Enhanced fluorescence with nanosecond dynamics in the solid state of metal ion complexes of alkoxy salophens†
Abstract
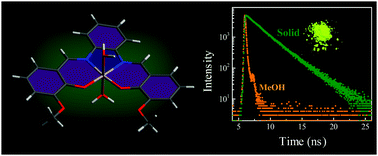
Salophen (N,N′-bis-(salicylidene)-o-phenylenediamine) is a Schiff base, which is weakly emissive in solution, but strongly emissive in the solid state. The trend is reversed in its aluminium complex (SalAl+), which is strongly emissive in solution, but feebly emissive in the solid state. Thus, SalAl+ is not attractive for application as a solid state emitter. In the present study, salophen derivatives containing –OCH3 and OC2H5 groups have been studied with the intent to perturb the structure and control the emission properties. The newly synthesized salophen derivatives are feebly emissive with ultrafast lifetime in solution. The mutiexponential nature of their fluorescence decay is ascribed to multiple ground state conformers of the enol and keto tautomers. In the solid phase, the ligands exhibit an order of magnitude increase in emission. Complexation with Al3+ (and Zn2+) does not bring about any significant increase in emissivity in solution, possibly because rotation/vibration of the alkoxy groups (with a contribution from axial water molecules) now provides the major nonradiative deactivation pathway. Such pathways are blocked in the solid state, leading to an increase in emission of up to 50 times in the aluminium complex. Thus, introduction of small functionalities is found to have profound effects on the suitability of a molecule as a solid state emitter.
- This article is part of the themed collection: New Frontiers in Indian Research


 Please wait while we load your content...
Please wait while we load your content...