Selective alkaline stripping of metal ions after solvent extraction by base-stable 1,2,3-triazolium ionic liquids†
Abstract
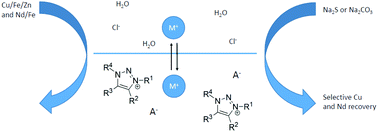
Novel 1,2,3-triazolium ionic liquids with a high base stability were synthesized for use in solvent extraction of first-row transition elements and rare earths from chloride media. The synthesis of these ionic liquids makes use of a recently reported, metal-free multicomponent reaction that allows full substitution of the 1,2,3-triazolium skeleton. The physical and chemical properties of these ionic liquids are compared with those of a trisubstituted analog. Peralkylation of the 1,2,3-triazolium skeleton leads to ionic liquids with superior properties, such as low viscosity, low solubility in water and higher thermal and base stability. Iodide and thiocyanate ionic liquids with peralkylated cations were applied to the solvent extraction of metal ions, and their stability in alkaline media was exploited in the selective stripping of the metals from the loaded ionic liquid phase by alkaline solutions. EXAFS and Raman spectroscopy were performed to gain insight into the extraction mechanism. The applicability of these extraction systems was demonstrated in separations relevant for the recovery of metals from ores and end-of-life products: Fe(III)/Cu(II)/Zn(II) (copper ores, brass scraps) and Fe(III)/Nd(III) (rare earth magnets).


 Please wait while we load your content...
Please wait while we load your content...