Electrocatalytic water oxidation by a molecular catalyst incorporated into a metal–organic framework thin film†
Abstract
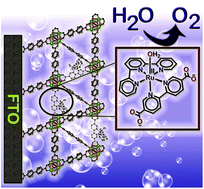
A molecular water oxidation catalyst, [Ru(tpy)(dcbpy)(OH2)](ClO4)2 (tpy = 2,2′:6′,2′′-terpyridine, dcbpy = 2,2′-bipyridine-5,5′-dicarboxylic acid) [1], has been incorporated into FTO-grown thin films of UiO-67 (UiO = University of Oslo), by post-synthetic ligand exchange. Cyclic voltammograms (0.1 M borate buffer at pH = 8.4) of the resulting UiO67-[RuOH2]@FTO show a reversible wave associated with the RuIII/II couple in the anodic scan, followed by a large current response that arises from electrocatalytic water oxidation beyond 1.1 V vs. Ag/AgCl. Water oxidation can be observed at an applied potential of 1.5 V over the timescale of hours with a current density of 11.5 μA cm−2. Oxygen evolution was quantified in situ over the course of the experiment, and the Faradaic efficiency was calculated as 82%. Importantly, the molecular integrity of [1] during electrocatalytic water oxidation is maintained even on the timescale of hours under turnover conditions and applied voltage, as evidenced by the persistence of the wave associated with the RuIII/II couple in the CV. This experiment highlights the capability of metal organic frameworks like UiO-67 to stabilize the molecular structure of catalysts that are prone to form higher clusters in homogenous phase.
- This article is part of the themed collection: Dalton Transactions Inorganic Symposia

 Please wait while we load your content...
Please wait while we load your content...