Highly selective oxidation of cyclohexene to 2-cyclohexene-1-one over polyoxometalate/metal–organic framework hybrids with greatly improved performances
Jinhui
Tong
*ab,
Wenhui
Wang
ab,
Lingdi
Su
ab,
Qing
Li
ab,
Fangfang
Liu
ab,
Wenmei
Ma
ab,
Ziqiang
Lei
ab and
Lili
Bo
*c
aKey Laboratory of Eco-Environment-Related Polymer Materials, Ministry of Education, PR China. E-mail: jinhuitong@126.com; Fax: (+) 86 931 7971533
bKey Laboratory of Gansu Polymer Materials, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou 730070, PR China
cCollege of Science, Gansu Agricultural University, Lanzhouv, P. R. China. E-mail: boxl6666@163.com; Fax: (+) 86 931 7631212
First published on 28th November 2016
Abstract
H3+xPMo12−xVxO40@MIL-100 (Fe) (x = 0, 1, 2) hybrids were prepared by encapsulation of polyoxometalates (POMs) within a metal–organic framework using a direct hydrothermal method. The as-prepared samples were well characterized by X-ray diffraction (XRD), Fourier transform infrared spectrophotometry (FT-IR), transmission electron microscopy (TEM), N2 adsorption–desorption, UV-vis diffused reflectance spectra and inductively coupled plasma atomic emission spectrometry (ICP-AES) analysis. The catalytic performances of the samples were tested in the oxidation of cyclohexene using H2O2 as green oxidant. The results have shown that both H4PMo11VO40@MIL-100 (Fe) and H5PMo10V2O40@MIL-100 (Fe) can effectively catalyze the allylic oxidation of cyclohexene to give 2-cyclohexen-1-one as the main product. In particular, when H4PMo11VO40@MIL-100 (Fe) was employed, 85% cyclohexene conversion, 91% selectivity for 2-cyclohexene-1-one and 715 h−1 of turnover frequency were obtained under optimized conditions. The catalyst can be reused at least five times without obvious loss of activity.
1. Introduction
Catalytic oxidation of alkenes into value-added oxygenated derivatives is a fundamental reaction in organic chemistry since the products obtained are valuable and resourceful commercial intermediates and undergo further reactions.1–4 In particular, allylic oxidation of cyclic olefins, represented by cyclohexene, produces a variety of oxygen-containing derivatives; for example, 2-cyclohexene-1-ol and 2-cyclohexene-1-one are important intermediates in the spice industry and in organic synthesis.5 Upon oxidation of cyclohexene, it undergoes accompanying olefinic oxidation at the double bond in addition to allylic oxidation. This leads to great challenges to obtain high selectivity for an allylic oxidation product.6 Recently, a variety of catalysts for the oxidation of cyclohexene have been reported in the literature, such as expanded graphite supported copper catalyst,7 Fe–Co doped graphitic carbon nitride,8 supported metal complex catalyst,9–11etc. Although there have been some achievements, the search for more stable and efficient catalysts is never-ending.Alternatively, polyoxometalates (POMs) have received much attention in oxidation chemistry12 based on their redox properties. These materials are discrete anionic metaloxygen clusters which exhibit diverse structures and tunable properties.13 In the past decades, numerous catalytic oxidation reactions by POMs have been discovered and mostly with alkenes as substrates.14–20 Although POMs have been widely used in oxidation catalysis,21 their applications are limited by their low specific surface area, low stability under catalytic conditions and difficult separation from most solvents. One of the important strategies to improve the properties of POMs is encapsulating the POMs in porous solid matrixes,15 such as molecular sieves22,23 and porous silica.13,24,25 However, the use of these materials is limited by several drawbacks, such as low POM loading, POM leaching, conglomeration of POM particles, and nonuniform active sites.26 A suitable support which can overcome the above drawbacks is desirable for immobilization of POMs.
Metal–organic frameworks, MOFs, a new class of crystalline porous materials with open frameworks, act as unique and outstanding candidates for carriers due to their high surface areas and uniform but tunable cavities.26 POM-based MOF catalysts have gained the attention of researchers in recent years.27 For example, Ni-containing polyoxometalate encapsulated in MOF 1 and 2 were used as highly efficient catalysts for visible-light-driven hydrogen evolution reaction;28 sandwich-type Eu-polyoxometalate supported on Al(III) and Cr(III) MIL-type MOFs were used as heterogeneous catalysts in desulfurization processes;29 hybrid materials of MIL-101/phosphotungstic acid were used as catalysts for the Baeyer condensation of benzaldehyde and 2-naphthol;30 hybrids of 12-tungstophosphoric heteropolyacid@MIL-100 (Fe) were used as catalysts for esterification and acetalization reactions;31 a direct hydrothermal synthesis was developed to prepare stable H3PMo12O40 insertion within MIL-100 (Fe);32 and H3PMo12O40@MIL-100 (Fe) hybrids were used for the photocatalytic selective oxidation of benzylic alcohols and the reduction of Cr(VI) under visible light irradiation.33 In all the cases, highly improved performances have been obtained from the hybrids compared with the corresponding POMs. All the exciting reports inspire us to develop new POM-MOF catalysts with much higher stability and activity.
Although POM-loaded MOFs have become more and more interesting, MIL-100 (Fe), a MOF derived from an iron salt and benzenetricarboxylic acid, is rarely used for catalytic studies because of its reportedly unknown crystal structure and poor local characterization.34 However, this is not a problem when the material is to be used as a support only. In the current work, hybrid materials of H3+xPMo12−xVxO40@MIL-100 (Fe) have been prepared by a one-pot hydrothermal route and used as catalysts for the allylic oxidation of cyclohexene. The samples have exhibited prominent catalytic activity and high reusability in selectively allylic oxidation of cyclohexene to produce 2-cyclohexene-1-one.
2. Experimental
2.1. Materials and methods
All reagents and solvents are of analytical grade and were used without further purification. The Fourier transform infrared (FT-IR) spectra of the samples were recorded on a ThennoNicolet NEXUS FT-IR instrument (KBr discs) in the 4000–400 cm−1 region. Solid UV-vis diffuse reflection spectra (UV-vis DRS) of the samples were measured with a Shimadzu UV-3100 spectrometer, and BaSO4 was used as an internal standard. X-ray diffraction (XRD) patterns were collected on a Shimadzu XRD-6000 diffractometer using a Cu Kα radiation source at 40 kV and 150 mA. TEM micrographs were obtained using a Hitachi H-600 microscope. BET surface area measurements were performed on a Micromeritics ASAP 2010 instrument at liquid nitrogen temperature. Metal content was determined by inductively coupled plasma spectrometry on a Perkin-Elmer ICP/6500 atomic emission spectrometer.2.2. Preparation of catalyst
2.3. Oxidation of cyclohexene
The liquid phase catalytic oxidation of cyclohexene was carried out in a round-bottom flask (50 mL) connected to a reflux condenser. In a typical reaction, cyclohexene (20 mmol) and catalyst (15 mg) were added to the glass flask. The reaction was initiated by adding 30 wt% H2O2 solution with vigorous stirring. The typical reaction temperature and time were 70 °C and 9 h, respectively. After reaction, the mixture was centrifuged to remove the catalyst, and the organic phase was analyzed using an HP 6890/5973 GC/MS instrument and quantified with an Agilent 6890 gas chromatograph using toluene as inner standard.3. Results and discussion
3.1. Catalyst characterization
Fig. 1 and 2 show the FT-IR spectra of H3+xPMo12−xVxO40, MIL-100 (Fe) and H3+xPMo12−xVxO40@MIL-100 (Fe). The POMs (curves 1a–1c) exhibit four infrared bands characteristic of the Keggin structure: 780–800 cm−1 (Mo–Oc–Mo), 860–880 cm−1 (Mo–Ob–Mo), 960–990 cm−1 (Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Od) and 1060–1080 cm−1 (P–Oa). The vibrational bands of MIL-100 (Fe) (curve 2a) around 1628, 1451, 1371, 761, and 709 cm−1 and the peaks of H3+xPMo12−xVxO40 located at 1062, 959, 864, and 812 cm−1 are all observed in the FT-IR spectra of H3+xPMo12−xVxO40@MIL-100 (Fe) (curves 2b–2d), which confirm the presence of MIL-100 (Fe) and H3+xPMo12−xVxO40 in the hybrid H3+xPMo12−xVxO40@MIL-100 (Fe). Moreover, for H3+xPMo12−xVxO40@MIL-100 (Fe), the νas(P–Oa) and νas(Mo–Oc) vibration bands (959 and 812 cm−1, respectively) were slightly shifted in comparison to those of free POM (967 and 782 cm−1, respectively). This shift discloses the confinement effect of POM inside the porous solid.
Od) and 1060–1080 cm−1 (P–Oa). The vibrational bands of MIL-100 (Fe) (curve 2a) around 1628, 1451, 1371, 761, and 709 cm−1 and the peaks of H3+xPMo12−xVxO40 located at 1062, 959, 864, and 812 cm−1 are all observed in the FT-IR spectra of H3+xPMo12−xVxO40@MIL-100 (Fe) (curves 2b–2d), which confirm the presence of MIL-100 (Fe) and H3+xPMo12−xVxO40 in the hybrid H3+xPMo12−xVxO40@MIL-100 (Fe). Moreover, for H3+xPMo12−xVxO40@MIL-100 (Fe), the νas(P–Oa) and νas(Mo–Oc) vibration bands (959 and 812 cm−1, respectively) were slightly shifted in comparison to those of free POM (967 and 782 cm−1, respectively). This shift discloses the confinement effect of POM inside the porous solid.
 | ||
| Fig. 2 FT-IR spectra of MIL-100 (Fe) (2a), H3PMo12O40@MIL-100 (Fe) (2b), H4PMo11VO40@MIL-100 (Fe) (2c) and H5PMo10V2O40@MIL-100 (Fe) (2d). | ||
The UV-vis diffuse reflectance spectra of the samples are presented in Fig. 3 and 4. All samples have shown absorption bands in the region of 200–400 nm. The absorption bands around 206 nm are associated with O → P transition and the wide energy bands appearing around 307 nm correspond to the ligand to metal charge transfer O2− → Mo6+ in the Keggin units (Fig. 3). It also can be seen from Fig. 4 that MIL-100 (Fe) has a peak near 340 nm (curve 4d). Compared with the corresponding POM and MIL-100 (Fe), the characteristic peaks of the hybrid material (curves 4a–4c) red shifted, indicating interactions between the two moieties.
 | ||
| Fig. 4 UV-vis DRS of H3PMo12O40@MIL-100 (Fe) (4a), H4PMo11VO40@MIL-100 (Fe) (4b), H5PMo10V2O40@MIL-100 (Fe) (4c), and MIL-100 (Fe) (4d). | ||
The XRD patterns of the as-synthesized POMs are shown in Fig. 5. All the samples present diffraction peaks typically at 2θ around 8° and 27°. The XRD patterns for the MIL-100 (Fe) and H3+xPMo12−xVxO40@MIL-100 (Fe) hybrids are illustrated in Fig. 6 and all samples present diffraction peaks typically at 2θ of 10.7°. However, no diffraction peaks for the original H3+xPMo12−xVxO40 can be observed in the H3+xPMo12−xVxO40@MIL-100 (Fe) hybrids. This is probably due to the following two reasons: (1) the size of H3+xPMo12−xVxO40 clusters is relatively smaller; (2) the H3+xPMo12−xVxO40 clusters have been encapsulated in the cavities of MIL-100 (Fe). A similar phenomenon was also observed in previous literature.35
 | ||
| Fig. 6 XRD of the samples: MIL-100 (Fe) (6a), H3PMo12O40@MIL-100 (Fe) (6b), H4PMo11VO40@MIL-100 (Fe) (6c), and H5PMo10V2O40@MIL-100 (Fe) (6d). | ||
The morphologies and elemental compositions of MIL-100 (Fe) and the hybrids H3+xPMo12−xVxO40@MIL-100 (Fe) were characterized by TEM/EDS. A representative image of MIL-100 (Fe) is shown in Fig. 7a and the images and EDS result of the sample H4PMo11VO40/MIL-100 (Fe) are shown in Fig. 7b–d. It is clear that the MIL-100 (Fe) displays a polyhedral morphology and the morphology and porous network of the MIL-101 (Fe) host remained intact after encapsulation of the POMs and no conglomeration of POM particles was observed. The EDS elemental analysis pattern of the sample H3+xPMo12−xVxO40@MIL-100 (Fe) (Fig. 7d) reveals the presence of Fe, P, Mo, and V elements in the H3+xPMo12−xVxO40@MIL-100 (Fe) hybrids.
The N2 adsorption–desorption isotherms of MIL-100 (Fe) and the hybrid samples are presented in Fig. 8 (inset is the pore size distribution plot). The pore size distribution curves of the as-prepared MIL-100 (Fe) and the hybrids display two different pore sizes centered at about 1.4 and 2.3 nm, demonstrating the presence of the two types of mesoporous cages in these samples. The BET surface areas and pore volumes of the samples are listed in Table 1. The BET surface area and pore volume for the prepared MIL-100 (Fe) are 1689 m2 g−1 and 1.02 cm3 g−1, respectively, close to the values reported in ref. 31. On the other hand, both specific surface areas and pore volumes of the hybrids lowered remarkably to ∼900 m2 g−1 and ∼0.5 cm3 g−1, respectively, after encapsulation of the POMs (Table 1).
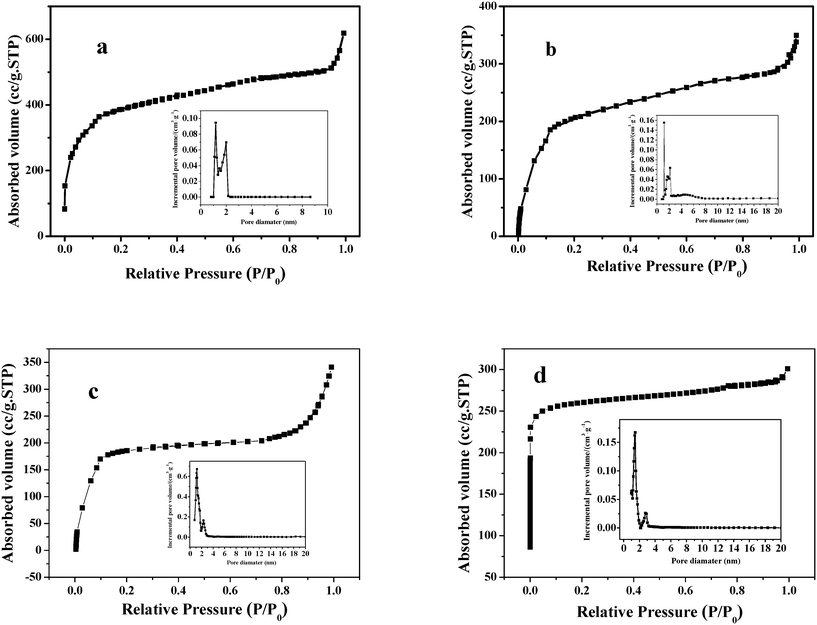 | ||
| Fig. 8 N2 adsorption–desorption isotherms and pore size distribution plots of MIL-100 (Fe) (a), H3PMo12O40/MIL-100 (Fe) (b), H4PMo11VO40/MIL-100 (Fe) (c) and H5PMo10V2O40/MIL-100 (Fe) (d). | ||
| Sample | BET surface area (m2 g−1) | Pore volume (cm3 g−1) | POM loading (×10−4) (mmol mg−1) |
|---|---|---|---|
| MIL-100 (Fe) | 1689 | 1.02 | — |
| H3PMo12O40@MIL-100 (Fe) | 908 | 0.35 | 1.92 |
| H4PMo11VO40@MIL-100 (Fe) | 931 | 0.42 | 1.76 |
| H5PMo10V2O40@MIL-100 (Fe) | 965 | 0.58 | 1.64 |
The POM loadings of the hybrid based on Mo and V determined by ICP-AES are also listed in Table 1. It can be seen that a higher POM loading leads to more decrease in specific surface area and pore volume. This confirms that the POM molecules were encapsulated in the pores of MIL-101 (Fe).
3.2. Catalytic performance of catalysts
The catalytic performances of the as-prepared catalysts in the oxidation of cyclohexene were investigated using H2O2 as oxidant and acetonitrile as solvent. The detectable products are epoxide, 2-cyclohexen-1-ol (denoted as -ol), 2-cyclohexen-1-one (denoted as -one) and 1,2-cyclohexanediol (denoted as -diol) (Scheme 1).The results of cyclohexene oxidation over different catalysts are summarized in Table 2. The result in the absence of catalyst is also listed in entry 1 for comparison. It was found that only 11% cyclohexene conversion was obtained in the absence of catalyst. Although the POMs and MIL-100 (Fe) have shown considerable catalytic activities, not more than 37% cyclohexene conversion was obtained (entries 2–5) while a greatly improved cyclohexene conversion of 72–85% was obtained in the cases of the POM/MIL-100 (Fe) hybrids (entries 6–8). The main reason for the greatly improved activity for the POM/MIL-100 (Fe) hybrids is that the porous structure of the hybrids can enhance the accessibility of the reactant molecules to active centers and the fast diffusion of products.5
| Entry | Catalyst | Conversion (%) | TOFb (h−1) | Selectivityc (%) | |||
|---|---|---|---|---|---|---|---|
| Epoxide | -ol | -one | -diol | ||||
| a Reaction conditions: cyclohexene 20 mmol, H2O2 (30 wt%) 40 mmol, catalyst 15 mg, acetonitrile 5 mL, 70 °C, 9 h. b TOF = mol of cyclohexene converted per mol of loaded POM per hour. c Selectivity = mol ratio of the given product to the total products. d From ref. 36. Reaction condition: 10 mL solution of n-heptane–TBHP (nTBHP = 10% ncyclohexene), 6 mmol cyclohexene, 50 mg catalyst, 333 K. e From ref. 37. Reaction conditions: 0.2 M cyclohexene, 0.4 M H2O2, 20 mg catalyst, 1.5 mL MeCN, 70 °C. | |||||||
| 1 | None | 11 | — | 99 | — | — | 1 |
| 2 | H3PMo12O40 | 31 | 84 | 68 | — | — | 32 |
| 3 | H4PMo11VO40 | 33 | 87 | — | — | 17 | 83 |
| 4 | H5PMo10V2O40 | 33 | 85 | — | — | 29 | 71 |
| 5 | MIL-100 (Fe) | 37 | 47 | — | 13 | 51 | 36 |
| 6 | H3PMo12O40@MIL-100 (Fe) | 72 | 556 | — | — | 46 | 54 |
| 7 | H4PMo11VO40@MIL-100 (Fe) | 85 | 715 | — | 9 | 91 | — |
| 8 | H5PMo10V2O40@MIL-100 (Fe) | 75 | 677 | — | 16 | 84 | — |
| 9d | PdCl2-ILs/CuBTC | 24 | 53.2 (-one) | ||||
| 10e | Ti-POM/MIL-101 | 39 | 32 (-ol + -one) | ||||
It is very interesting that the encapsulation greatly influenced the product distribution. The POMs are in favor of producing 1,2-cyclohexanediol (entries 3–4) except for H3PMo12O40, over which 68% selectivity for the main product epoxide has been obtained (entry 2). In the case of MIL-100 (Fe), only 51% selectivity for the main product 2-cyclohexen-1-one has been obtained. However, the POM/MIL-100 (Fe) hybrids are in favor of producing 2-cyclohexen-1-one and higher than 84% selectivity has been obtained (entries 7–8) except for H3PMo12O40@MIL-100 (Fe) over which 54% selectivity for the main product 1,2-cyclohexanediol has been obtained (entry 6). In particular, the hybrid H4PMo11VO40@MIL-100 (Fe) has obtained the highest value of 85%, 91% and 715 h−1 of cyclohexene conversion, 2-cyclohexene-1-one selectivity and turnover frequency (TOF), respectively. This is a more exciting result than that reported for metal–organic framework CuBTC immobilized PdCl2 with the aid of ionic liquids (ILs)36 (entry 9) and titanium-monosubstituted Keggin heteropolyanions electrostatically bound to the chromium terephthalate polymer matrix MIL-101 (entry 10).37
According to the literature,38,39 the oxidation of cyclohexene with H2O2 initially forms 2-cyclohexene-1-hydroperoxide as shown in Scheme 2 (step 1). 2-Cyclohexene-1-hydroperoxide is not stable and can form epoxide and 2-cyclohexene-1-ol by epoxidation of cyclohexene (step 2), decompose to 2-cyclohexene-1-ol and 2-cyclohexene-1-one in the presence of catalyst (step 3), or decompose to 2-cyclohexene-1-one and water (step 4). The conversion of cyclohexene is controlled by the rate of step 1 in Scheme 2 and the product distribution is controlled by the rate ratio of step 2 to steps 3 and 4. That is, the encapsulation greatly changed the rate ratio of step 2 to steps 3 and 4, and thus the distribution of the products.
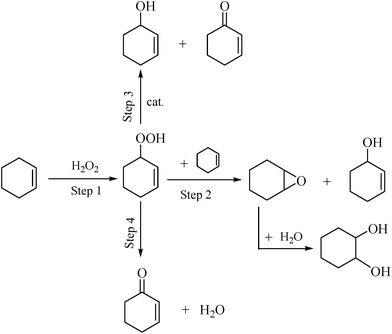 | ||
| Scheme 2 Proposed process of cyclohexene oxidation.38,39 | ||
Most importantly, the POMs have been heterogenized after being encapsulated in MIL-100 (Fe). That is, the POM/MIL-100 (Fe) hybrids can be recovered and are reusable. In consideration of the high cyclohexene conversion, 2-cyclohexene-1-one selectivity and TOF, H4PMo11VO40@MIL-100 (Fe) was chosen as the preferable catalyst to optimize the following reaction conditions.
| Entry | Amount of catalyst (mg) | Conversion (%) | TOFb (h−1) | H2O2 efficiency (%) | Selectivityc (%) | |
|---|---|---|---|---|---|---|
| -ol | -one | |||||
| a Reaction conditions: cyclohexene 20 mmol, H2O2 (30 wt%) 40 mmol, acetonitrile 5 mL, 70 °C, 9 h. b TOF = mol of cyclohexene converted per mol of loaded POM per hour. c Selectivity = mol ratio of the given product to the total products. | ||||||
| 1 | 5 | 26 | 656 | 56 | 20 | 80 |
| 2 | 10 | 53 | 669 | 73 | 16 | 84 |
| 3 | 15 | 85 | 715 | 81 | 9 | 91 |
| 4 | 20 | 89 | 561 | 74 | 14 | 86 |
| 5 | 25 | 92 | 464 | 69 | 18 | 82 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 2.5
2 to 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the results are shown in Table 4. It is clear that cyclohexene conversion increased with the increase of H2O2. As a result, when the molar ratio of H2O2/cyclohexene increased from 1
1, and the results are shown in Table 4. It is clear that cyclohexene conversion increased with the increase of H2O2. As a result, when the molar ratio of H2O2/cyclohexene increased from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 2.5
2 to 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, cyclohexene conversion increased from 26% to 88%. The selectivity for 2-cyclohexene-1-one increased from 42% to 91% when the molar ratio of H2O2/cyclohexene increased from 1
1, cyclohexene conversion increased from 26% to 88%. The selectivity for 2-cyclohexene-1-one increased from 42% to 91% when the molar ratio of H2O2/cyclohexene increased from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 2
2 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, but then decreased to 87% when the molar ratio of H2O2/cyclohexene increased further to 2.5
1, but then decreased to 87% when the molar ratio of H2O2/cyclohexene increased further to 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The second highest values of 81% and 715 h−1 for H2O2 efficiency and TOF, respectively, were also obtained when the molar ratio of H2O2/cyclohexene was 2
1. The second highest values of 81% and 715 h−1 for H2O2 efficiency and TOF, respectively, were also obtained when the molar ratio of H2O2/cyclohexene was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Based on the above facts, the molar ratio of 2
1. Based on the above facts, the molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was chosen for the following investigations.
1 was chosen for the following investigations.
| Entry | H2O2/cyclohexene (mol/mol) | Conversion (%) | TOFb (h−1) | H2O2 efficiency (%) | Selectivityc (%) | ||
|---|---|---|---|---|---|---|---|
| -ol | -one | -diol | |||||
| a Reaction conditions: cyclohexene 20 mmol, H4PMo11VO40@MIL-100 (Fe) 15 mg, acetonitrile 5 mL, 70 °C, 9 h. b TOF = mol of cyclohexene converted per mol of loaded POM per hour. c Selectivity = mol ratio of the given product to the total products. | |||||||
| 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
26 | 218 | 84 | 40 | 42 | 18 |
| 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
42 | 353 | 77 | 39 | 61 | — |
| 3 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
70 | 589 | 80 | 22 | 78 | — |
| 4 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
85 | 715 | 81 | 9 | 91 | — |
| 5 | 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
88 | 740 | 70 | 13 | 87 | — |
| Entry | Reaction time (h) | Conversion (%) | TOFb (h−1) | H2O2 efficiency (%) | Selectivityc (%) | ||
|---|---|---|---|---|---|---|---|
| -ol | -one | -diol | |||||
| a Reaction conditions: cyclohexene 20 mmol, H2O2 (30 wt%) 40 mmol, H4PMo11VO40@MIL-100 (Fe) 15 mg, acetonitrile 5 mL, 70 °C. b TOF = mol of cyclohexene converted per mol of loaded POM per hour. c Selectivity = mol ratio of the given product to the total products. | |||||||
| 1 | 3 | 51 | 1287 | 72 | 18 | 61 | 21 |
| 2 | 5 | 56 | 848 | 74 | 22 | 68 | 10 |
| 3 | 7 | 78 | 844 | 77 | 11 | 75 | 4 |
| 4 | 9 | 85 | 715 | 81 | 9 | 91 | — |
| 5 | 11 | 88 | 606 | 73 | 6 | 94 | — |
| Entry | Reaction temperature (°C) | Conversion (%) | TOFb (h−1) | H2O2 efficiency (%) | Selectivityc (%) | ||
|---|---|---|---|---|---|---|---|
| -ol | -one | -diol | |||||
| a Reaction conditions: cyclohexene 20 mmol, H2O2 (30 wt%) 40 mmol, H4PMo11VO40@MIL-100 (Fe) 15 mg, acetonitrile 5 mL, 9 h. b TOF = mol of cyclohexene converted per mol of loaded POM per hour. c Selectivity = mol ratio of the given product to the total products. | |||||||
| 1 | 40 | 32 | 269 | 60 | — | 5 | 95 |
| 2 | 50 | 53 | 446 | 70 | — | 34 | 66 |
| 3 | 60 | 67 | 563 | 75 | 15 | 62 | 23 |
| 4 | 70 | 85 | 715 | 81 | 9 | 91 | — |
3.3. Reusability of the catalyst
To investigate the reusability of the catalyst H4PMo11VO40@MIL-100 (Fe), the catalyst was separated by centrifugation after the first run, dried at 150 °C under vacuum and then subjected to the second run under the same conditions. After five consecutive cycles under our optimized conditions, the catalytic activity of the sample hardly dropped. The average values for cyclohexene conversion, the selectivity to 2-cyclohexene-1-ol, 2-cyclohexene-1-one and TOF were 83%, 10%, 90% and 698 h−1, respectively, which were nearly the same as those of the fresh one (Fig. 9). To estimate the stability of the catalyst, the loading of POM and the porous structure of the catalyst were investigated again after five recycle runs. The values of POM loading, BET surface area and pore volume are 1.70 mmol mg−1, 854 m2 g−1 and 0.37 cm3 g−1, which are 3.04%, 8.20% and 10.78% lower than those of the fresh one, respectively. The porous structure of the five-run reused catalyst hardly changed except for a 0.3 nm decrease in pore diameter (centered at 1.0 nm and 1.9 nm) compared with the fresh one as depicted in Fig. 10. The results confirmed that the catalyst has high stability and reusability. | ||
| Fig. 9 Reusability of the catalyst H4PMo11VO40@MIL-100 (Fe). Reaction conditions: cyclohexene 20 mmol, H2O2 (30 wt%) 40 mmol, H4PMo11VO40@MIL-100 (Fe) 15 mg, acetonitrile 5 mL, 70 °C, 9 h. | ||
 | ||
| Fig. 10 N2 adsorption–desorption isotherm and pore size distribution plot of H4PMo11VO40/MIL-100 (Fe) after five runs. | ||
4. Conclusions
Polyoxometalate catalysts have been successfully heterogenized through a simple one-pot hydrothermal method by encapsulation of H3+xPMo12−xVxO40 in the metal–organic framework MIL-100 (Fe). The hybrids have shown greatly improved catalytic activities and stabilities in challenging the allylic oxidation of cyclohexene using H2O2 as green oxidant compared with the corresponding moieties. In particular, the hybrid H4PMo11VO40@MIL-100 (Fe) has obtained the average values of 83% and 90% for cyclohexene conversion and 2-cyclohexene-1-one, respectively, after five cycles. This is a most outstanding example of the preparation of POMs/MOF hybrids and selective allylic oxidation of cyclohexene to produce 2-cyclohexene-1-one with high selectivity.Acknowledgements
The authors are grateful to the National Natural Science Foundation of China (51302222, 21363021, 21202133), the Natural Science Foundation of Gansu Province (1308RJYA017), and the Program for Changjiang Scholars and Innovative Research Team in University (IRT15R56) for financial support.References
- D. E. De Vos, S. de Wildeman, B. F. Sels, P. J. Grobet and P. A. Jacobs, Angew. Chem., Int. Ed., 1999, 38, 980–983 CrossRef CAS.
- C. Yin, Z. Yang, B. Li, F. Zhang, J. Wang and E. Ou, Catal. Lett., 2009, 131, 440–443 CrossRef CAS.
- I. Y. Skobelev, A. B. Sorokin, K. A. Kovalenko, V. P. Fedin and O. A. Kholdeeva, J. Catal., 2013, 298, 61–69 CrossRef CAS.
- Y. Cao, H. Yu, F. Peng and H. Wang, ACS Catal., 2014, 4, 1617–1625 CrossRef CAS.
- G. Zou, D. Jing, W. Zhong, F. Zhao, L. Mao, Q. Xu, J. Xiao and D. Yin, RSC Adv., 2016, 6, 3729–3734 RSC.
- D. R. Godhani, H. D. Nakum, D. K. Parmar, J. P. Mehta and N. C. Desai, J. Mol. Catal. A: Chem., 2016, 415, 37–55 CrossRef CAS.
- R. Zhang and R. Tang, J. Mater. Sci., 2016, 51, 5802–5810 CrossRef CAS.
- D. Yang, T. Jiang, T. Wu, P. Zhang, H. Han and B. Han, Catal. Sci. Technol., 2016, 6, 193–200 Search PubMed.
- S. Urus, H. Achguzel and M. Incesu, Chem. Eng. J., 2016, 296, 90–101 CrossRef CAS.
- M. Bazarganipour and M. Salavati-Niasari, Chem. Eng. J., 2016, 286, 259–265 CrossRef CAS.
- L. Q. Xu, J. C. Chen, S. S. Qian, A. K. Zhang, G. D. Fu, C. M. Li and E.-T. Kang, Macromol. Chem. Phys., 2015, 216, 417–426 CrossRef CAS.
- W. Adam, M. Herold, C. L. Hill and C. R. Saha-Moller, Eur. J. Org. Chem., 2002, 941–946 CrossRef CAS.
- J. A. F. Gamelas, F. Oliveira, M. G. Evtyugina, I. Portugal and D. V. Evtuguin, Appl. Catal., A, 2016, 509, 8–16 CrossRef CAS.
- U. Jameel, M. Q. Zhu, X. Z. Chen and Z. F. Tong, J. Mater. Sci., 2016, 51, 2181–2198 CrossRef CAS.
- A. Dolbecq, E. Dumas, C. R. Mayer and P. Mialane, Chem. Rev., 2010, 110, 6009–6048 CrossRef CAS PubMed.
- X. J. Song, W. C. Zhu, K. G. Li, J. Wang, H. L. Niu, H. C. Gao, W. X. Gao, W. X. Zhang, J. H. Yu and M. J. Jia, Catal. Today, 2016, 259, 59–65 CrossRef CAS.
- M. R. Farsani, F. Jalilian, B. Yadollahi and H. A. Rudbari, Appl. Organomet. Chem., 2015, 29, 7–11 CrossRef.
- F. Bentaleb, O. Makrygenni, D. Brouri, C. C. Diogo, A. Mehdi, A. Proust, F. Launay and R. Villanneau, Inorg. Chem., 2015, 54, 7607–7616 CrossRef CAS PubMed.
- A. Mouret, L. Leclercq, A. Muhlbauer and V. Nardello-Rataj, Green Chem., 2014, 16, 269–278 RSC.
- V. Mirkhani, M. Moghadam, S. Tangestaninejad, I. Mohammadpoor-Baltork, E. Shams and N. Rasouli, Appl. Catal., A, 2008, 334, 106–111 CrossRef CAS.
- M. Sun, J. Zhang, P. Puta, V. Caps, F. D. R. Lefebvre, J. Pelletier and J.-M. Basset, Chem. Rev., 2014, 114, 981–1019 CrossRef CAS PubMed.
- D. J. Thompson, Y. Zhang and T. Ren, J. Mol. Catal. A: Chem., 2014, 392, 188–193 CrossRef CAS.
- L. Suo, R. Q. Meng, D. M. Zheng, L. X. Wu and L. H. Bi, Appl. Organomet. Chem., 2014, 28, 845–851 CrossRef CAS.
- M. Li, M. Zhang, A. Wei, W. Zhu, S. Xun, Y. Li, H. Li and H. Li, J. Mol. Catal. A: Chem., 2015, 406, 23–30 CrossRef CAS.
- N. Wu, B. Li, W. Ma and C. Han, Microporous Mesoporous Mater., 2014, 186, 155–162 CrossRef CAS.
- D. Y. Du, J. S. Qin, S. L. Li, Z. M. Su and Y. Q. Lan, Chem. Soc. Rev., 2014, 43, 4615–4632 RSC.
- G. Férey, Chem. Soc. Rev., 2008, 37, 191–214 RSC.
- X.-J. Kong, Z. Lin, Z.-M. Zhang, T. Zhang and W. Lin, Angew. Chem., 2016, 55, 6411–6416 CrossRef CAS PubMed.
- C. M. Granadeiro, L. S. Nogueira, D. Juliao, F. Mirante, D. Ananias, S. S. Balula and L. Cunha-Silva, Catal. Sci. Technol., 2016, 6, 1515–1522 CAS.
- L. Bromberg, Y. Diao, H. M. Wu, S. A. Speakman and T. A. Hatton, Chem. Mater., 2012, 24, 1664–1675 CrossRef CAS.
- F. M. Zhang, Y. Jin, J. Shi, Y. J. Zhong, W. D. Zhu and M. S. El-Shall, Chem. Eng. J., 2015, 269, 236–244 CrossRef CAS.
- R. Canioni, C. Roch-Marchal, F. Secheresse, P. Horcajada, C. Serre, M. Hardi-Dan, G. Ferey, J. M. Greneche, F. Lefebvre, J. S. Chang, Y. K. Hwang, O. Lebedev, S. Turner and G. Van Tendeloo, J. Mater. Chem., 2011, 21, 1226–1233 RSC.
- R. Liang, R. Chen, F. Jing, N. Qin and L. Wu, Dalton Trans., 2015, 44, 18227–18236 RSC.
- A. Micek-Ilnicka and B. Gil, Dalton Trans., 2012, 41, 12624–12629 RSC.
- J. S. Li, Y. J. Tang, C. H. Liu, S. L. Li, R. H. Li, L. Z. Dong, Z. H. Dai, J. C. Bao and Y. Q. Lan, J. Mater. Chem. A, 2016, 4, 1202–1207 CAS.
- Q. X. Luo, M. Ji, S. E. Park, C. Hao and Y. Q. Li, RSC Adv., 2016, 6, 33048–33054 RSC.
- N. V. Maksimchuk, M. N. Timofeeva, M. S. Melgunov, A. N. Shmakov, Y. A. Chesalov, D. N. Dybtsev, V. P. Fedin and O. A. Kholdeeva, J. Catal., 2008, 257, 315–323 CrossRef CAS.
- L. Yanyong, M. Kazuhisa, I. Megumu, N. Hitoshi, K. Masahiko and T. Keizou, Chem. Lett., 2004, 33, 200–201 CrossRef.
- M. N. Timofeeva, S. H. Jhung, Y. K. Hwang, D. K. Kim, V. N. Panchenko, M. S. MeIgunov, Y. A. Chesalov and J. S. Chang, Appl. Catal., A, 2007, 317, 1–10 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |





