 Open Access Article
Open Access ArticleFast flip–flop of halogenated cobalt bis(dicarbollide) anion in a lipid bilayer membrane
Tatyana I.
Rokitskaya
 *a,
Irina D.
Kosenko
b,
Igor B.
Sivaev
*a,
Irina D.
Kosenko
b,
Igor B.
Sivaev
 b,
Yuri N.
Antonenko
a and
Vladimir I.
Bregadze
b
b,
Yuri N.
Antonenko
a and
Vladimir I.
Bregadze
b
aBelozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, Moscow 119991, Russian Federation. E-mail: rokitskaya@genebee.msu.ru
bA. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, 28 Vavilov Str., 119991, Moscow, Russian Federation
First published on 7th September 2017
Abstract
Transmembrane translocation (flip–flop) of cobalt bis(dicarbollide) (COSAN) anions, elicited by application of a voltage-jump across the lipid bilayer membrane, manifested itself in monoexponential electrical current transients in the microsecond time scale. Halogenation of COSAN led to multi-fold acceleration of the flip–flop, the effect increasing with the molecular weight of the halogens. The exception was a fluorinated analog which exhibited slowing of the translocation kinetics. Measurements of the fluorescence ratio of the dye di-4-ANEPPS in lipid vesicles showed significant differences in the adsorption of studied hydrophobic anions. Based on these data, it can be concluded that COSAN and COSAN-F2 were located on the surface of the lipid membrane in the cisoid conformation increasing the dipole potential of the lipid membrane, while other halogenated COSAN analogs were adsorbed in the transoid conformation. Differences in the flip–flop kinetics of COSAN analogs were attributed to variation in the molecular volume of the anions and their orientation on the membrane surface.
1. Introduction
Biological membranes are formed by self-assembly of lipid molecules, with their polar head groups exposed to the aqueous environment and their fatty acid tails orientated internally, to create a hydrophobic barrier. In addition to passive diffusion, the permeability of cellular membranes is determined by protein channels and carriers which orchestrate the highly regulated selective transport of ions and metabolites of living cells. In contrast to small inorganic ions, which have poor passive permeability through lipid bilayers, some large organic ions were shown to permeate readily through lipid membranes especially anions with delocalized charge.1–4 The anion of cobalt bis(dicarbollide) [3,3′-Co(1,2-C2B9H11)2]− (COSAN, Fig. 1, left structure on the top) consists of the two dicarbollide ligands sandwiching the central cobalt atom and has a dispersed net charge but a hydrophobic surface of two dicarbollide moieties due to weakly polarized B–H and C–H bonds.5,6 Surprisingly, COSAN molecules are surface-active7 and form a monolayer at the water–air interface with the long axis normal to the surface.8 Furthermore cobalt bis(dicarbollide) exhibits a self-association in water9 that is comparable to classical micellization.10 The origin of the aggregation is still controversial according to the literature. One explanation assumes formation of the intermolecular dihydrogen B–H⋯H–C bonds in COSAN solutions that results in their aggregation11 whereas the other one is based on non-specific hydrophobic action.10 The combination of hydrophobic semi-cages and the hydrophilic center of COSAN together with the rigid ends (θ shape12) makes it form small monolayer vesicles in aqueous solution at a critical concentration of tenths of micromoles with a radius of about 20 nm. It has been shown that the vesicles of COSAN permeate through artificial13 and natural14 membranes. Iodinated cobalt bis(dicarbollide) [8,8′-I2-3,3′-Co(1,2-C2B9H10)2]− (COSAN-I2, Fig. 1, right structure on the top) accumulates in the cells of Dictyostelium14 in spite of its lower (compared to COSAN) permeability through artificial lipid membranes. Importantly, the permeation of COSAN is not accompanied by disruption of membrane integrity. The above properties raise a question about the mechanism of the permeability of COSAN and its halogenated derivatives. | ||
| Fig. 1 Chemical structure of cobalt bis(dicarbollide) (COSAN) and its halogenated analogs studied in the present work. | ||
The mechanism of the transmembrane translocation of tetraphenylborate and some other hydrophobic anions was studied several decades ago by measuring relaxation of the electrical current across a planar bilayer lipid membrane (BLM) after applying a voltage.3,15 The approach can be applied to hydrophobic cations as well16,17 provided they possess a high affinity to the lipid–water interface. The transport of hydrophobic ions across the membrane involves three stages: adsorption to the membrane–solution interface, transition through an energy barrier to the opposite interface, and desorption into the aqueous solution. In this work a comparison of the kinetics of the current relaxation of COSAN and their halogenated derivatives allowed us to reveal differences in the process of their transmembrane permeation which were discussed in connection to the molecular volume of the anions and their orientation on the membrane surface.
2. Materials and methods
Materials
Salts of cobalt bis(dicarbollide) Na[3,3′-Co(1,2-C2B9H11)2],18 fluorinated cobalt bis(dicarbollide) ((Bu4N)[8,8′-F2-3,3′-Co(1,2-C2B9H10)2]),19 chlorinated cobalt bis(dicarbollide) ((Me3NH)[8,8′-Cl2-3,3′-Co(1,2-C2B9H10)2]),20 brominated cobalt bis(dicarbollide) ((Me3NH)[8,8′-Br2-3,3′-Co(1,2-C2B9H10)2])20 and iodinated cobalt bis(dicarbollide) ((Me4N)[8,8′-I2-3,3′-Co(1,2-C2B9H10)2])20 (Fig. 1) were synthesized as described in the literature.Planar bilayer
BLMs were formed from a 2% solution of diphytanoylphosphatidylcholine (DPhPC) (Avanti Polar Lipids, Alabaster, AL) in n-decane on a hole in a Teflon partition separating two compartments of a cell containing aqueous buffer solutions.21 The cell with the 0.8 mm diameter hole was used in current relaxation experiments. Hydrophobic anions were added from stock solutions in ethanol to the bathing solutions at both sides of the BLM and routinely incubated for at least 5 min with constant stirring. The solution consisted of 100 mM KCl, 10 mM TRIS, and 10 mM MES, pH 6.0. All experiments were carried out at room temperature (23–25 °C).In the current relaxation experiments voltages were applied to BLMs by means of a Siglent3408 waveform generator (square form of the waves) with Ag–AgCl electrodes placed into the solutions on the two sides of the BLM via agar bridges. The electric current (I) was recorded using a Keithley 428 amplifier (Cleveland, Ohio, USA), digitized using an NI-DAQmx (National Instruments, Austin, TX) and analyzed with a personal computer with the use of WinWCP Strathclyde Electrophysiology Software designed by J. Dempster (University of Strathclyde, UK). At the beginning of each experiment we recorded the capacitance response of the unmodified membrane (control record of the current after applying a voltage wave of the square form). The record in the presence of the studied anions was analyzed after subtraction of the control record.
The electric current was recorded under voltage-clamp conditions by means of a patch-clamp amplifier (Warner Instruments, Hamden, CT, model BC-525C) as stationary current measurements (at a moment of time >20 s after application of 5 mV).
Fluorescence measurements
4-(2-[6-(Dibutylamino)-2-naphthalenyl]ethenyl)-1-(3-sulfopropyl)-pyridinium hydroxide inner salt (di-4-ANEPPS) was obtained from Sigma Aldrich. In vesicles as well as in an aqueous solution dye it showed a single long wavelength fluorescence emission band regardless of the excitation wavelength.To prepare liposomes, the lipid (10 mg phosphatidylcholine, eggPC, Avanti Polar Lipids) in a chloroform suspension was dried in a round-bottom flask under a stream of nitrogen. The lipid was then resuspended in 1 ml buffer (100 mM KCl, 30 mM Tris pH 7.6). The suspension was vortexed and then freeze-thawed three times. Unilamellar liposomes were prepared by extrusion through 0.1 μm-pore size nucleopore polycarbonate membranes using an Avanti Mini-Extruder. Measurements with the vesicles were performed in the buffer containing 100 mM KCl, 30 mM Tris pH 7.6. In the presence of liposomes (1.3 mM of lipid in solution), after addition of 0.5 μM di-4-ANEPPS 15 min equilibration time was left to allow for dye disaggregation and incorporation into the membranes. The excitation spectrum was measured at emission wavelength 670 nm.22 The ratio of the fluorescence intensities detected at two excitation wavelengths on the blue (420 nm) and red (520 nm) flanks of the excitation spectrum was measured as a function of the concentration of the studied hydrophobic anions.
3. Results
As shown in the scheme in Fig. 2, the anions of COSAN bind symmetrically to the lipid membrane in the absence of the applied voltage. The application of voltage leads to transmembrane redistribution (flip) of the compounds manifesting itself in the initially high electrical current relaxing to a low level due to a depletion of the compound at one of the interfaces. Fig. 2 shows the COSAN-mediated current relaxation curve upon the application of 50 mV at t = 0. Low steady-state COSAN current after several milliseconds pointed to a slow binding process from the aqueous phase to the interface compared to the rate of the translocation. At t = 4.2 ms the potential was switched to zero while the current exhibited a similar relaxation but of the opposite sign (Fig. 2). This process was a result of a return (flop) of COSAN molecules to the initial symmetrical distribution at two sides of the membrane (right part of the scheme in Fig. 2).Current relaxations mediated by 0.1 μM COSAN (black curve), COSAN-F2 ([8,8′-F2-3,3′-Co(1,2-C2B9H10)2]−, pink), COSAN-Cl2 ([8,8′-Cl2-3,3′-Co(1,2-C2B9H10)2]−, green), COSAN-Br2 ([8,8′-Br2-3,3′-Co(1,2-C2B9H10)2]−, red) and COSAN-I2 (blue) are presented in Fig. 2, inset. The BLM was made from diphytanoylphosphatidylcholine (DPhPC) and the voltage-jump was 50 mV. The data are represented by straight lines in semi-log plots pointing to their monoexponential character following the equation I(t) = I∞ + (I0 − I∞)·exp(−t/τ). The following τ values were derived: 660 μs, COSAN; 900 μs, COSAN-F2; 50.4 μs, COSAN-Cl2; 36.7 μs, COSAN-Br2; and 27.7 μs, COSAN-I2. Therefore, the rate of the flip–flop increased in the series: COSAN-F2 < COSAN < COSAN-Cl2 < COSAN-Br2 < COSAN-I2. It can be noted that the magnitude of the initial current of halogenated COSAN compounds exceeded substantially that of parent COSAN and COSAN-F2 (Fig. 2, inset). This finding can be understood bearing in mind that the area below the current relaxation curve corresponded to the total transfer of electrical charge (Q), i.e. the total number of COSAN anions capable to flip upon voltage-jump. Thus, the increase in the rate of the flip must lead to the increase in the amplitude of the initial current assuming a similar surface concentration of the compounds.
The dependence of Q on voltage is shown in Fig. 3A. As expected, Q increased with voltage for all five compounds having a tendency to saturation. The value of Q slightly increased in the series: COSAN ≈ COSAN-Cl2 ≈ COSAN-I2 < COSAN-Br2 < COSAN-F2 apparently not parallel to the increase in affinity to the lipid–water interface.14 Panel B of Fig. 3 displays the dependence of the characteristic time τ on the BLM voltage (V). As shown for tetraphenylborate and several other anions3,4 the dependence of τ on dimensionless voltage 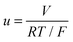 (F, R are the Faraday and gas constants, T is absolute temperature, V refers to membrane voltage) can be described by
(F, R are the Faraday and gas constants, T is absolute temperature, V refers to membrane voltage) can be described by
 | (1) |
 | ||
| Fig. 3 (A) Voltage-dependence of the transferred electrical charge for 0.1 μM COSAN-I2, COSAN-Br2, COSAN-Cl2, COSAN-F2 and COSAN in the bathing solution. (B) Voltage dependence of the relaxation time τ for COSAN-I2, COSAN-Br2, COSAN-Cl2, COSAN-F2 and COSAN (concentration of all compounds was 0.1 μM). Solid curves are best fits according to eqn (1). | ||
| DPhPC | k i , s−1 | β |
|---|---|---|
| COSAN | 580 | 0.78 |
| COSAN-F2 | 410 | 0.77 |
| COSAN-Cl2 | 7430 | 0.71 |
| COSAN-Br2 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
0.67 |
| COSAN-I2 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.67 |
The translocation could be described as a transfer from one energy well at the membrane–water interface to another across an energy barrier in the interior part of the membrane. It can be noted that the higher value of ki for COSAN-I2 compared to COSAN was in apparent contradiction with the data of Verdia-Baguena et al.13 on the direct measurements of permeabilities of these two compounds through the planar lipid membrane by ICP-MS. Fig. 4 shows the concentration-dependence of the BLM steady-state current induced by COSAN (closed circles) or COSAN-I2 (open circles). In agreement with Verdia-Baguena et al.13 the steady-state current corresponding to ion penetration from one aqueous solution to another was lower in case of COSAN-I2 at concentrations higher than 10 μM. However, the COSAN-I2-mediated current was higher than that of COSAN at the lower concentrations (Fig. 4). Because 10 μM was shown to be critical concentration of vesicle formation for COSAN,10 one can speculate that the aggregation of COSAN-I2 suppressed the BLM current to a greater extent than that of COSAN. The suppression could be a consequence of the reduced monomer concentration and/or poor interaction of COSAN-I2 vesicles with the lipid membrane. The process of COSAN-I2 desorption into the aqueous phase could proceed via the transfer of monomers or as a separation of COSAN-I2 aggregates in the lipid membrane and subsequent vesicle budding. Images of COSAN vesicles penetrating into lipid vesicles have been shown earlier.13
 | ||
| Fig. 4 Steady-state electrical current mediated by COSAN (closed circles) and COSAN-I2 (open circles) at an applied voltage of 5 mV. | ||
The large difference between the rate of translocation of COSAN-F2 and the other halogenated COSAN analogs points to some special feature of COSAN-F2 binding to the lipid membranes. It has been shown earlier that the addition of hydrophobic anion tetraphenylborate (TPB) to liposomes with fluorescent styrylpiridinium dye RH421 led to a shift of the excitation spectrum of fluorescence in the long-wavelength region,23 which was associated with the effect of TPB on the membrane potential. Using the dual wavelength ratiometric approach with the voltage sensitive fluorescence probe di-4-ANEPPS24 we analyzed the qualitative changes of the lipid membrane potential caused by the adsorption of halogenated COSANs. This dye is more sensitive to changes in the dipole potential and adsorbed faster on the lipid membrane compared with RH421 and di-8-ANEPPS.22,25 We assume that under the conditions of excessive lipid concentrations (1.3 mM) over the concentrations of the hydrophobic anions (<80 μM) COSAN analogs adsorbed completely on the surface of the lipid membrane and that the change of the electrical potential profile in the membrane related to surface potential should be equal for all derivatives. It can be suggested therefore that the variations in the fluorescence ratio for different anions (Fig. 5A) were caused by the different effects of the compounds on the dipole potential of the lipid membrane. Fig. 5A shows the dependence of the fluorescence ratio (R420/520) of the dye di-4-ANEPPS on the concentration of COSAN and its halogenated derivatives. R420/520 is strongly reduced in the presence of COSAN-Cl2, COSAN-Br2 and COSAN-I2 while in the case of COSAN and especially COSAN-F2 the decrease of R420/520 was substantially less. The reason for these differences can be based on the differences in the dipole potential of the compounds as well as on the differences in their effects on the dipole potential of the lipid membrane. This point will be further discussed below.
4. Discussion
The rate of flip–flop of penetrating ions depends on different factors, such as sign and value of charge, radius of charge delocalization and other features. It has been shown previously that halogenation of tetraphenylborate increased the translocation rate of tetrakis(4-chlorophenyl)borate as well as the fluorinated analog about 1000-fold compared to unsubstituted TPB.15 This dramatic effect was accounted for by the smearing of the negative charge across a larger sphere, i.e., the increase of the effective diameter of TPB analogues upon halogenation which should decrease the Born electrostatic component of the transmembrane free energy profile.26 In the present study we revealed the following series of the rates of the translocation for halogenated COSAN: COSAN-F2 < COSAN < COSAN-Cl2 < COSAN-Br2 < COSAN-I2. In part, this dependence correlates with the increase in the molecular volumes of the halogens that may be essential for the radius of charge delocalization. However, COSAN-F2 clearly did not conform to this tendency. Remarkably, the same series was observed in the experiments with the dipole potential sensor di-4-ANEPPS (Fig. 5). This finding points to an involvement of the dipole moment of the compounds in the mechanism of their translocation through the membrane.The membrane potential of the lipid membrane consists of three major parts: the surface potential, resulting from the accumulation of charge on the membrane interfaces, the transmembrane potential, determined by the imbalance of charge in the aqueous solutions, and the dipolar component, which originates due to the nonrandom arrangements of lipid dipoles and water molecules at the membrane surface.27 Several hydrophobic compounds with dipole moments can significantly change the dipole potential of the lipid membrane adsorbing at the lipid bilayer surface.28–30 In addition hydrophobic compounds carrying electrical charge can change the surface potential of the membrane in the process of adsorption.
Each of the dicarbollide ligands possesses a significant dipole moment, directed perpendicular to their common axis of rotation in the molecule (scheme in Fig. 5B). The calculated energy difference between the rotational conformers in the case of unsubstituted dicarbollide ligands (COSAN) is not high (approx. 10–12 kJ mol−1)31 allowing their facile interconversion in solution. As a result, the dicarbollide complexes could produce different conformations depending on small external factors. The interaction between the anions and cations observed in the solid state can lead to the stabilization of certain conformations of rotation. A search for COSAN in the Cambridge Structural Database revealed structures containing cisoid conformation (about 80%, D = 5.4), gauche conformation (about 15%, D = 3.1) or transoid conformation (about 5%, D = 0)32 (scheme in Fig. 5B). Introduction of the halogen atoms X opposite to the carbons position of the pentagonal face of the dicarbollide ligand results in the additional stabilization of the transoid conformation due to formation of the intramolecular hydrogen C–H⋯X–B bonds between the dicarbollide ligands. The introduction leads to exclusively transoid conformation for X = Br33 and X = I,34,35 and preferential transoid (80%, gauche 20%) conformations for X = Cl36–39 in the solid state. However in the case of X = F (COSAN-F2), the C–H⋯F–B distances are too long to form the intramolecular hydrogen bonds resulting in the domination of the cisoid conformation in a solid state.19
Based on the results shown in Fig. 5A and on the literature data concerning the effects of dipole modifiers on the changes of the fluorescence ratio (R420/520) of di-8-ANEPPS22 we can conclude that the binding of COSAN and COSAN-F2 to liposomes led to an increase in the dipole potential of the lipid membrane. This conclusion is based on the ability of anionic TPB lacking dipole potential to decrease R420/52023 similar to the effects of COSAN-Cl2, COSAN-Br2 and COSAN-I2. Most likely, the adsorption of COSAN and COSAN-F2 on the lipid membrane favors the preferential cisoid conformation imposed by the electric field of the membrane, and the ordered orientation of their dipole moments should contribute to an increase in the value of the membrane dipole potential (Fig. 5C) similar to 6-ketocholestanol upon its binding.22 However, COSAN-Cl2, COSAN-Br2 and COSAN-I2 could retain a transoid conformation upon adsorption on the lipid membrane (Fig. 5C). Possibly, the differences in the reside depth of the anions in the hydrophobic layer of the lipid phase (parameter β, Table 1) can also be related to the presence/absence of the dipole moment of the anions. The slowing of the translocation rate of COSAN-F2 compared to COSAN could be attributed to several factors. One of them could be the increase of the dipole moment of the fluorinated analogue due to the high electronegativity of fluorine. Also, fluorination leads frequently to an increase in hydrophobicity40 as was in the case for the fluorinated derivative of the anionic tungsten carbonyl complex [W(CO5)SC6H5]−.41 Interestingly, the fluorination of the phenyl ring in this anionic complex led to a slowing of its translocation across the lipid membrane by at least one order of magnitude.41
The presence of the dipole moment of the anion COSAN localized on the surface of the lipid membrane (Fig. 5C) should provide an additional potential barrier to penetration through the membrane compared to the analogs lacking a dipole moment because the process of penetration must include a stage of reorientation of its dipole. It has been shown recently that the orientation of anion carriers (dithioureido decalins) at the membrane surface contributes significantly to the rate of chloride permeation through the lipid membrane.42 The bound transporter and chloride complex oriented their polar regions towards the membrane interface and are able to exhibit effective polar interactions with the phospholipid head groups or with bound water molecules. These polar interactions must be broken before the transporter/complex can pass through the membrane, and therefore contribute a substantial potential barrier to the translocation. Recently, the role of orientational and conformational motion in the process of passive transbilayer diffusion of the solutes43 and steroids44 has been pointed out.
It is well known, that the increase of the dipole potential of the lipid membrane promotes the translocation of hydrophobic anions.45 From this point of view, one would expect that the rates of translocation of COSAN and COSAN-F2 should be higher than for other halogenated analogues (Fig. 5). However, it can be suggested that the concentrations of anions in the experiments on current relaxation were too small (100 nM in aqueous solution) to form a regular layer of oriented dipoles on the membrane surface. In fact, the number of COSAN in one monolayer was about NCOSAN = Q/e ≈ 1 nC/1.6 × 10−19 C ≈ 6 × 109 (e – the charge of the electron), while the number of the lipid molecules was Nlipid = 5 × 1013 Å2/60 Å2 ≈ 1012 (the area of BLM was S = πR2 = π × (0.4 mm)2 ≈ 0.5 mm2 = 5 × 1013 Å2, and the area per lipid molecules was 60 Å2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46). Therefore, the number of COSAN anions adsorbed on the lipid membrane was more than two orders of magnitude lower than the lipid molecules suggesting their minor contribution to the dipole potential of the membrane.
46). Therefore, the number of COSAN anions adsorbed on the lipid membrane was more than two orders of magnitude lower than the lipid molecules suggesting their minor contribution to the dipole potential of the membrane.
Thus, we found that the rate of flip–flop of most halogenated derivatives of COSAN increased with increasing molecular weight of the halogen and the volume of the penetrating anion. In contrast, the fluorinated derivative exhibited slowing down of transmembrane penetration. We hypothesize that these analogs acquire a dipole moment in the process of adsorption on the surface of the lipid membrane which complicates the permeation due to the rotational component of the transmembrane diffusion. Further work is required to confirm or deny the role of the dipole potential in the mechanisms of COSAN penetration through lipid membranes. In general, the understanding of the mechanism of the drug permeation through lipid membranes could promote work on the increase of bioavailability of different therapeutic compounds. For example, conjugates of COSAN with synthetic and natural porphyrins are studied in relation to the photodynamic and boron neutron capture therapy (BNCT) of tumors.47–49 Of note, the rate of the flip–flop of COSAN and its analogs were substantially faster compared to the tetraphenylborate anion and its derivatives.4,15,50
Abbreviations
| COSAN | Cobalt bis(dicarbollide) |
| COSAN-F2 | Fluorinated cobalt bis(dicarbollide) |
| COSAN-Cl2 | Chlorinated cobalt bis(dicarbollide) |
| COSAN-Br2 | Brominated cobalt bis(dicarbollide) |
| COSAN-I2 | Iodinated cobalt bis(dicarbollide) |
| BLM | Bilayer lipid membrane |
| DPhPC | Diphytanoylphosphatidylcholine |
| TPB | Tetraphenylborate |
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors are grateful to Dr Galina Korshunova and Dr Elena Kotova for help in the present work and fruitful discussions. This work was supported in part by the Russian Foundation for Basic Research grants 15-04-01688, 15-04-01755 and 16-03-00724.References
- E. A. Liberman and V. P. Topaly, Biochim. Biophys. Acta, 1968, 163, 125–136 CrossRef CAS.
- O. H. LeBlanc, Biochim. Biophys. Acta, 1969, 193, 350–360 CrossRef CAS.
- B. Ketterer, B. Neumcke and P. Lauger, J. Membr. Biol., 1971, 5, 225–245 CrossRef CAS PubMed.
- O. S. Andersen and M. Fuchs, Biophys. J., 1975, 15, 795–830 CrossRef CAS PubMed.
- M. F. Hawthorne and G. B. Dunks, Science, 1972, 178, 462–471 CAS.
- I. B. Sivaev and V. I. Bregadze, Collect. Czech. Chem. Commun., 1999, 64, 783–805 CrossRef CAS.
- A. Popov and T. Borisova, J. Colloid Interface Sci., 2001, 236, 20–27 CrossRef CAS PubMed.
- P. M. Gassin, L. Girard, G. Martin-Gassin, D. Brusselle, A. Jonchere, O. Diat, C. Vinas, F. Teixidor and P. Bauduin, Langmuir, 2015, 31, 2297–2303 CrossRef CAS PubMed.
- P. Matejicek, P. Cigler, K. Prochazka and V. Kral, Langmuir, 2006, 22, 575–581 CrossRef CAS PubMed.
- M. Uchman, V. Dordovic, Z. Tosner and P. Matejicek, Angew. Chem., Int. Ed., 2015, 54, 14113–14117 CrossRef CAS PubMed.
- C. Vinas, M. Tarres, P. Gonzalez-Cardoso, P. Farras, P. Bauduin and F. Teixidor, Dalton Trans., 2014, 43, 5062–5068 RSC.
- P. Bauduin, S. Prevost, P. Farras, F. Teixidor, O. Diat and T. Zemb, Angew. Chem., Int. Ed., 2011, 50, 5298–5300 CrossRef CAS PubMed.
- C. Verdia-Baguena, A. Alcaraz, V. M. Aguilella, A. M. Cioran, S. Tachikawa, H. Nakamura, F. Teixidor and C. Vinas, Chem. Commun., 2014, 50, 6700–6703 RSC.
- M. Tarres, E. Canetta, E. Paul, J. Forbes, K. Azzouni, C. Vinas, F. Teixidor and A. J. Harwood, Sci. Rep., 2015, 5, 7804 CrossRef CAS PubMed.
- R. Benz, Biophys. J., 1988, 54, 25–33 CrossRef CAS PubMed.
- G. B. Melikyan, B. N. Deriy, D. C. Ok and F. S. Cohen, Biophys. J., 1996, 71, 2680–2691 CrossRef CAS PubMed.
- T. I. Rokitskaya, S. S. Klishin, I. I. Severina, V. P. Skulachev and Y. N. Antonenko, J. Membr. Biol., 2008, 224, 9–19 CrossRef CAS PubMed.
- M. F. Hawthorne, D. C. Young, T. D. Andrews, D. V. Howe, R. L. Pilling, L. F. Warren Jr. and P. A. Wegner, J. Am. Chem. Soc., 1968, 90, 879–896 CrossRef CAS.
- A. N. Gashti, J. C. Huffman, A. Edwards, G. Szekeley, A. R. Siedle, J. A. Karty, J. P. Reilly and L. J. Todd, J. Organomet. Chem., 2000, 614–615, 120–124 CrossRef CAS.
- L. Matel, F. Macásek, P. Rajec, S. Heřmánek and J. Plešek, Polyhedron, 1982, 1, 511–519 CrossRef CAS.
- P. Mueller, D. O. Rudin, H. T. Tien and W. C. Wescott, J. Phys. Chem., 1963, 67, 534–535 CrossRef CAS.
- R. J. Clarke and D. J. Kane, Biochim. Biophys. Acta, 1997, 1323, 223–239 CrossRef CAS.
- R. J. Clarke, A. Zouni and J. F. Holzwarth, Biophys. J., 1995, 68, 1406–1415 CrossRef CAS PubMed.
- L. M. Loew, L. B. Cohen, J. Dix, E. N. Fluhler, V. Montana, G. Salama and J. Y. Wu, J. Membr. Biol., 1992, 130, 1–10 CrossRef CAS PubMed.
- V. S. Sokolov, A. N. Gavrilchik, A. O. Kulagina, I. N. Meshkov, P. Pohl and Y. G. Gorbunova, J. Photochem. Photobiol., B, 2016, 161, 162–169 CrossRef CAS PubMed.
- A. Parsegian, Nature, 1969, 221, 844–846 CrossRef CAS PubMed.
- L. G. Wang, Annu. Rev. Biochem., 2012, 81, 615–635 CrossRef CAS PubMed.
- O. S. Andersen, in Renal Function, ed. G. H. Giebisch and E. F. Purcell, Josiah Macy, Jr. Foundation, New York, 1978, pp. 71–99 Search PubMed.
- P. Pohl, T. I. Rokitskaya, E. E. Pohl and S. M. Saparov, Biochim. Biophys. Acta, 1997, 1323, 163–172 CrossRef CAS.
- J. C. Franklin and D. S. Cafiso, Biophys. J., 1993, 65, 289–299 CrossRef CAS PubMed.
- M. Buhl, D. Hnyk and J. Machacek, Chem. – Eur. J., 2005, 11, 4109–4120 CrossRef PubMed.
- E. J. Juarez-Perez, R. Nuriez, C. Vinas, R. Sillanpaa and F. Teixidor, Eur. J. Inorg. Chem., 2010, 2385–2392 CrossRef CAS.
- O. Kazheva, G. Alexandrov, A. Kravchenko, V. Starodub, I. Lobanova, I. Sivaev, V. Bregadze, L. Buravov and O. Dyachenko, Solid State Sci., 2008, 10, 1734–1739 CrossRef CAS.
- P. Sivý, A. Preisinger, O. Baumgartner, F. Valach, B. Koren and L. Matel, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1986, 42, 28–30 CrossRef.
- O. N. Kazheva, G. G. Alexandrov, A. V. Kravchenko, V. A. Starodub, I. A. Lobanova, I. B. Sivaev, V. I. Bregadze, L. V. Titov, L. I. Buravov and O. A. Dyachenko, J. Organomet. Chem., 2009, 694, 2336–2342 CrossRef CAS.
- N. I. Kirillova, A. S. Zhdanov, A. I. Gusev, V. N. Kirin, S. P. Knyazev and T. V. Sokolova, Organomet. Chem. USSR, 1989, 2, 448–450 Search PubMed.
- P. K. Hurlburt, R. L. Miller, K. D. Abney, T. M. Foreman, R. J. Butcher and S. A. Kinkhead, Inorg. Chem., 1995, 34, 5215–5219 CrossRef CAS.
- O. N. Kazheva, A. V. Kravchenko, G. G. Alexandrov, I. B. Sivaev, V. I. Bregadze, I. D. Kosenko, I. A. Lobanova and L. I. Buravov, Russ. Chem. Bull., 2014, 63, 1322–1329 CrossRef CAS.
- O. N. Kazheva, G. G. Alexandrov, A. V. Kravchenko, I. B. Sivaev, V. A. Starodub, I. D. Kosenko, I. A. Lobanova, V. I. Bregadze, L. I. Buravov and O. A. Dyachenko, J. Chem. Eng. Chem. Res., 2015, 2, 497–503 CAS.
- M. P. Krafft and J. G. Riess, Biochimie, 1998, 80, 489–511 CrossRef CAS PubMed.
- M. Kurschner, K. Nielsen, J. R. G. von Langen, W. A. Schenk, U. Zimmermann and V. L. Sukhorukov, Biophys. J., 2000, 79, 1490–1499 CrossRef CAS PubMed.
- S. J. Edwards, I. Marques, C. M. Dias, R. A. Tromans, N. R. Lees, V. Felix, H. Valkenier and A. P. Davis, Chem. – Eur. J., 2016, 22, 2004–2011 CrossRef CAS PubMed.
- G. Parisio, M. Stocchero and A. Ferrarini, J. Chem. Theory Comput., 2013, 9, 5236–5246 CrossRef CAS PubMed.
- G. Parisio, M. M. Sperotto and A. Ferrarini, J. Am. Chem. Soc., 2012, 134, 12198–12208 CrossRef CAS PubMed.
- E. A. Liberman and V. P. Topaly, Biofizika, 1969, 14, 452–461 CAS.
- B. A. Cornell, J. Middlehurst and F. Separovic, Biochim. Biophys. Acta, 1980, 598, 405–410 CrossRef CAS.
- E. Hao, T. J. Jensen, B. H. Courtney and M. G. Vicente, Bioconjugate Chem., 2005, 16, 1495–1502 CrossRef CAS PubMed.
- P. Kubat, K. Lang, P. Cigler, M. Kožišek, P. Matejiček, P. Janda, Z. Zelinger, K. Prochazka and V. Kral, J. Phys. Chem. B, 2007, 111, 4539–4546 CrossRef CAS PubMed.
- A. V. Efremenko, A. A. Ignatova, M. A. Grin, I. B. Sivaev, A. F. Mironov, V. I. Bregadze and A. V. Feofanov, Photochem. Photobiol. Sci., 2014, 13, 92–102 CAS.
- D. Y. Malkov and V. S. Sokolov, Biochim. Biophys. Acta, 1996, 1278, 197–204 CrossRef.
| This journal is © the Owner Societies 2017 |


