O(9)–Yb(2)–O(7) | 67.00(5) |
| Yb(2)–O(13) | 2.2356(14) | O(9)–Yb(2)–O(8) | 131.62(5) |
| Yb(2)–O(9) | 2.2625(14) | O(7)–Yb(2)–O(8) | 64.62(5) |
| Yb(2)–O(7) | 2.2727(14) | O(9)–Yb(2)–O(10) | 63.72(5) |
| Yb(2)–O(8) | 2.4796(14) | O(7)–Yb(2)–O(10) | 130.22(5) |
| Yb(2)–O(10) | 2.5173(14) | O(8)–Yb(2)–O(10) | 163.61(5) |
| Zn(1)–Yb(1) | 3.3415(2) | ||
| Zn(2)–Yb(2) | 3.3977(2) | ||
| Yb(1)–Yb(2) | 3.53628(12) | ||
| 4 | |||
| Yb(1)–O(3) | 2.1759(18) | O(3)–Yb(1)–O(1) | 80.81(7) |
| Yb(1)–O(1) | 2.1910(17) | O(3)–Yb(1)–O(5) | 150.37(6) |
| Yb(1)–O(5) | 2.2747(17) | O(1)–Yb(1)–O(5) | 79.60(6) |
| Yb(1)–O(7) | 2.3078(17) | O(3)–Yb(1)–O(7) | 87.62(6) |
| Yb(1)–N(2) | 2.464(2) | O(1)–Yb(1)–O(7) | 87.99(6) |
| Yb(1)–N(4) | 2.480(2) | O(5)–Yb(1)–O(7) | 69.72(6) |
| Yb(1)–N(1) | 2.500(2) | O(9)–Yb(2)–O(11) | 86.89(6) |
| Yb(1)–N(3) | 2.515(2) | O(9)–Yb(2)–O(7) | 121.43(6) |
| Yb(1)–Yb(2) | 3.78559(18) | O(11)–Yb(2)–O(7) | 150.46(6) |
| Yb(2)–O(9) | 2.1724(18) | O(9)–Yb(2)–O(5) | 161.47(6) |
| Yb(2)–O(11) | 2.1799(17) | O(11)–Yb(2)–O(5) | 81.47(6) |
| Yb(2)–O(7) | 2.2776(17) | O(7)–Yb(2)–O(5) | 68.99(6) |
| Yb(2)–O(5) | 2.3466(17) | O(9)–Yb(2)–O(1S) | 81.92(8) |
| Yb(2)–O(1S) | 2.408(2) | O(11)–Yb(2)–O(1S) | 81.10(7) |
| Yb(2)–N(5) | 2.455(2) | O(7)–Yb(2)–O(1S) | 93.89(7) |
| Yb(2)–N(6) | 2.467(2) | O(5)–Yb(2)–O(1S) | 82.07(7) |
| Yb(2)–O(8) | 3.056(2) | ||
Photophysical properties
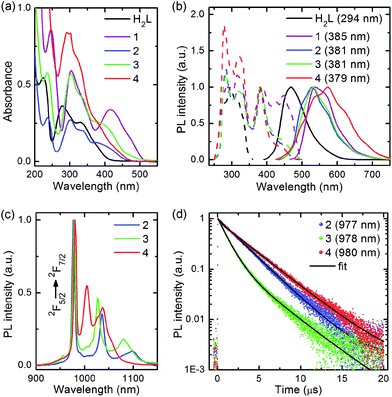
UV-vis absorption spectra of H2L and 1–5 were obtained in a MeCN solution at 2 × 10−5 M. The Schiff-base showed a π–π* band peaked at 280 nm, whereas the zinc dimer 1 revealed an additional strong broad band assigned to the LMCT in the 370–500 nm region. Binding of the ZnL chromophore to Yb[OCH(CF3)2]3 caused a hypsochromic shift of the LMCT band and enhanced the optical density in the range of favourable two-photon absorption for 2. Interestingly, although the anionic isopropoxide moiety, but not aqua, occupies the axial position in the coordination polyhedron of the Zn atom in the heterometallic product 3, the spectrum of 3 was virtually identical to that of 2. In contrast, the spectral line shape of the homometallic derivative 4 had no explicit absorption shoulder in the range of interest (Fig. 5a, Table 2). Spectrum of 5 was similar to that reported by H. Wang et al.46| λ abs (nm) | λ ex (nm) |
λ
em![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (nm) a (nm) |
τ
1, τ2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (μs) b (μs) |
〈τ〉 (μs) | |
|---|---|---|---|---|---|
| a Excitation wavelength is given in parentheses. b Contribution of an exponential function with corresponding lifetime to overall PL decay is given in parentheses. | |||||
| H 2 L | 224, 280 | 295 | 470 (294) | — | — |
| 1 | 245, 303, 415 | 284, 315, 385, 447 | 535 (385) | — | — |
| 2 | 236, 301 | 283, 315, 381 | 529, 977, 1035, 1099 (381) | 2.7 (1.0) | 2.7 |
| 3 | 235, 302 | 283, 315, 381 | 531, 978, 1027, 1180 (381) | 1.1 (0.83), 3.4 (0.17) | 2.0 |
| 4 | 291 | 278, 322, 378 | 573, 980, 1004, 1037 (379) | 3.3 (0.9), 3.7 (0.1) | 3.3 |
Excitation of the Schiff-base approximately at 300 nm produced blue fluorescence. The complexes 1–4 exhibited visible emission exclusively from the LMCT state under 380 nm irradiation under ambient conditions (Fig. 5b, Table 2), whereas 5 showed an additional signal of room temperature phosphorescence at 630 nm (Fig. S1†). PL spectra of the solids 2–4 perfectly matched their emission spectra in MeCN. On the contrary, the LMCT emission band for dissolved 1 and 5 underwent a remarkable blue shift as compared to the luminescence of the solid samples (Fig. S1†); apparently, this could be explained based on the solvation of the metal centres by the MeCN molecules. Short-wavelength excited (λex ∼260–355 nm) luminescence of 1–5 contained a blue fluorescence signal of moderate intensity along with a strong LMCT band (Fig. S2†) that manifests the incomplete 1S1 → LMCT energy transfer. In addition to visible light, 2–4 generated NIR emission corresponding to the 2F5/2 → 2F7/2 transition of Yb3+ (Fig. 5c, Table 2). For each ytterbium complex, the NIR PL spectra measured in the solid state and MeCN solution (Fig. S3†) demonstrated a very similar Stark splitting referring to the effect of the electrostatic ligand field on the energies of the f orbitals. This implies that no major structural changes occur in the coordination environment of Yb atoms during the dissolution of 2–4. Metal-centred emission decay for 2–4 (Fig. 5d, Table 2) was measured via time-resolved spectroscopy and fitted using a multi-exponential expression:
It has been found that PL of 2 decays mono-exponentially with τ = 2.7 μs, whereas radiative relaxation for compounds 3 and 4 has a bi-exponential character due to energy exchange between two closely located luminescent centres in one molecule. Therefore, their PL kinetics were described by average lifetime (2.0 μs for 3 and 3.3 μs for 4), which is given as follows:
Since the lifetime of 4f excited states of lanthanide complexes depends on the geometry and chemical composition of the nearest environment around Ln3+, non-radiative relaxation introduced by ligands or free solvent molecules containing C–H or O–H oscillators, and Ln–Ln energy exchange, it is interesting to assess the contribution of these factors towards the PL decay. Coordination polyhedra of 2 and 3 are close to the D5h point group containing 20 elements of symmetry and consist of seven oxygen atoms. Thus, two hydroxyl groups and Yb–Yb energy transfer in molecule 3 reduce the NIR PL decay time by 26%. The complex 4 has coordination polyhedra close to the D2d point group of order 16. On the one hand, the decrease in the number of symmetry elements shortens the lifetime of f–f emission since electrostatic field from lower symmetry coordination environment enforces the formation of mixed parity states of Ln3+.47 On the other hand, the presence of two and four N atoms in the polyhedra of 4, contrary to the oxygen surroundings of Yb3+ in 2 and 3, increases the decay time of metal-centred emission according to the data on the luminescence of Eu3+ benzimidazole-, benzoxazole-, and benzothiazole-substituted pyridine-2-carboxylates obtained by Bünzli and co-workers.48–50 Considering that all the coordination polyhedra on the basis of their chemical inhomogeneity and/or distorted geometries could not be precisely described by the point groups, the impact of ligand-field symmetry on NIR PL decay of 2–4 is not pronounced. On the contrary, the chemical composition of the rare-earth centre surroundings, Yb–Yb energy exchange, and the presence of luminescence quenchers in the first coordination sphere of ytterbium atom have a significant influence on the observed radiative relaxation of the 2F5/2 excited state.
To investigate the mechanism of sensitization of Yb3+ emission, energies of 1S1 (≈28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 570 cm−1), LMCT (≈22
570 cm−1), LMCT (≈22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 cm−1), and 3T1 (≈15
200 cm−1), and 3T1 (≈15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 870 cm−1) states of 2–4 were determined from the absorption spectra (Fig. 5a) and low-temperature phosphorescence of the Gd derivative (Fig. S4†). Efficient energy transfer process in lanthanide complexes occurs mostly via Förster or Dexter mechanisms, and there are two main conditions for its successful accomplishment: (i) spectral overlap (ΩDA) between the ligand triplet state emission and rare-earth ion absorption and (ii) energy gap between 3T1 and resonant level of Ln3+ exceeding 1500 cm−1, which prevents backward lanthanide-to-ligand energy transfer.51 Although the latter condition is fairly fulfilled for vast majority of Yb derivatives, there are numerous ytterbium complexes exhibiting NIR luminescence even in case of zero spectral overlap ΩDA.52–55 Taking into account that ΔE{3T1 − 2F5/2} ≈ 5400 cm−1 for 2–4, but the triplet state phosphorescence does not extend to wavelengths longer than 750 nm (Fig. S4†) in the region where Yb3+ absorbs light, it is surprising to find out the bright metal-centred PL of 2–4. Therefore, a vanishing role of the triplet excited state in the ligand–ytterbium energy transfer was speculated and approved by measuring the luminescence intensity of the solid samples in the 77–373 K temperature range and in the experiments with aerated MeCN solutions. If the 3T1 → Yb3+* energy pathway is the most probable, the thermally activated non-radiative relaxation of the triplet state should weaken the f–f emission. Moreover, in the aerated solution, energy transfer from the 3T1 state of the ytterbium complex towards the triplet oxygen molecule should give rise to noticeable oxygen-dependent behaviour of Yb3+ luminescence along with appearance of an additional peak at 1270 nm associated with emission of the produced singlet oxygen.56 As expected, neither significant thermally/oxygen initiated changes in the PL spectra of the Yb complexes nor the signal of singlet oxygen were detected. These observations together with the fact that 2–4 generate f–f and LMCT emission attenuating and increasing synchronously under photoexcitation allow us to speculate that the 2F5/2 state of Yb3+ ion is populated through reversible ligand-to-lanthanide electron transfer. In this process, an excited organic ligand (anionic or neutral) reduces Yb3+ to Yb2+ ion, and further, the radical ligand (neutral or cationic) oxidizes the divalent ytterbium, resulting in the formation of excited Yb3+* ion, which subsequently emits light.55,57–60 An energy diagram describing the sensitization of luminescence of the complexes 2–5 is shown in the Fig. S5.†
870 cm−1) states of 2–4 were determined from the absorption spectra (Fig. 5a) and low-temperature phosphorescence of the Gd derivative (Fig. S4†). Efficient energy transfer process in lanthanide complexes occurs mostly via Förster or Dexter mechanisms, and there are two main conditions for its successful accomplishment: (i) spectral overlap (ΩDA) between the ligand triplet state emission and rare-earth ion absorption and (ii) energy gap between 3T1 and resonant level of Ln3+ exceeding 1500 cm−1, which prevents backward lanthanide-to-ligand energy transfer.51 Although the latter condition is fairly fulfilled for vast majority of Yb derivatives, there are numerous ytterbium complexes exhibiting NIR luminescence even in case of zero spectral overlap ΩDA.52–55 Taking into account that ΔE{3T1 − 2F5/2} ≈ 5400 cm−1 for 2–4, but the triplet state phosphorescence does not extend to wavelengths longer than 750 nm (Fig. S4†) in the region where Yb3+ absorbs light, it is surprising to find out the bright metal-centred PL of 2–4. Therefore, a vanishing role of the triplet excited state in the ligand–ytterbium energy transfer was speculated and approved by measuring the luminescence intensity of the solid samples in the 77–373 K temperature range and in the experiments with aerated MeCN solutions. If the 3T1 → Yb3+* energy pathway is the most probable, the thermally activated non-radiative relaxation of the triplet state should weaken the f–f emission. Moreover, in the aerated solution, energy transfer from the 3T1 state of the ytterbium complex towards the triplet oxygen molecule should give rise to noticeable oxygen-dependent behaviour of Yb3+ luminescence along with appearance of an additional peak at 1270 nm associated with emission of the produced singlet oxygen.56 As expected, neither significant thermally/oxygen initiated changes in the PL spectra of the Yb complexes nor the signal of singlet oxygen were detected. These observations together with the fact that 2–4 generate f–f and LMCT emission attenuating and increasing synchronously under photoexcitation allow us to speculate that the 2F5/2 state of Yb3+ ion is populated through reversible ligand-to-lanthanide electron transfer. In this process, an excited organic ligand (anionic or neutral) reduces Yb3+ to Yb2+ ion, and further, the radical ligand (neutral or cationic) oxidizes the divalent ytterbium, resulting in the formation of excited Yb3+* ion, which subsequently emits light.55,57–60 An energy diagram describing the sensitization of luminescence of the complexes 2–5 is shown in the Fig. S5.†
To evaluate the influence of ZnL and {[(CF3)2HCO]ZnL}− chromophore groups on the intensity of Yb3+ emission, luminescence spectra of 2–4 in MeCN (O.D. = 0.1 at 405 nm) were obtained under 405 nm laser diode excitation. Although the highest integral intensity was detected for [(H2O)Yb2(L)3] (4), the complex {(H2O)Zn(μ-L)Yb[OCH(CF3)2]3} (2) revealed an approximately three- and two-fold enhancement of the 980 nm band with respect to 3 and 4 (Fig. S3†). Therefore, taking into consideration that two-photon microscopes are usually equipped with silicon detectors having poor responsivity at wavelengths above 1000 nm, compound 2 is the best candidate for 2P microscopy among the ytterbium complexes studied herein.
Moreover, two-photon excitation (TPE) of NIR PL of the solids 2–4 was performed using a femtosecond Ti:Sa laser. An optimal excitation wavelength was chosen to be 750 nm because at longer wavelengths, the intrinsic one-photon absorption of Yb3+ began to obviously increase due to the strong Stark splitting of the 2F5/2 and 2F7/2 energy levels. The incident laser power varied from 1 to 100 mW. It was found that TPE at the LMCT absorption band produces desirable metal-centered emission of ytterbium ion at ca. 980 nm (Fig. 6a). The experimental data showing the dependence of the intensity of NIR PL on the incident laser power in the log–log scale nicely fit to the linear relationship with the slopes of 1.99 (2), 1.67 (3), and 1.63 (4) (Fig. 6b).
 | ||
| Fig. 6 Energy diagram describing the TPE metal-centred luminescence of the complexes 2–4 (a) and intensity of TPE NIR PL of 2–4versus incident laser power relationship in the log–log scale (b). | ||
The complex 2 exhibited the most efficient two-photon excited luminescence; this is in line with the strong absorption of the 375 nm LMCT state of the ZnL chromophore, efficient ZnL → Yb3+ energy transfer, and absence of luminescence quenchers in the first coordination sphere of the Yb atom.
Conclusions
In summary, five complexes: [ZnL]2 (1), {(H2O)Zn(μ-L)Yb[OCH(CF3)2]3} (2), {[(CF3)2HCO]Zn(μ-L)Yb[OCH(CF3)2](μ-OH)}2 (3), and [(H2O)Ln2(L)3] (Ln = Yb (4) and Gd (5)) were synthesized. The complexes 1–4 were structurally characterized. The ytterbium derivatives 2–4 showed bright NIR metal-centred luminescence under one- and two-photon excitation at the LMCT absorption band. The compound 2 exhibited the most efficient one- (λex = 381 nm) and two-photon (λex = 750 nm) excited f–f emission owing to substantial absorption of the LMCT state of the ZnL chromophore in the UV-to-vis spectral region, efficient ZnL → Yb3+ energy transfer, and absence of luminescence quenchers bound to the Yb atom. The moderate solubility of 2 in DMSO affords ground for its utilization in bioimaging.11Acknowledgements
This work was supported by Russian Foundation for Basic Research (grant no. 16-33-00056). A.P.P. acknowledges financial support from Ministry of Education and Science of Russian Federation (Project 16.8939.2017/8.9) and Russian Foundation for Basic Research (grant no. 17-03-00621).References
- J. F. Herbst, J. Magn. Magn. Mater., 1991, 100, 57 CrossRef CAS.
- C. Kränkel, D.-T. Marzahl, F. Moglia, G. Huber and P. W. Metz, Laser Photonics Rev., 2016, 10, 548 CrossRef.
- M. Nakazawa, Opt. Rev., 2014, 21, 862 CrossRef.
- H. Xu, R. Chen, Q. Sun, W. Lai, Q. Su, W. Huang and X. Liu, Chem. Soc. Rev., 2014, 43, 3259 RSC.
- S. V. Eliseeva and J.-C. G. Bunzli, Chem. Soc. Rev., 2010, 39, 189 RSC.
- J.-C. G. Bunzli and S. V. Eliseeva, New J. Chem., 2011, 35, 1165 RSC.
- M. Shang, C. Li and J. Lin, Chem. Soc. Rev., 2014, 43, 1372 RSC.
- G. Piszczek, I. Gryczynski, B. P. Maliwal and J. R. Lakowicz, J. Fluoresc., 2002, 12, 15 CrossRef CAS.
- T. Zhang, X. Zhu, C. C. W. Cheng, W. M. Kwok, H. L. Tam, J. Hao, D. W. J. Kwong, W. K. Wong and K. L. Wong, J. Am. Chem. Soc., 2011, 133, 20120 CrossRef CAS PubMed.
- A. D'Aléo, A. Bourdolle, S. Brustlein, T. Fauquier, A. Grichine, A. Duperray, P. L. Baldeck, C. Andraud, S. Brasselet and O. Maury, Angew. Chem., Int. Ed., 2012, 51, 6622 CrossRef PubMed.
- A. T. Bui, A. Grichine, S. Brasselet, A. Duperray, C. Andraud and O. Maury, Chem. – Eur. J., 2015, 21, 17757 CrossRef CAS PubMed.
- D. Zare, Y. Suffren, L. Guénée, S. V. Eliseeva, H. Nozary, L. Aboshyan-Sorgho, S. Petoud, A. Hauser and C. Piguet, Dalton Trans., 2015, 44, 2529 RSC.
- R. Jia, H.-F. Li, P. Chen, T. Gao, W.-B. Sun, G.-M. Li and P.-F. Yan, CrystEngComm, 2016, 18, 917 RSC.
- L.-Y. Zhang, K. Li, M. Pan, Y.-N. Fan, H.-P. Wang and C.-Y. Su, New J. Chem., 2016, 40, 5379 RSC.
- A. Chandra, K. Singh, S. Singh, S. Sivakumar and A. K. Patra, Dalton Trans., 2016, 45, 494 RSC.
- F.-F. Chen, H.-B. Wei, Z.-Q. Bian, Z.-W. Liu, E. Ma, Z.-N. Chen and C.-H. Huang, Organometallics, 2014, 33, 3275 CrossRef CAS.
- W.-K. Wong, X. Yang, R. A. Jones, J. H. Rivers, V. Lynch, W.-K. Lo, D. Xiao, M. M. Oye and A. L. Holmes, Inorg. Chem., 2006, 45, 4340 CrossRef CAS PubMed.
- W.-K. Lo, W.-K. Wong, W.-Y. Wong, J. Guo, K.-T. Yeung, Y.-K. Cheng, X. Yang and R. A. Jones, Inorg. Chem., 2006, 45, 9315 CrossRef CAS PubMed.
- W.-Y. Bi, X.-Q. Lü, W.-L. Chai, T. Wei, J.-R. Song, S.-S. Zhao and W.-K. Wong, Inorg. Chem. Commun., 2009, 12, 267 CrossRef CAS.
- W. Bi, T. Wei, X. Lü, Y. Hui, J. Song, S. Zhao, W.-K. Wong and R. A. Jones, New J. Chem., 2009, 33, 2326 RSC.
- X. Yang, D. Schipper, A. Liao, J. M. Stanley, R. A. Jones and B. J. Holliday, Polyhedron, 2013, 52, 165 CrossRef CAS.
- P. Chen, H. Chen, P. Yan, Y. Wang and G. Li, CrystEngComm, 2011, 13, 6237 RSC.
- W.-B. Sun, P.-F. Yan, S.-D. Jiang, B.-W. Wang, Y.-Q. Zhang, H.-F. Li, P. Chen, Z.-M. Wang and S. Gao, Chem. Sci., 2016, 7, 684 RSC.
- T. D. Pasatoiu, A. M. Madalan, M. Zamfirescu, C. Tiseanu and M. Andruh, Phys. Chem. Chem. Phys., 2012, 14, 11448 RSC.
- D. C. Bradley, J. S. Ghotra and F. A. Hart, J. Chem. Soc., Dalton Trans., 1973, 1021 RSC.
- D. M. Kuzyaev, T. V. Balashova, M. E. Burin, G. K. Fukin, R. V. Rumyantcev, A. P. Pushkarev, V. A. Ilichev, I. D. Grishin, D. L. Vorozhtsov and M. N. Bochkarev, Dalton Trans., 2016, 45, 3464 RSC.
- SAINT. Data Reduction and Correction Program. Version 8.37A, Bruker AXS Inc., Madison, Wisconsin, USA, 2012 Search PubMed.
- Data Collection. Reduction and Correction Program. Version 1.171.37.35, CrysAlisPro – Software Package Agilent Technologies, 2012 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A, 2015, 71, 3 CrossRef PubMed.
- G. M. Sheldrick, SHELXTL, Version 2014/7, Structure Determination Software Suite, Bruker AXS, Madison, WI, 2000 Search PubMed.
- G. M. Sheldrick, SADABS-2014/5. Bruker/Siemens Area Detector Absorption Correction Program, Bruker AXS Inc., Madison, Wisconsin, USA, 2001 Search PubMed.
- SCALE3 ABSPACK: Empirical absorption correction, CrysAlisPro – Software Package, Agilent Technologies, 2012 Search PubMed.
- D. M. Kuzyaev, R. V. Rumyantsev, G. K. Fukin and M. N. Bochkarev, Russ. Chem. Bull., 2014, 63, 848 CrossRef CAS.
- W.-Y. Bi, X.-Q. Lü, W.-L. Chai, J.-R. Song, W.-K. Wong, X.-P. Yang and R. A. Jones, Z. Anorg. Allg. Chem., 2008, 634, 1795 CrossRef CAS.
- D. Olea-Román, N. Bélanger-Desmarais, M. Flores-Álamo, C. Bazán, F. Thouin, C. Reber and S. E. Castillo-Blum, Dalton Trans., 2015, 44, 17175 RSC.
- C. Yu, Z. Zhang, L. Liu, H. Li, Y. He, X. Lü, W.-K. Wong and R. A. Jones, New J. Chem., 2015, 39, 3698 RSC.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Cryst., 1976, 32, 751 CrossRef.
- S. S. Batsanov, Russ. J. Inorg. Chem., 1991, 36, 3015 CAS.
- M. V. Escárcega-Bobadilla, G. A. Zelada-Guillén, S. V. Pyrlin, M. Wegrzyn, M. M. D. Ramos, E. Giménez, A. Stewart, G. Maier and A. W. Kleij, Nat. Commun., 2013, 4, 2648 Search PubMed.
- M. M. Belmonte, S. J. Wezenberg, R. M. Haak, D. Anselmo, E. C. Escudero-Adan, J. Benet-Buchholz and A. W. Kleij, Dalton Trans., 2010, 39, 4541 RSC.
- C. Janiak, J. Chem. Soc., Dalton Trans., 2000, 3885 RSC.
- Yu. V. Zefirov and P. M. Zorky, Russ. Chem. Rev., 1995, 64, 415 CrossRef.
- T. D. Pasatoiu, J.-P. Sutter, A. M. Madalan, F. Z. C. Fellah, C. Duhayon and M. Andruh, Inorg. Chem., 2011, 50, 5890 CrossRef CAS PubMed.
- T.-Q. Liu, P.-F. Yan, F. Luan, Y.-X. Li, J.-W. Sun, C. Chen, F. Yang, H. Chen, X.-Y. Zou and G.-M. Li, Inorg. Chem., 2015, 54, 221 CrossRef CAS PubMed.
- V. A. Blatov, A. P. Shevchenko and D. M. Proserpio, Cryst. Growth Des., 2014, 14, 3576 CAS.
- H. Wang, C. Liu, T. Liu, S. Zeng, W. Cao, Q. Ma, C. Duan, J. Dou and J. Jiang, Dalton Trans., 2013, 42, 15355 RSC.
- B. M. Walsh, Judd–Ofelt theory: principles and practices, in Advances in Spectroscopy for Lasers and Sensing, ed. B. Di Bartolo and O. Forte, Springer, Printed in the Netherlands, 2006, pp. 403–433 Search PubMed.
- N. M. Shavaleev, R. Scopelliti, F. Gumy and J.-C. G. Bünzli, Inorg. Chem., 2009, 48, 6178 CrossRef CAS PubMed.
- N. M. Shavaleev, R. Scopelliti, F. Gumy and J.-C. G. Bünzli, Inorg. Chem., 2009, 48, 5611 CrossRef CAS PubMed.
- N. M. Shavaleev, S. V. Eliseeva, R. Scopelliti and J.-C. G. Bünzli, Chem. – Eur. J., 2009, 15, 10790 CrossRef CAS PubMed.
- J.-C. G. Bünzli and S. V. Eliseeva, Photophysics of Lanthanoid Coordination Compounds, in Comprehensive Inorganic Chemistry II, ed. J. Reedijk and K. Poeppelmeier, vol. 8, Elsevier, Oxford, 2013, pp. 339–398 Search PubMed.
- S. Biju, Y. K. Eom, J.-C. G. Bunzli and H. K. Kim, J. Mater. Chem. C, 2013, 1, 6935 RSC.
- Z. Li, H. Zhang and J. Yu, Thin Solid Films, 2012, 520, 3663 CrossRef CAS.
- C. Reinhard and H. U. Gudel, Inorg. Chem., 2002, 41, 1048 CrossRef CAS PubMed.
- V. A. Ilichev, A. P. Pushkarev, R. V. Rumyantcev, A. N. Yablonskiy, T. V. Balashova, G. K. Fukin, D. F. Grishin, B. A. Andreev and M. N. Bochkarev, Phys. Chem. Chem. Phys., 2015, 17, 11000 RSC.
- A. Watkis, R. Hueting, T. J. Sørensen, M. Tropiano and S. Faulkner, Chem. Commun., 2015, 51, 15633 RSC.
- W. D. Horrocks Jr., P. J. Bolender, W. D. Smith and R. M. Supkowski, J. Am. Chem. Soc., 1997, 119, 5972–5973 CrossRef.
- T. Ala-Kleme, K. Haapakka and M. Latva, Anal. Chim. Acta, 1999, 395, 205 CrossRef CAS.
- A. Y. Zhong, L. Si, H. He and A. G. Sykes, Dalton Trans., 2011, 40, 11389 RSC.
- P. Pushkarev, V. A. Ilichev, T. V. Balashova, D. L. Vorozhtsov, M. E. Burin, D. M. Kuzyaev, G. K. Fukin, B. A. Andreev, D. I. Kryzhkov, A. N. Yablonskiy and M. N. Bochkarev, Russ. Chem. Bull., 2013, 62, 392 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Details of crystallographic, collection and refinement data for 1–4, PL spectra of 1–5 and energy diagram for 2–5. CCDC 1537230–1537232. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7dt01340j |
| This journal is © The Royal Society of Chemistry 2017 |