Ion-solvation structure and battery electrode characteristics of nonflammable organic electrolytes based on tris(trifluoroethyl)phosphate dissolving lithium salts†
Abstract
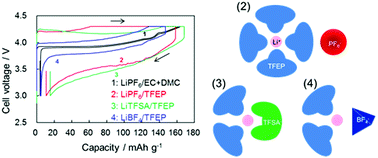
The structure and properties of lithium salt solutions based on tris(2,2,2-trifluoroethyl)phosphate (TFEP) solvent have been studied to design a safer electrolyte system for large-sized lithium-ion battery applications. Influences of the ionic structure on the polarization behavior of the LiCoO2 (LCO) positive electrode were investigated. The ionic conductivity and viscosity of the solution consisting of lithium salts dissolved in TFEP, LiX/TFEP (X = PF6, BF4 and TFSA) (TFSA = (CF3SO2)2N), were measured. The results suggest that the ion-solvation structure greatly depends on the anionic species in the salt. Spectroscopic measurements also support the conclusion that the Li+-solvation structure varies with the lithium salts. The differences in the ionic structure of LiX/TFEP influence the electrochemical oxidation potential of the solution and the polarization behavior of the LCO electrode. The overvoltage for Li-desertion/insertion from/into LCO in LiX/TFEP, being much higher than that observed in conventional LIB electrolyte solutions, shows the order of BF4 < PF6 < TFSA. The addition of ethylene carbonate (EC) to LiX/TFEP increases the ionic conductivity, which is probably caused by changes in the Li+-solvation structure in TFEP. The overvoltage for the Li-desertion/insertion of LCO is much lowered by the addition of EC to LiX/TFEP.


 Please wait while we load your content...
Please wait while we load your content...