The selective oxidation of substituted aromatic hydrocarbons and the observation of uncoupling via redox cycling during naphthalene oxidation by the CYP101B1 system†
Abstract
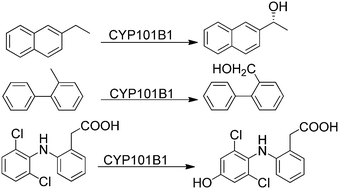
The cytochrome P450 monooxygenase enzyme CYP101B1, from Novosphingobium aromaticivorans DSM12444, efficiently and selectively oxidised a range of naphthalene and biphenyl derivatives. Methyl substituted naphthalenes were better substrates than ethylnaphthalenes and naphthalene itself. The highest product formation activity for a singly substituted alkylnaphthalene was obtained with 2-methylnaphthalene. The oxidation of alkylnaphthalenes was regioselective for the benzylic methyl or methine C–H bonds. The products from 1- and 2-ethylnaphthalene oxidation were highly enantioselective with a single stereoisomer being generated in significant excess. The disubstituted substrate, 2,7-dimethylnaphthalene, had a higher product formation activity than either 1- and 2-methylnaphthalene. Methyl substituted biphenyls were also better substrates than biphenyl and had similar biocatalytic parameters to 1-methylnaphthalene. CYP101B1 catalysed oxidation of 2- and 3-methylbiphenyl was selective for attack at the methyl C–H bonds. The exception was the turnover of 4-methylbiphenyl which generated 4′-(4-methylphenyl)phenol as the major product (70%) with 4-biphenylmethanol making up the remainder. The drug molecule diclofenac was also regioselectively oxidised to 4′-hydroxydiclofenac by CYP101B1. The activity of the CYP101B1 system with naphthalene was more complex and the rate of NADH oxidation increased over time but very little product, 1-naphthol, was generated. Addition of samples of 1-naphthol and 2-naphthol and low concentrations of 1,4-naphthoquinone induced rapid NADH oxidation activity in the in vitro turnovers in both the presence and absence of the cytochrome P450 enzyme. Hydrogen peroxide was generated in these reactions in absence of the P450 enzymes demonstrating that the ferredoxin and ferredoxin reductase in combination with quinones from naphthol oxidation and oxygen can undergo redox cycling giving rise to a form of uncoupling of the reducing equivalents.
- This article is part of the themed collection: 2017 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...