Effect of the solvation state of electron in dissociative electron attachment reaction in aqueous solutions†
Abstract
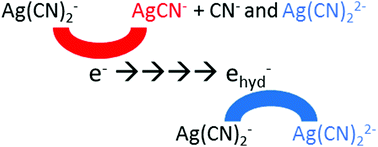
It is generally considered that the pre-solvated electron and the solvated electron reacting with a solute yield the same product. Silver cyanide complex, Ag(CN)2−, is used as a simple probe to demonstrate unambiguously the existence of a different reduction mechanism for pre-hydrated electrons. Using systematic multichannel transient absorption measurements at different solute concentrations from millimolar to decimolar, global data analysis and theoretical calculations, we present the dissociative electron attachment on Ag(CN)2−. The short-lived silver complex, Ag0(CN)22−, formed by hydrated electron with nanosecond pulse radiolysis, can be observed at room temperature. However, at higher temperatures only the free silver atom, Ag0, is detected, suggesting that Ag0(CN)22− dissociation is fast. Surprisingly, pulse radiolysis measurements on Ag(CN)2− reduction, performed by a 7 ps electron pulse at room temperature, show clearly that a new reduced form of silver complex, AgCN−, is produced within the pulse. This species, absorbing at 560 nm, is not formed by the hydrated electron but exclusively by its precursor. DFT calculations show that the different reactivity of the hydrated and pre-hydrated electrons can be due to the formation of different electronic states of Ag0(CN)22−: the prehydrated electron can form an excited state of this complex, which mainly dissociates into Ag0CN− + CN−.


 Please wait while we load your content...
Please wait while we load your content...