Co3O4 morphology in the preferential oxidation of CO†
Abstract
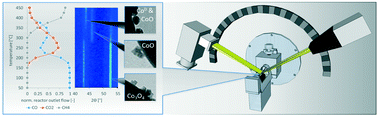
The preferential oxidation (PrOx) of carbon monoxide is an effective process for the removal of trace amounts of CO in a hydrogen-rich gas stream originating from steam reforming or gasification processes. CO can act as catalyst poison in various downstream processes such as the ammonia synthesis or PEM fuel cells for power generation. The effect on activity and selectivity of different cobalt oxide morphologies (cubes, sheets and belts) in Co3O4/SiO2 model catalysts was studied against conventional near-spherical nanoparticles. With a combination of offline and specialized in situ characterisation techniques the stability and catalytic performance of the model catalysts was monitored. With TEM and XRD, the prepared nanosheets and nanobelts were identified as superstructures constituted by small crystallites with similar catalytic activity to conventional nanoparticles. The nanocubes however, consisting of single crystals or at least large crystalline domains, display a superior surface specific CO oxidation activity which is attributed to the preferential exposure of {001} planes. Catalytic sites on these plains seem to support the formation of the Co3+/2+ redox pair required for the underlying Mars-van Krevelen mechanism.


 Please wait while we load your content...
Please wait while we load your content...