Self-assembled morphologies of an amphiphilic Y-shaped weak polyelectrolyte in a thin film
Abstract
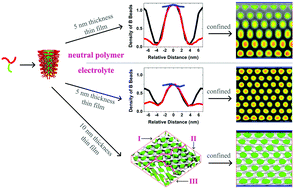
Different from the self-assembly of neutral polymers, polyelectrolytes self-assemble into smaller aggregates with a more loosely assembled structure, which results from the repulsive forces acting between similar electrical compositions with the introduction of ions. The Y-shaped weak polyelectrolytes self-assemble into a core–shell type cylindrical structure with a hexagonal arrangement in a thin film, whose thickness is smaller than the gyration radius of the polymer chain. The corresponding formation mechanism consists of enrichment of the same components, adjustment of the shape of the aggregate, and the subsequent separation into individual aggregates. With the increase in the thickness of the thin film until it exceeds the gyration radius of the polymer chain, combined with the greater freedom of movement along the direction of thin film thickness, the self-assembled structure changes into a micellar structure. Under confinement, the repulsive force to the polymeric components is weakened by the repulsive forces among polyelectrolyte components with like charges, and this helps in generating aggregates with more uniform size and density distribution. In particular, when the repulsive force between the walls and the core forming components is greater than that between the walls and the shell forming components, such asymmetric confinement produces a crossed-cylindrical structure with nearly perpendicular arrangement of two cylinder arrays. Similarly, a novel three-crossed cylinder morphology is self-assembled upon removal of confinement.


 Please wait while we load your content...
Please wait while we load your content...