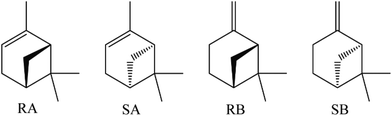
The electrochemical discrimination of pinene enantiomers by a cyclodextrin metal–organic framework†
Cheng-Hua
Deng
 a,
Tao
Li
a,
Jing-Huo
Chen
a,
Jian-Gong
Ma
a,
Tao
Li
a,
Jing-Huo
Chen
a,
Jian-Gong
Ma
 *a and
Peng
Cheng
*a and
Peng
Cheng
 ab
ab
aCollege of Chemistry, and Key Laboratory of Advanced Energy Materials Chemistry (MOE), Nankai University, Tianjin 300071, China. E-mail: mvbasten@nankai.edu.cn
bCollaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Tianjin 300071, China
First published on 28th March 2017
Abstract
Pinene is a family of bicyclic monoterpenes found in nature, which exhibits important applications in chemical industry and biomedicine; however, the discrimination methods used for pinene enantiomers are still rare. The alpha- and beta-pinene enantiomers were recognized and discriminated via an electrochemical method for the first time based on a cyclodextrin metal–organic framework (CD-MOF) as an electrochemical chiral sensor.
Pinene is a family of bicyclic monoterpenes existing in nature, which is found in conifers, limes, and numerous other plants,1 and exhibits important applications in chemical industry and biomedicine including the production of perfumes, turpentine, antiseptics, bronchodilators, and anticatarrhals as well as anti-cancer agents.2–6 There are two isomers of pinene, namely alpha- and beta-pinene, and both alpha- and beta-pinene have two chiral isomers: (1R)-(+)-alpha-pinene (RA), (1S)-(−)-alpha-pinene (SA), (1R)-(+)-beta-pinene (RB), and (1S)-(−)-beta-pinene (SB), the structures of which are illustrated in Fig. 1. Each pinene enantiomer has its distinctive value; as a result, the recognition, discrimination, and separation of pinene isomers are one of the imperative areas for pinene applications; however, the corresponding analytical methods are still rare.7–10 Previous reports on pinene enantiomer recognition discrimination mainly focus on the NMR technique, which firstly requires reacting the pinenes with other chiral derivation agents.7,8 Moreover, high performance liquid chromatography (HPLC) could be a good tool in pinene enantiomers separation with the assistance of an appropriate chiral stationary phase (CSP).9,10 The complexity of the pretreatments, high cost of the instruments and accessories, dosage of analytes, and the low solubility of pinenes limited the further development of current methods. The electrochemical technique has been recently developed as a promising method for the detection of enantiomers, owing to its advantages of short detection times, high recognition accuracy, high stability, and sensitivity as well as low cost in comparison with other traditional methods.11–13
In this report, we present the recognition and discrimination of pinene enantiomers via an electrochemical method for the first time. Each enantiomer of either alpha- or beta-pinene exhibited a typical cyclic voltammetry (CV) response with a metal–organic framework (MOF) sensor.
MOFs are a type of two/three dimensional porous materials, which have attracted significant attention for their high permanent porosity coupled with structural tenability in which organic struts link metal-containing clusters and show wide application potentials including gas adsorption and separation, catalysis, chromatography separation, luminescent sensing, and drug delivery.14–27 Most recently, MOFs have been applied as electrochemical sensors due to their absorption properties and interactions with target molecules, whereas the use of chiral MOFs for the electrochemical discrimination of enantiomers has been rarely reported.28–30 For the discrimination of pinene enantiomers, we selected a gamma-cyclodextrin MOF (CD-MOF-1, 1)31 as the sensor since it has been used as a CSP in HPLC for pinene.10
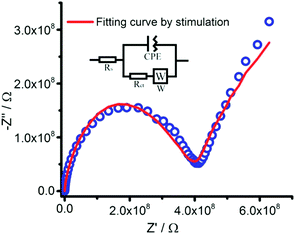
First, the electrochemical properties of 1 were fully characterized. The proton conductivity of 1 was investigated using electrochemistry impedance spectroscopy (EIS) analysis after pressing powder 1 into a round piece covered with conductive silver paint.32 The Nyquist plot of the EIS spectra is shown in Fig. 2. Because 1 contains hydroxyl groups from gamma-cyclodextrin, protons can be easily released into the nanochannels and thereby exhibit proton conduction. The EIS stimulation circuit reflects that the charge transfer resistance (Rct) value of 1 was 357 MΩ. The electron transfer rate constant (Ket) at the electrode interface was 244.85, which could be estimated using EIS and the following equation:33–35
The electrochemical behavior of 1 was investigated using CV measurements after coating 1 on a bare clean pretreated platinum electrode (PE, Φ = 1 mm) and glassy carbon electrode (GCE, Φ = 3 mm) with Nafion D-521, named as 1-PE and 1-GCE, respectively. A platinum wire electrode was used as the auxiliary electrode and a silver ion electrode was used as the reference electrode. An acetonitrile solution containing 0.5 mM 1-butyl-3-methylimidazolium tetrafluoroborate ([Bmim]BF4) ([Bmim]BF4/CH3CN) was used as the electrolyte. The transmission electron microscopy (TEM) image in Fig. 3a shows the uniform distribution of 1 particles on Nafion D-521 and 1 was assembled as cubic nanocrystals. The scanning electron microscopy (SEM) image reveals the similar cubic crystal of 1 in the Nafion D-521 environment (Fig. 3b). Both 1-PE and 1-GCE exhibit stable electrochemical signals for more than ten runs, as shown in Fig. S4 and S5,† respectively. When using 1-PE or 1-GCE as the working electrode, the oxidation peak of 1 was observed around Epa = 1.3 V versus Ag/AgNO3. Due to the low conductivity of 1, at a high electric potential, both 1-PE and 1-GCE do not exhibit as high current density as bare PE and 1-GCE, respectively. Around −0.5 V to −1 V, the reaction peak of 1-PE was influenced by the electrochemical behavior of platinum in the [Bmim]BF4/CH3CN electrolyte.36
 | ||
| Fig. 3 (a) TEM image of dried 1 and the Nafion D-521 mixture. (b) SEM image of dried 1 (marked in orange for clear) and the Nafion D-521 mixture. | ||
All the pinene enantiomers were dissolved in the [Bmim]BF4/CH3CN electrolyte at selected concentrations for the electrochemical tests. For the alpha-pinene enantiomers, both RA and SA exhibited unconspicuous shoulder peaks, which were hardly discriminated for the bare PE (Fig. S10†). Fig. 4a shows the CV response of the alpha-pinene enantiomers (RA and SA) sharing the same concentration at 1-PE with the scan rate of 10 mV s−1. In comparison with the blank 1-PE, a new sharp and obvious oxidation peak was observed for both RA and SA, indicating the interaction between the alpha-pinene enantiomers with the framework of 1. The CV behavior of the alpha-pinene enantiomer molecules influenced by the scan rate reflected that both ipa and ipc were directly proportional to the square root of the scan rate (v1/2); thus the electrochemical process under 1-PE should be the diffusion process (Fig. S7 and S8†).37,38 Under the circumstance of PE, when 1 was coated on PE, the alpha-pinene enantiomer molecules diffused into the nanochannels of 1 during the electrochemical process. The enantiomers RA and SA could differently interact with the chiral framework, which resulted in different electro-redox processes, as revealed by the CV measurements.
The current density of the oxidation peak I for RA was observed at Epa = 1.263 V, whereas the corresponding peak for SA was observed at Epa = 1.220 V. These two peaks are not far from each other, however, their peak current densities are significantly different. The oxidation peak current density of RA is 1070.25341 μA cm−2 and that for SA is only 415.15569 μA cm−2. The difference between the peak current density of RA and SA can be attributed to the different manner and strength of the interaction between the enantiomers and the framework. Peak I for either RA or SA present distinct gradual increases upon increasing the concentration in the range from 0.5 to 5.0 mM (Fig. S6†). ipa of each isomer exhibited a nearly linear variation with concentration (Fig. 4b), following the equations
| ipa-RA = 211.12062cRA + 18.41422, R2 = 0.98967 |
| ipa-SA = 48.25041cSA + 175.62834, R2 = 0.98513 |
When 1-PE was applied as a sensor for discriminating the beta-pinene enantiomers, both RB and SB presented no obvious oxidation peak and could be hardly distinguished, as shown in Fig. S9.† Considering the impact of the electrodes on the sensor, we modified the sensor using GCE instead of PE to obtain 1-GCE, which was subsequently applied for recognizing and discriminating the beta-pinene enantiomers.
Fig. 5a shows the CV response of RB and SB at 1-GCE at the scan rate of 10 mV s−1, in which an oxidation current density peak I around Epa = 1.512 V was observed for both RB and SB, respectively. However, the peak of RB was significantly sharper with higher current density than that for SB. The peak I for each enantiomer gradually increased upon increasing the concentration in the range from 0.5 mM to 5.0 mM (Fig. S11†), where ipa exhibited a nearly linear variation with the concentration (Fig. 5b), following the equations
| ipa-RB = 125.88385cRB − 68.41541, R2 = 0.95867 |
| ipa-SB = 57.15374cSB + 31.53884, R2 = 0.94793 |
We also attempted the application of 1-GCE to discriminate the alpha-pinene enantiomers; during this process, CV curves with different shapes and current densities were observed for RA and SA (Fig. S15†) and thus the alpha-pinene enantiomers could be distinguished at 1-PE as well.
The excellent reliability of 1-PE and 1-GCE was observed as well. An electrode of either 1-PE or 1-GCE was used to test one enantiomer at first, which exhibited steady signals for at least five cycles and then the electrode was washed in acetonitrile. After this, the washed electrodes were used for the electrochemical test of another enantiomer for at least five cycles, which gave the constant characteristic signals, indicating that both 1-PE and 1-GCE are reliable electrochemical sensors with repeatable signals for the discrimination of pinene enantiomers, as shown in Fig. S16 and S17,† respectively. After the electrochemical tests, the 1-Nafion D521 coating material was carefully scratched from the electrode, the SEM image of which confirmed that the cubic nanocrystals of 1 were stable during the tests (Fig. S18†).
Conclusions
In summary, we successfully discriminated pinene enantiomers via an electrochemical method for the first time. Both the alpha-pinene and beta-pinene enantiomers present corresponding typical CV signals using the chiral CD-MOF as a electrochemical sensor. Taking advantage of the unique ability of the MOF material, the electrochemical method exhibits a promising way for the detection of enantiomers with a short detection time, small sample amount, high stability and sensitivity as well as low cost, which should be beneficial for areas dealing with chiral molecules such as biology, pharmacy, and chemical engineering.This project was financially supported by the NFSC (21671110, 21671111 and 91422302).
Notes and references
- E. Russo, Br. J. Pharmacol., 2011, 163, 1344 CrossRef CAS PubMed.
- J. B. Conant and G. H. Carlson, J. Am. Chem. Soc., 1929, 51, 3464 CrossRef CAS.
- K. Satoh, A. Nakahara, K. Mukunoki, H. Sugiyama, H. Saito and M. Kamigaito, Polym. Chem., 2014, 5, 3222 RSC.
- A. Milewska, A. M. B. Osuna, I. M. Fonseca and M. N. Ponte, Green Chem., 2005, 7, 726 RSC.
- D. E. Lincoln and B. M. Lawrence, Phytochemistry, 1984, 23, 933 CrossRef CAS.
- H. C. Brown, S. A. Weissman, P. T. Perumal and U. P. Dhokte, J. Org. Chem., 1990, 55, 1217 CrossRef CAS.
- H. Dodziuka, W. Koźmińskib, O. Lukinc and D. Sybilska, J. Mol. Struct., 2000, 523, 205 CrossRef.
- H. Dodziuk, A. Ejchart, O. Lukin and M. O. Vysotsky, J. Org. Chem., 1999, 64, 1503 CrossRef CAS PubMed.
- A. Bielejewska, K. Duszczyk and D. Sybilska, J. Chromatogr. A, 2001, 931, 81 CrossRef CAS PubMed.
- K. J. Hartlieb, J. M. Holcroft, P. Z. Moghadam, N. A. Vermeulen, M. M. Algaradah, M. S. Nassar, Y. Y. Botros, R. Q. Snurr and J. F. Stoddart, J. Am. Chem. Soc., 2016, 138, 2292 CrossRef CAS PubMed.
- M. Trojanowicz and M. Kaniewska, Electroanalysis, 2009, 21, 229 CrossRef CAS.
- E. L. Izke, J. Pharm. Sci., 2007, 96, 1659 CrossRef PubMed.
- E. Zor, I. H. Patir, M. Bingol and M. Ersoz, Biosens. Bioelectron., 2013, 42, 321 CrossRef CAS PubMed.
- H. Li, M. Eddaoudi, M. O'Keeffe and O. M. Yaghi, Nature, 1999, 402, 276 CrossRef CAS.
- M. Eddaoudi, D. B. Moler, H. Li, B. L. Chen, T. M. Reineke, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2001, 34, 319 CrossRef CAS PubMed.
- G. Férey, C. Mellot-draznieks, C. Serre, F. Millange, J. Dutour, S. Surblé and I. Margiolaki, Science, 2005, 309, 2040 CrossRef PubMed.
- A. G. Slater and A. I. Cooper, Science, 2015, 348, 8075 CrossRef PubMed.
- J. R. Li, R. J. Kuppler and H. C. Zhou, Chem. Soc. Rev., 2009, 38, 1477 RSC.
- H. Furukawa, K. E. Cordova, M. O′Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- P. Nugent, Y. Belmabkhout, S. D. Burd, A. J. Cairns, R. Luebke, K. Forrest, T. Pham, S. Q. Ma, B. Space, L. Wojtas, M. Eddaoudi and M. J. Zaworotko, Nature, 2013, 495, 80 CrossRef CAS PubMed.
- J. S. Seo, D. M. Whang, H. Y. Lee, S. I. Jun, J. H. Oh, Y. J. Jeon and K. M. Kim, Nature, 2000, 404, 982 CrossRef CAS PubMed.
- P. García-garcía, M. Müller and A. Corma, Chem. Sci., 2014, 5, 2979 RSC.
- Z. Y. Gu and X. P. Yan, Angew. Chem., Int. Ed., 2010, 49, 1477 CrossRef CAS PubMed.
- Y. Cui, B. Chen and G. Qian, Coord. Chem. Rev., 2014, 273, 76–86 CrossRef.
- P. Horcajada, R. Gref, T. Baati, P. K. Alla, G. Maurin, P. Couvreur, G. Férey, R. E. Morris and C. Serre, Chem. Rev., 2011, 112, 1232 CrossRef PubMed.
- L. Q. Ma, C. Abney and W. B. Lin, Chem. Soc. Rev., 2009, 38, 1248 RSC.
- B. L. Chen, S. C. Xiang and G. D. Qian, Acc. Chem. Res., 2010, 43, 1115 CrossRef CAS PubMed.
- X. Q. Wu, J. G. Ma, H. Li, D. M. Chen, W. Gu, G. M. Yang and P. Cheng, Chem. Commun., 2015, 51, 9161 RSC.
- X. Wang, Q. X. Wang, Q. H. Wang, F. Gao, F. Gao, Y. Z. Yang and H. X. Guo, ACS Appl. Mater. Interfaces, 2014, 6, 11573 CAS.
- Y. Wang, Y. C. Wu, J. Xie and X. Y. Hu, Sens. Actuators, B, 2013, 177, 1161 CrossRef CAS.
- R. A. Smaldone, R. S. Forgan, H. Furukawa, J. J. Gassensmith, A. M. Z. Slawin, O. M. Yaghi and J. F. Stoddart, Angew. Chem., Int. Ed., 2010, 49, 8630 CrossRef CAS PubMed.
- J. J. Gassensmith, J. Y. Kim, J. M. Holcroft, O. K. Farha, J. F. Stoddart, J. T. Hupp and N. C. Jeong, J. Am. Chem. Soc., 2014, 136, 8277 CrossRef CAS PubMed.
- S. Chen, J. Phys. Chem. B, 2000, 104, 663 CrossRef CAS.
- B. Díaz, J. Światowska, V. Maurice, A. Seyeux, B. Normand, E. Härkönen, M. Ritala and P. Marcus, Electrochim. Acta, 2011, 56, 10516 CrossRef.
- P. K. Vabbina, A. Kaushik, N. Pokhrela, S. Bhansalia and N. Pala, Biosens. Bioelectron., 2015, 63, 124 CrossRef CAS PubMed.
- U. Schröder, J. D. Wadhawan, R. G. Compton, F. Marken, P. A. Z. Suarez, C. S. Consorti, R. F. Souza and J. Dupont, New J. Chem., 2000, 24, 1009 RSC.
- A. J. Bard and L. R. Faulkner, Electrochemical Methods: Fundamental and Applications, John Wiley, New York, 2001 Search PubMed.
- X. Wang, A. Sumboja, M. F. Lin, J. Yan and P. S. Lee, Nanoscale, 2012, 4, 7266–7272 RSC.
- A. Murthy and A. Manthiram, J. Phys. Chem. C, 2012, 116, 3827 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis procedure & measurement of CD-MOF-1, working electrode modifying strategy, and other relevant data. See DOI: 10.1039/c7dt00808b |
| This journal is © The Royal Society of Chemistry 2017 |