Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Interconnected hierarchical NiCo2O4 microspheres as high-performance electrode materials for supercapacitors†
Ming
Cheng
a,
Hongsheng
Fan
a,
Yuanjun
Song
b,
Yimin
Cui
 a and
Rongming
Wang
a and
Rongming
Wang
 *b
*b
aDepartment of Physics, Beihang University, Beijing 100191, P. R. China
bBeijing Key Laboratory for Magneto-Photoelectrical Composite and Interface Science, School of Mathematics and Physics, University of Science and Technology Beijing, Beijing 100083, P. R. China. E-mail: rmwang@ustb.edu.cn
First published on 21st June 2017
Abstract
Herein, interconnected hierarchical NiCo2O4 microspheres (IH-NiCo2O4) were prepared via a solvothermal method followed by an annealing treatment. IH-NiCo2O4 possesses large tunnels and abundant mesopores, which are in favor of their applications in energy storage field. When employed as an electrode material for supercapacitors, IH-NiCo2O4 exhibits a high specific capacitance of 1822.3 F g−1 at a current density of 2 A g−1, an excellent rate property of 68.6% capacity retention at 20 A g−1, and an 87.6% specific capacitance retention of its initial value after 7000 cycles at a high current density of 10 A g−1, superior to those of IH-Co3O4. Furthermore, an optimal asymmetric supercapacitor (ASC) was also constructed with IH-NiCo2O4 as the positive electrode and graphene as the negative electrode. The ASC delivers a high energy density of 39.4 Wh kg−1 at a power density of 800 W kg−1. Even at a high power density of 8000 W kg−1, the energy density still reaches 27.2 Wh kg−1. Moreover, the ASC shows a good cycling stability with 80.1% specific capacitance retention after 5000 cycles at 6 A g−1. The excellent electrochemical performance of IH-NiCo2O4 makes it a promising electrode material in energy storage field.
Introduction
Owing to the inevitable exhaustion of fossil fuels, ever-deteriorating environment, and fast-growing energy demand, the development of renewable, environmentally friendly, and efficient energy storage technologies has attracted significant attention.1–3 Among various energy storage systems, supercapacitors have occupied an indispensable position due to their high power density, improved safety, and long-life stability.4,5 Supercapacitors are commonly divided into two categories: electrical double-layer capacitors (EDLCs) and pseudocapacitors.6 Considering the energy storage mechanisms, pseudocapacitors that are mainly based on reversible Faradaic redox reactions deliver higher energy density than the typical EDLCs, which only relies on charge accumulation at the surface of electrode materials.7,8 However, currently, pseudocapacitors still suffer from low energy density and poor long-life cycling that limit their practical applications. Considering this, numerous efforts have been devoted towards exploring attractive electrode materials that can provide higher energy density and longer life span without sacrificing high power density.2,9–14Among the pseudocapacitive materials, spinel NiCo2O4 has drawn significant interest due to its many advantages such as rich redox reactions involving different cations, environmental friendliness, low-cost, and abundant resources.15–18 Moreover, owing to the co-existence of two different metal cations in a single crystal structure, NiCo2O4 can synergistically enhance the electrochemical performance as compared to the corresponding nickel oxides and cobalt oxides.15,19–22 For example, An et al. demonstrated that the porous NiCo2O4 nanowires exhibited higher capacitance than Co3O4 with a similar structure.19 More recently, Hu et al. also reported that Co3O4/NiCo2O4 double-shelled nanocages showed improved pseudocapacitance than the single-component Co3O4/Co3O4 double-shelled nanocages.23 These excellent features disclose the development potential of NiCo2O4 as a promising electrode material for supercapacitors.
As is well-known, the electrochemical performance of electrode materials also strongly depends on their microstructures, which influence the porosity and specific surface area.9,24–27 NiCo2O4 materials with diverse structures,28,29 such as porous nanowires,19 mesoporous nanosheets,4 hierarchical hollow nanocubes,18 hierarchical tetragonal microtubes,30 hollow spheres,31 and urchin-like microspheres,32 have been synthesized, and they exhibit a distinct difference in their capacitance performance. Therefore, it is a practical strategy to explore an appropriate structural NiCo2O4 to achieve high supercapacitance performance. Considering the similar structural requirements of electrode materials for Li-ion-based batteries and supercapacitors, the structure with excellent performance for one can be applied in the other one. For example, yolk-shelled Ni–Co mixed oxide nanoprisms,15 NiCo2O4 complex hollow spheres,31 Mn-based mixed metal oxide hierarchical tubular structures,20 and UNF@NiCo2O4 composites22 have been demonstrated to possess excellent performance in both fields. Thus, a material with large tunnels and mesopores, which has been proved to exhibit high performance for Li–O2 batteries,33 is a promising alternative electrode material for supercapacitors.
Herein, we synthesized an IH-NiCo2O4 with large tunnels and mesopores via a facile solvothermal process followed by an annealing treatment in an air atmosphere at 200 °C. IH-NiCo2O4 exhibits a high specific capacitance of 1822.3 F g−1 at 1 A g−1, much higher than that of IH-Co3O4. After 7000 continuous cycles at 10 A g−1, IH-NiCo2O4 retains an 87.6% specific capacitance retention of its initial value. Furthermore, an optimized asymmetric supercapacitor IH-NiCo2O4//graphene was fabricated and showed high capacitance, excellent rate property, and good cycling stability. These results indicate that IH-NiCo2O4 is a promising pseudocapacitance material for supercapacitors.
Experimental
Chemicals
Cobaltous nitrate hexahydrate (Co(NO3)2·6H2O, ≥96%), nickel(II) nitrate hexahydrate (Ni(NO3)2·6H2O, ≥98%), isopropanol (IPA, C3H8O, ≥99.6%), ethylene glycol (EG, C2H6O2, ≥99%), and ethanol (C2H6O, ≥99.6%) were purchased from Beijing Chemical Reagents Company. Deionized water was purchased from Beijing Deionized Water Company. All chemicals were of analytical grade and used as received without further purification. Graphene was purchased from Nanjing XFNANO. Materials Tech Co., Ltd.Preparation of materials
In a typical synthesis of nickel–cobalt glycolate, first, 0.194 g Co(NO3)2·6H2O and 0.097 g Ni(NO3)2·6H2O were placed in a 100 mL Teflon container. Second, 25 mL IPA, 35 mL EG, and 1 mL deionized water were added one after another to a container under stirring for 10 min after each addition. The container was then transferred to a stainless steel autoclave and kept in an electrical oven at 190 °C for 12 h. After being naturally cooled down to room temperature, the precipitate was separated via centrifugation, washed several times, and dried in an oven at 80 °C overnight. Then, the precursor was calcined at 200 °C in air for 2 h at a heating rate of 1 °C min−1 to obtain IH-NiCo2O4. To prepare IH-Co3O4, 0.291 g Co(NO3)2·6H2O was added to a Teflon container and mixed with 30 mL IPA, 30 mL EG, and 1 mL deionized. The subsequent process was similar to that of IH-NiCo2O4.Characterization of materials
The crystal structure, morphologies, chemical compositions, and chemical state of the as-synthesized products were studied via X-ray diffraction (XRD, X'Pert Pro MPD system, Cu Kα, λ = 0.154 nm), X-ray photoemission spectroscopy (XPS, ESCALAB MK II, Al Kα photon source, C 1s 284.8 eV), field-emission scanning electron microscopy (FESEM, HITACHI, S4800 and Carl Zeiss Supra 55 Ultra SEM), and transmission electron microscopy (TEM, JEOL 2200F with a field emission gun and an accelerating voltage of 200 kV). The specimen for the TEM study was prepared by dispersing a drop of alcohol solution containing the as-synthesized product onto a porous carbon film supported on a copper grid, and it was dried in a vacuum drying oven. Thermogravimetric and differential scanning calorimetry analyses were performed using a thermal analysis instrument (NETZSCH STA 449F3). The specific surface areas of the products were determined via N2 adsorption–desorption isotherm measurements at 77 K (Micrometrics, ASAP 2020).Electrochemical measurements
For the preparation of the working electrodes, the as-synthesized active materials were mixed with carbon black and polyvinylidene fluoride (PVDF) at a mass ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in N-methyl-2-pyrrolidinone. The mixture was coated onto a clean nickel foam current collector (area ∼1 cm2) and dried in a vacuum oven at 80 °C for 12 h. The dried electrode was then pressed using a hydraulic press at a pressure of 10 MPa to serve as the working electrode. The electrochemical tests were carried out in both three-electrode and two-electrode configurations with 6 M KOH aqueous solution as an electrolyte. In the three-electrode electrochemical cell, the loading masses of IH-NiCo2O4 and IH-Co3O4 are about 2.1 mg cm−2 and 2.31 mg cm−2, respectively. A Pt wire and a saturated calomel electrode were used as the counter and reference electrodes, respectively. In the two-electrode electrochemical system, an asymmetric supercapacitor was constructed by taking IH-NiCo2O4 as the positive electrode and graphene as the negative electrode. Electrochemical measurements were performed using an electrochemical workstation (CHI660D) via cyclic voltammetry (CV) and chronopotentiometry (CP). Electrochemical impedance spectroscopy (EIS) measurements were also carried out using this apparatus over a frequency range of 100 kHz–0.01 Hz at open circuit voltage with an AC amplitude of 5 mV.
1 in N-methyl-2-pyrrolidinone. The mixture was coated onto a clean nickel foam current collector (area ∼1 cm2) and dried in a vacuum oven at 80 °C for 12 h. The dried electrode was then pressed using a hydraulic press at a pressure of 10 MPa to serve as the working electrode. The electrochemical tests were carried out in both three-electrode and two-electrode configurations with 6 M KOH aqueous solution as an electrolyte. In the three-electrode electrochemical cell, the loading masses of IH-NiCo2O4 and IH-Co3O4 are about 2.1 mg cm−2 and 2.31 mg cm−2, respectively. A Pt wire and a saturated calomel electrode were used as the counter and reference electrodes, respectively. In the two-electrode electrochemical system, an asymmetric supercapacitor was constructed by taking IH-NiCo2O4 as the positive electrode and graphene as the negative electrode. Electrochemical measurements were performed using an electrochemical workstation (CHI660D) via cyclic voltammetry (CV) and chronopotentiometry (CP). Electrochemical impedance spectroscopy (EIS) measurements were also carried out using this apparatus over a frequency range of 100 kHz–0.01 Hz at open circuit voltage with an AC amplitude of 5 mV.
Moreover, specific capacitance (C), energy density (E), and power density (P) were calculated from the discharge curves based on the following equations:16,17,19,34
| C = IΔt/ΔV | (1) |
| E = CΔV2/2 | (2) |
| P = E/Δt | (3) |
Results and discussion
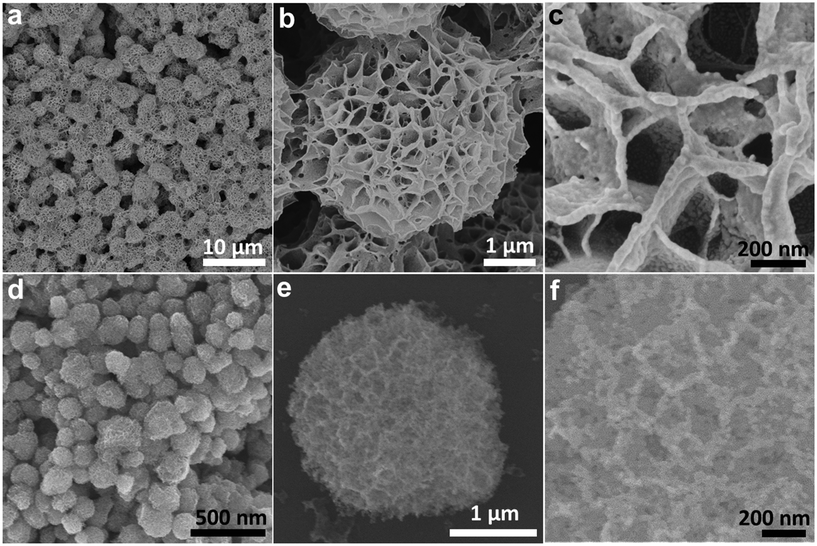
The IH-NiCo2O4 microspheres were prepared by a facile solvothermal process followed by an annealing treatment in an air atmosphere. First, nickel–cobalt glycolate was synthesized as the precursor for the NiCo2O4 microspheres. Fig. 1(a–c) shows the scanning electron microscopy (SEM) images of nickel–cobalt glycolate. It can be observed that nickel–cobalt glycolate possesses an interconnected hierarchical spherical structure with a diameter of about 1 μm. Large tunnels can also be observed. The structure of the prepared precursor is sensitive to the solvent composition. Fig. S1† shows the SEM images of the precursors prepared using different solvent compositions. It can be seen that only the precursors synthesized with 25 mL IPA and 35 mL EG have a uniform interconnected hierarchical structure. The amount of water also significantly affects the morphology of the precursors (Fig. S2†). If no water is added to the system, only solid precursors would be obtained. If less amount of water is added, the size of the tunnels of the product would be too small. However, if more amount of water is added, only flower-like products would be formed. Thermogravimetric analysis (TGA) of the nickel–cobalt glycolate precursor is shown in Fig. S3.† A rapid weight loss is observed between ∼180 and ∼240 °C with a strong exothermic peak at ∼235 °C, due to the decomposition of the precursor to NiCo2O4. After calcination in air at 200 °C for 2 h, the nickel–cobalt glycolate microspheres can be successfully converted into IH-NiCo2O4 with the same structure, as shown in Fig. 1(d–f). If the calcination temperature is too high, the interconnected hierarchical structure would severely shrink (Fig. S4†). The crystallographic structure of the as-prepared IH-NiCo2O4 was confirmed by XRD (Fig. S5a†). Obviously, the peaks at 31.1°, 36.7°, 44.6°, 59.1°, 64.4°, and 77° were detected, corresponding to the (220), (311), (400), (511), (440), and (533) lattice planes of the typical spinel phase NiCo2O4 (JCPDF no. 73-1702). The atomic ratio of Ni to Co was measured to be 0.51 via EDS (Fig. S5b†), which is close to the stoichiometric ratio of NiCo2O4.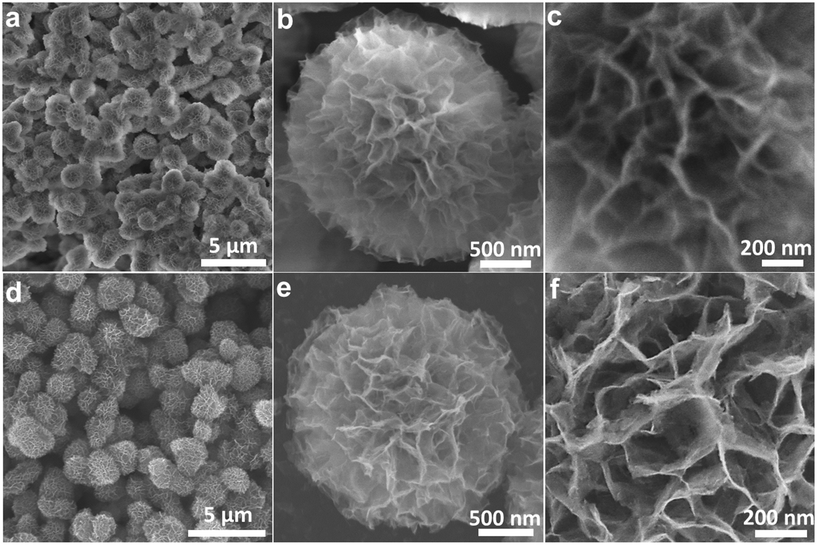 | ||
| Fig. 1 Typical SEM images of (a–c) the nickel–cobalt glycolate precursor and (d–f) the IH-NiCo2O4 microspheres. | ||
Moreover, the IH-Co3O4 microspheres were also fabricated via the similar process. Fig. 2(a–c) shows the SEM images of cobalt glycolate. The interconnected hierarchical structure of cobalt glycolate was also clearly disclosed. Through annealing treatment, cobalt glycolate transformed into Co3O4 phase (Fig. S6a†). Fig. 2(d–f) illustrates that the IH-Co3O4 microspheres still retain the interconnected hierarchical structure but have shrunk only with simplex mesopores. The results indicate that the Co element partially substituted by nickel is beneficial for the complete preservation of the interconnected hierarchical structure.
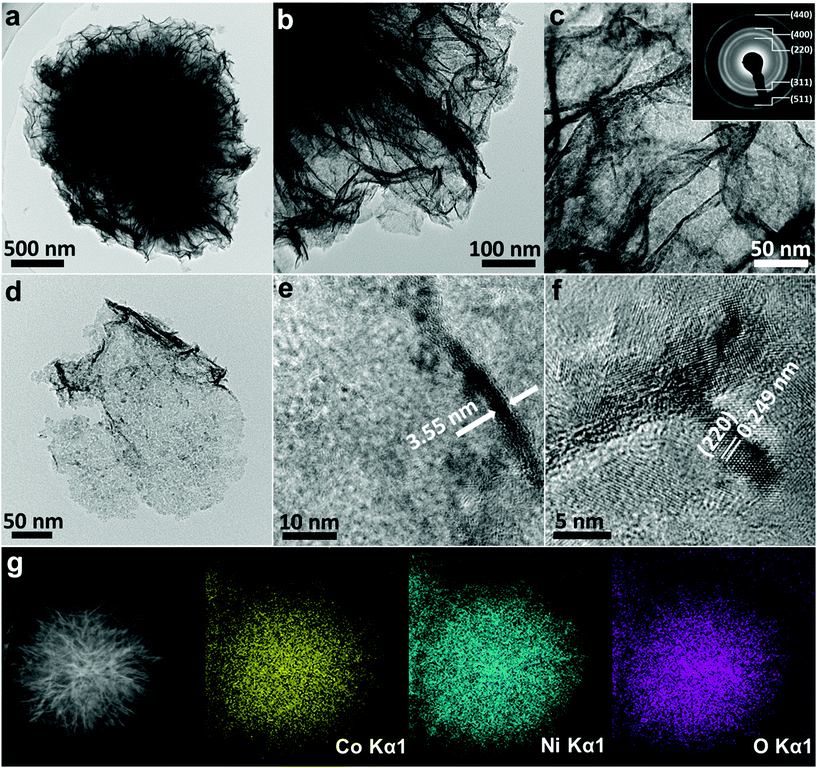
To further explore the microstructure of the IH-NiCo2O4 sample, detailed TEM measurements were conducted. Fig. 3a and b shows the TEM images of a single NiCo2O4 sphere, demonstrating the self-supported hierarchical structure. Fig. 3c proves that the sample possesses an interconnected character and is composed of ultrathin nanosheets. Fig. 3d shows the TEM image of an individual ultrathin nanosheet exfoliated from the sphere. It can be seen that the nanosheet has an obvious nano/mesoporous structure with interconnected nanoparticles. The thickness of the nanosheet was calculated to be sub-5 nm, as shown in Fig. 3e. Note that the interconnected hierarchical structure of NiCo2O4 facilitates electrolyte diffusion and exposure of active sites. The inset in Fig. 3c is the corresponding selected area electron diffraction (SAED) pattern, which shows the polycrystalline nature of the IH-NiCo2O4 sample. The diffraction rings can be assigned to the (220), (311), (400), (511), and (440) planes of NiCo2O4. The HRTEM lattice image in Fig. 4f shows that the comprised nanoparticle has lattice fringes with an interplane spacing of 0.249 nm, corresponding to the (220) planes of NiCo2O4. To further demonstrate the structural features of the NiCo2O4 sample, the high angle annular dark field scanning TEM (HAADF-STEM) image and corresponding element mappings were obtained, as shown in Fig. 4g. The interconnected hierarchical structure with ultrathin nanosheets of NiCo2O4 is confirmed, and Co, Ni, and O elements are homogeneously distributed over the whole NiCo2O4. In addition, the TEM results of Co3O4 are also presented in Fig. S7.† It can be observed that the Co3O4 microsphere is self-supported by interconnected nanoparticles, and no ultrathin nanosheets are observed. This structure provides Co3O4 with abundant simplex mesopores with a size of less than 5 nm. The HRTEM image of the Co3O4 nanoparticle confirms its good crystallinity, consistent with the XRD results (Fig. S6a†).
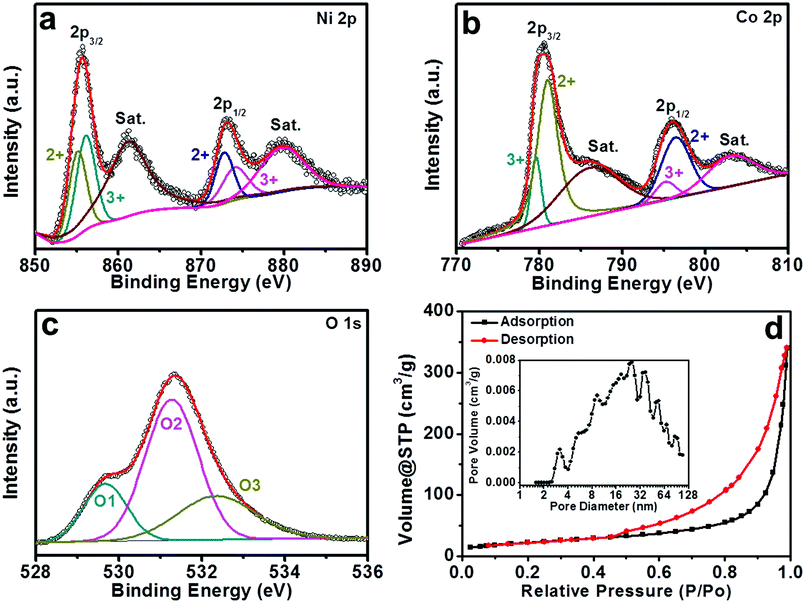
The more detailed elemental composition and electron structure of the as-prepared IH-NiCo2O4 were further characterized by X-ray photoelectron spectroscopy (XPS) measurements. The elements Ni, Co, and O were clearly detected by XPS, as shown in Fig. 4. Furthermore, a Gaussian fitting method was employed to comprehensively analyze the oxidation state of the elements. As shown in Fig. 4a, Ni2+ and Ni3+ were completely characterized via the Ni 2p emission spectrum, exhibiting two shake-up satellites.12,17,35 The fitting peaks at 855.1 eV and 872.8 eV correspond to Ni2+, whereas the peaks at 856.1 eV and 874.1 eV are ascribed to Ni3+.11,12 In addition, two strong shakeup-type peaks of nickel at 861.2 and 879.7 eV were also found.32,35 Moreover, for the spectrum of Co 2p shown in Fig. 4b, two kinds of Co species can be found; the fitting peaks at 781.0 and 796.6 eV are assigned to Co2+, and the peaks located at 779.6 and 795.2 are attributed to Co3+.30,36 The high-resolution spectrum of the O 1s region elucidates three oxygen contributions, which have been named O1, O2, and O3, as shown in Fig. 4c.12,32,33 The fitting peak of O1 at 529.7 eV is attributed to a typical metal–oxygen bond.19 The peak O2 at 531.3 eV is usually regarded as the low-oxygen-coordinated defect site and the surface-adsorbed oxygen species.19 In addition, the peak O3 at 531.8 eV is commonly ascribed to the multiplicity of physic-/chemisorbed water at/within the interface of the material.19,33 The abovementioned results show that the surface of the as-prepared IH-NiCo2O4 has a composition containing Ni2+, Ni3+, Co2+, and Co3+. Moreover, the XPS data of the obtained Co3O4 were also obtained and are shown in Fig. S6,† which confirm Co2+ and Co3+ in the surface of Co3O4.
Generally, the electrochemical performances of active materials are intimately associated with their mesoporous characteristics.9,24,26 To confirm the mesoporous structure of the obtained microspheres, the specific surface area (SSA) and pore size distribution analysis were performed by nitrogen adsorption and desorption experiments. Fig. 4d displays the N2 adsorption–desorption isotherm of the as-prepared IH-NiCo2O4. The loop, which is well consistent with the type IV curve, demonstrates the existence of mesopores in the sample.19 The linear increase in N2 uptake in the medium P/P0 region indicates capillary condensation and multilayer adsorption in the mesopores.25 The representative hysteresis loop in the high P/P0 region reveals that the IH-NiCo2O4 microspheres mainly consist of mesopores.16 The SSA of the IH-NiCo2O4 microspheres is calculated to be 81.27 m2 g−1 by the Brunauer–Emmett–Teller (BET) method. The Barrett–Joyner–Halenda (BJH) pore size distribution plot shown in the inset of Fig. 4d reveals that abundant mesopores exist in IH-NiCo2O4, which is consistent with its hierarchical structure. The large SAA and abundant mesopores can facilitate substantial contact between the electrolyte and the active sites. Furthermore, the SSA of IH-Co3O4 is also calculated to be 273.51 m2 g−1 with a narrow pore diameter distribution around 3.8 nm, as shown in Fig. S6d,† corresponding to the TEM results (Fig. S7†).
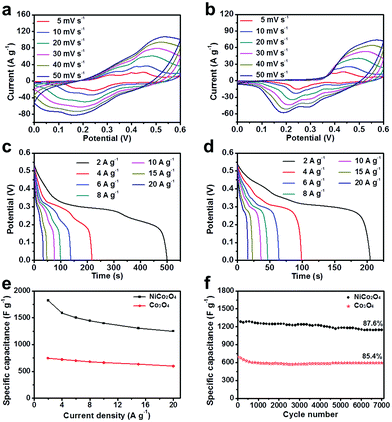
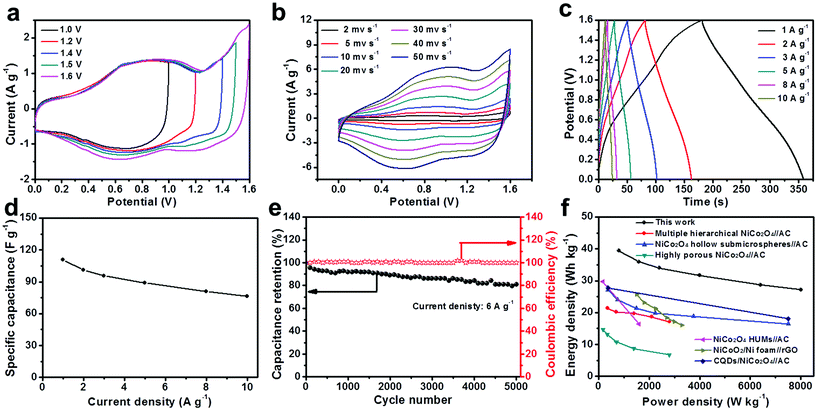
The electrochemical performance of the as-prepared samples employed as positive electrodes for supercapacitors was evaluated in a 6 M KOH aqueous solution. Fig. 5a presents the CV curves of the IH-NiCo2O4 electrode at different scan rates ranging from 5 to 50 mV s−1. Distinct redox peaks can be observed, which demonstrate the pseudocapacitive performance of the IH-NiCo2O4 sample. These pairs of redox peaks are associated with the surface reversible redox reactions of Ni2+/Ni3+ and Co3+/Co4+.32,35 In addition, with the increasing scan rate, the anodic/cathodic peak continually shifts in the positive/negative direction.37Fig. 5b shows the CV plots of the IH-Co3O4 electrode, which exhibits the pseudocapacity based on the redox reactions of Co2+/Co3+ and Co3+/Co4+.2,27 Moreover, the integral area of IH-NiCo2O4 is higher than that of IH-Co3O4, indicating higher specific capacitance. To further evaluate the specific capacitance of the two samples, galvanostatic charge and discharge tests were carried out at various current densities, and the typical discharge curves are shown in Fig. 5c and d. Based on the galvanostatic discharge curves at the current densities of 2, 4, 6, 8, 10, 15, and 20 A g−1, the specific capacitances of IH-NiCo2O4 were calculated to be 1822.3, 1588.0, 1504.0, 1446.3, 1397.1, 1303.5, and 1250.9 F g−1, respectively, whereas the specific capacitances of only IH-Co3O4 were 754.3, 719.8, 699.0, 679.8, 662.2, 633.3, and 596.4 F g−1 at the corresponding current densities, as shown in Fig. 5e. This high specific capacitance of IH-NiCo2O4 is also superior to those of the previously reported high-performance Ni–Co oxide materials such as yolk-shelled Ni0.37Co oxide nanoprisms (1563 F g−1 at 2 A g−1),15 NiCo2O4 microtubes (1387.9 F g−1 at 2 A g−1),30 UNF@NiCo2O4 composites (1460 F g−1 at 2 A g−1),22 and porous NiCo2O4 nanowires (1481 F g−1 at 0.5 A g−1).19 A comparison with previously reported NiCo2O4 nanomaterials is also given in Table S1,† which further confirms the excellent performance of the as-prepared IH-NiCo2O4.
To identify the electrical conductivity of the two samples, electrochemical impedance spectra (EIS) were obtained over a frequency range from 100 kHz to 0.01 Hz under open-circuit conditions. Typical Nyquist plots of the two samples are presented in Fig. S8.† It can be seen that all the EIS curves show a partial semicircle in the high-frequency region and an inclined line in the low-frequency region with the transition between two regions. The EIS data can be fitted by an equivalent circuit model (Fig. S8†), including a bulk system resistance Rs, a charge-transfer resistance Rct, a pseudo-capacitance element Cps from the redox process, and a Cdl representing the double-layer capacitance.38–40 The Rs values of IH-NiCo2O4 and IH-Co3O4 are both measured to be 0.40 Ω, indicating that the two electrodes possess nearly equal solution resistance. It is widely known that the charge-transfer resistance Rct controls the electron transfer kinetics of the redox reaction at the electrode interface and is a decisive parameter for supercapacitors.26 The Rct of the IH-NiCo2O4 electrode is measured to be 0.54 Ω, lower than that of the IH-Co3O4 electrode (4.56 Ω). The lower charge-transfer resistance of the IH-NiCo2O4 electrode benefits faster ion/electron transfer. Moreover, the straight sloping line in the low-frequency region shows the Warburg impedance that represents diffusive resistances of the electrolyte into the interior of the electrode pores and OH− into the host materials or active sites. The IH-NiCo2O4 electrode has a more vertical line in the low-frequency range, suggesting better capacitive behavior and lower electrolyte diffusion impedance.41,42 These results demonstrate the excellent supercapacitive performance of the IH-NiCo2O4 sample.
It is widely accepted that the long-term cycling life at high current density is an important parameter to evaluate the electrochemical performance of supercapacitors in practical application. To evaluate the cycling stability, consecutive charging/discharging measurements at a constant current density of 10 A g−1 were performed, as shown in Fig. 5f. Impressively, cycling is very stable for the IH-NiCo2O4 electrode with an 87.6% of the initial specific capacitance retention after 7000 cycles. In addition, the IH-Co3O4 electrode also exhibited an 85.4% of the initial specific capacitance after 7000 cycles. The enhanced cycling stability of the IH-NiCo2O4 is probably attributed to the unique interconnected hierarchal mesoporous structure, which is expected to improve the structural integrity. Degradation of IH-NiCo2O4 occurred due to the fact that during the continuous cycles at high current density, repeated intercalation and deintercalation of OH− ions at the interface of electrode/electrolyte led to structural collapse to some extent, as shown in Fig. S9.†
To further evaluate the practical application of the IH-NiCo2O4 microspheres in energy storage field, an asymmetric supercapacitor (ASC) was fabricated using IH-NiCo2O4 and graphene as the cathode and the anode, respectively. The electrochemical performance of the graphene electrode was also tested in a 6 M KOH aqueous solution (Fig. S10†). The CV curves of the graphene electrode exhibit typical rectangular shapes, indicating an electrical double-layer capacitance behavior. Based on the discharge curve obtained at 1 A g−1, the specific capacitance of the graphene electrode is calculated to be 197.8 F g−1. To balance the charge between the positive and negative electrodes, the mass ratio of the positive electrode to the negative electrode is optimized to be 0.22 based on the CV studies of the IH-NiCo2O4 and the graphene electrodes at a scan rate of 10 mV s−1 (Fig. S10d†). A series of CV measurements of the asymmetric supercapacitor with different voltage windows at 10 mV s−1 was performed to estimate the best operating potential of the ASC (Fig. 6a). As expected, the asymmetric supercapacitor has a stable potential window up to 1.6 V without obvious polarization curves. Therefore, an operation potential window of 1.6 V was appropriately chosen to investigate the electrochemical performance of the ASC.
Fig. 6b shows the CV curves of the ASC at different scan rates ranging from 2 to 50 mV s−1 within the potential window of 0–1.6 V in a 6 M KOH aqueous electrolyte. The capacitance contribution from both the electric double-layer capacitance and pseudocapacitance can be clearly observed from the distorted rectangular CV curves at each scan rate. Fig. 6c shows the typical galvanostatic charge/discharge curves of the asymmetric supercapacitor measured at different current densities in a potential window of 0–1.6 V. The specific capacitances of the asymmetric supercapacitor calculated from galvanostatic discharge curves are 110.8, 101.2, 95.7, 89.1 80.8, and 76.5 F g−1 at the current densities of 1, 2, 3, 5, 8, and 10 A g−1 (Fig. 6d). Note that the ASC exhibits good rate capability with 69% of the capacitance retained at a high current density of 10 A g−1. Moreover, a long cycling-life test was carried out for the IH-NiCo2O4//graphene ASC by repeated charging/discharging measurements at a constant current density of 6 A g−1 between 0 and 1.6 V (Fig. 6e). It can be seen that the coulombic efficiency of the IH-NiCo2O4//graphene ASC approaches and then maintains a value of 100%, and as much as 80% of the initial specific capacitance retention is presented after 5000 cycles. These results suggest that the IH-NiCo2O4//graphene ASC possesses excellent electrochemical stability.
Moreover, to evaluate the energy storage ability of the IH-NiCo2O4//graphene ASC, the Ragone plots based on the galvanostatic discharge curves are displayed in Fig. 6f. The assembled ASC delivers a high energy density of 39.4 Wh kg−1 at a power density of 800 W kg−1 and still holds 27.2 Wh kg−1 at a higher power density of 8000 W kg−1. This high-performance ASC is superior to many previously reported systems (Fig. 6f) such as multiple hierarchical NiCo2O4//AC,16 NiCo2O4 hollow submicrospheres//AC,43 highly porous NiCo2O4//AC,17 NiCo2O4 HUMs//AC,32 NiCoO2/Ni foam//rGO,37 and CQDs/NiCo2O4//AC.34 The abovementioned results suggest that IH-NiCo2O4 is a promising alternative electrode material for supercapacitor applications.
Conclusions
In summary, we reported the fabrication of IH-NiCo2O4 microspheres via a typical solvothermal process followed by an annealing treatment. When only Co-based salt was introduced into a similar system, the corresponding IH-Co3O4 was obtained. TEM techniques and N2 adsorption and desorption experiments reveal their mesoporous structure with abundant mesopores. Due to the unique structure of large tunnels and mesopores and the synergetic effect of two different metal cations in a single crystal structure, when IH-NiCo2O4 is employed as an electrode material for supercapacitors, it shows a high specific capacitance of 1822.3 F g−1 at a current density of 2 A g−1 and a capacitance retention of 87.6% at 10 A g−1 after 7000 cycles, which outperforms IH-Co3O4. Moreover, an IH-NiCo2O4//graphene ASC was further fabricated and showed high specific capacitance, good cycling performance, and excellent reversibility of the Faradaic reaction. These results suggest that IH-NiCo2O4 prepared through a facile synthesis strategy can be a promising alternative material for energy storage devices.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 51371015, 51331002, 11674023, 51571006, 21601013) and Beijing Municipal Science and Technology Project (No. Z17111000220000).Notes and references
- G. Zhang and X. W. Lou, Adv. Mater., 2013, 25, 976 CrossRef CAS PubMed.
- X. Zhou, X. Shen, Z. Xia, Z. Zhang, J. Li, Y. Ma and Y. Qu, ACS Appl. Mater. Interfaces, 2015, 7, 20322 CAS.
- G. Q. Zhang, H. B. Wu, H. E. Hoster, M. B. Chan-Park and X. W. Lou, Energy Environ. Sci., 2012, 5, 9453 CAS.
- G. Gao, H. B. Wu, S. Ding, L. M. Liu and X. W. Lou, Small, 2015, 11, 804–808 CrossRef CAS PubMed.
- B. D. Boruah and A. Misra, J. Mater. Chem. A, 2016, 4, 17552 CAS.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845 CrossRef CAS PubMed.
- Q. Liao, N. Li, S. Jin, G. Yang and C. Wang, ACS Nano, 2015, 9, 5310 CrossRef CAS PubMed.
- N. Yu, H. Yin, W. Zhang, Y. Liu, Z. Tang and M. Q. Zhu, Adv. Energy Mater., 2015, 6, 1501 Search PubMed.
- X. Chen, M. Cheng, D. Chen and R. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 3892 CAS.
- Y. Xu, X. Gao, W. Chu, Q. Li, T. Li, C. Liang and Z. Lin, J. Mater. Chem. A, 2016, 4, 10248 CAS.
- L. Ye, L. Zhao, H. Zhang, P. Zan, G. Shuai, W. Shi, B. Han, H. Sun, X. Yang and T. Xu, J. Mater. Chem. A, 2016, 5, 1603 Search PubMed.
- L. Liu, H. Zhang, L. Fang, Y. Mu and Y. Wang, J. Power Sources, 2016, 327, 135 CrossRef CAS.
- W. Zeng, L. Wang, H. Shi, G. Zhang, K. Zhang, H. Zhang, F. Gong, T. Wang and H. Duan, J. Mater. Chem. A, 2016, 4, 8233 CAS.
- X. Xiong, B. Zhao, D. Ding, D. Chen, C. Yang, Y. Lei and M. Liu, NPG Asia Mater., 2016, 8, e300 CrossRef.
- L. Yu, B. Guan, W. Xiao and X. W. D. Lou, Adv. Energy Mater., 2015, 5, 1500981 CrossRef.
- S. Wang, S. Sun, S. Li, F. Gong, Y. Li, Q. Wu, P. Song, S. Fang and P. Wang, Dalton Trans., 2016, 45, 7469 RSC.
- R. Ding, L. Qi, M. Jia and H. Wang, Electrochim. Acta, 2013, 107, 494 CrossRef CAS.
- C. Zheng, C. Cao, R. Chang, J. Hou and H. Zhai, Phys. Chem. Chem. Phys., 2016, 18, 6268 RSC.
- C. An, Y. Wang, Y. Huang, Y. Xu, L. Jiao and H. Yuan, Nano Energy, 2014, 10, 125 CrossRef CAS.
- Y. Guo, L. Yu, C.-Y. Wang, Z. Lin and X. W. D. Lou, Adv. Funct. Mater., 2015, 25, 5184 CrossRef CAS.
- C. Yuan, J. Li, L. Hou, X. Zhang, L. Shen and X. W. D. Lou, Adv. Funct. Mater., 2012, 22, 4592 CrossRef CAS.
- J. Pu, Z. Liu, Z. Ma, J. Wang, L. Zhang, S. Chang, W. Wu, Z. Shen and H. Zhang, J. Mater. Chem. A, 2016, 4, 17394 CAS.
- H. Hu, B. Guan, B. Xia and X. W. Lou, J. Am. Chem. Soc., 2015, 137, 5590 CrossRef CAS PubMed.
- Y. Zhang, M. Ma, J. Yang, C. Sun, H. Su, W. Huang and X. Dong, Nanoscale, 2014, 6, 9824–9830 RSC.
- Z. Chen, Z. Wan, T. Yang, M. Zhao, X. Lv, H. Wang, X. Ren and X. Mei, Sci. Rep., 2016, 6, 25151 CrossRef CAS PubMed.
- M. Cheng, S. Duan, H. Fan and R. Wang, CrystEngComm, 2016, 18, 6849 RSC.
- X. Zhang, Y. Zhao and C. Xu, Nanoscale, 2014, 6, 3638 RSC.
- D. P. Dubal, P. Gomez-Romero, B. R. Sankapal and R. Holze, Nano Energy, 2015, 11, 377 CrossRef CAS.
- Z. Wu, Y. Zhu and X. Ji, J. Mater. Chem. A, 2014, 2, 14759 CAS.
- F.-X. Ma, L. Yu, C.-Y. Xu and X. W. Lou, Energy Environ. Sci., 2016, 9, 862 CAS.
- L. Shen, L. Yu, X. Y. Yu, X. Zhang and X. W. Lou, Angew. Chem., Int. Ed., 2015, 54, 1868 CrossRef CAS PubMed.
- Y. Lei, Y. Wang, W. Yang, H. Yuan and D. Xiao, RSC Adv., 2015, 5, 7575 RSC.
- L. Liu, J. Wang, Y. Hou, J. Chen, H. K. Liu, J. Wang and Y. Wu, Small, 2016, 12, 602 CrossRef CAS PubMed.
- Y. Zhu, Z. Wu, M. Jing, H. Hou, Y. Yang, Y. Zhang, X. Yang, W. Song, X. Jia and X. Ji, J. Mater. Chem. A, 2015, 3, 866 CAS.
- M. J. Pang, S. Jiang, G. H. Long, Y. Ji, W. Han, B. Wang, X. L. Liu, Y. L. Xi, F. Z. Xu and G. D. Wei, RSC Adv., 2016, 6, 67839 RSC.
- S. H. Yue, H. Tong, L. Lu, W. W. Tang, W. L. Bai, F. Q. Jin, Q. W. Han, J. P. He, J. Liu and X. G. Zhang, J. Mater. Chem. A, 2017, 5, 689 CAS.
- X. Xu, H. Zhao, J. Zhou, R. Xue and J. Gao, J. Power Sources, 2016, 329, 238 CrossRef CAS.
- S. Duan and R. Wang, NPG Asia Mater., 2014, 6, e122 CrossRef CAS.
- H. Pang, C. Z. Wei, X. X. Li, G. C. Li, Y. H. Ma, S. J. Li, J. Chen and J. S. Zhang, Sci. Rep., 2014, 4, 8 Search PubMed.
- W. Du, Z. Wang, Z. Zhu, S. Hu, X. Zhu, Y. Shi, H. Pang and X. Qian, J. Mater. Chem. A, 2014, 2, 9613 CAS.
- H. Pang, Y. Liu, J. Li, Y. Ma, G. Li, Y. Ai, J. Chen, J. Zhang and H. Zheng, Nanoscale, 2013, 5, 503 RSC.
- H. Pang, Z. Yan, W. Wang, J. Chen, J. Zhang and H. Zheng, Nanoscale, 2012, 4, 5946 RSC.
- Y. Zhu, J. Wang, Z. Wu, M. Jing, H. Hou, X. Jia and X. Ji, J. Power Sources, 2015, 287, 307 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7dt01289f |
| This journal is © The Royal Society of Chemistry 2017 |