Temperature sensitive synthesis of γ-Al2O3 support with different morphologies for CoMo/γ-Al2O3 catalysts for hydrodesulfurization of thiophene and 4,6-dimethyldibenzothiophene†
Abstract
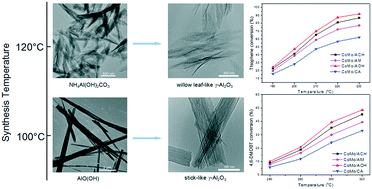
Two different kinds of γ-Al2O3 precursors: stick-like ammonium aluminum carbonate hydroxide and willow leaf-like boehmite could be selectively synthesized via a facile hydrothermal method by just adjusting reaction temperature. After heat treatment, γ-Al2O3 with two different morphologies (stick-like and willow leaf-like) were synthesized and used as supports for CoMo hydrodesulfurization catalysts. Fourier transform infrared spectroscopy of pyridine adsorption and NH3-temperature-programmed desorption showed that stick-like γ-Al2O3 (ACH) and willow leaf-like γ-Al2O3 (AOH) exhibited a lower Lewis acidity than commercial γ-Al2O3. X-ray diffraction indicated that CoMo oxidic catalysts supported on ACH and AOH contained more β-CoMoO4 phase. X-ray photoelectron spectroscopy and high resolution transmission electron microscopy revealed that after presulfurization, more CoMoS active sites and multilayered (Co)MoS2 slabs formed on ACH and AOH supports, thereby creating catalysts with a higher hydrodesulfurization activity in the hydrodesulfurization of thiophene and 4,6-dimethyldibenzothiophene.


 Please wait while we load your content...
Please wait while we load your content...