Excited state characterization of carbonyl containing carotenoids: a comparison between single and multireference descriptions†
Abstract
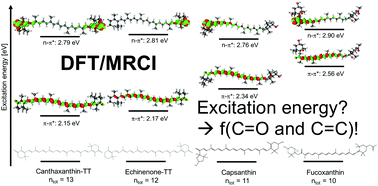
Carotenoids can play multiple roles in biological photoreceptors thanks to their rich photophysics. In the present work, we have investigated six of the most common carbonyl containing carotenoids: echinenone, canthaxanthin, astaxanthin, fucoxanthin, capsanthin and capsorubin. Their excitation properties are investigated by means of a hybrid density functional theory (DFT) and multireference configuration interaction (MRCI) approach to elucidate the role of the carbonyl group: the bright transition is of ππ* character, as expected, but the presence of a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O moiety reduces the energy of nπ* transitions which may become closer to the ππ* transition, in particular as the conjugation chain decreases. This can be related to the presence of a low-lying charge transfer state typical of short carbonyl-containing carotenoids. The DFT/MRCI results are finally used to benchmark single-reference time-dependent DFT-based methods: among the investigated functionals, the meta-GGA (and in particular M11L and MN12L) functionals show to perform the best for all six investigated systems.
O moiety reduces the energy of nπ* transitions which may become closer to the ππ* transition, in particular as the conjugation chain decreases. This can be related to the presence of a low-lying charge transfer state typical of short carbonyl-containing carotenoids. The DFT/MRCI results are finally used to benchmark single-reference time-dependent DFT-based methods: among the investigated functionals, the meta-GGA (and in particular M11L and MN12L) functionals show to perform the best for all six investigated systems.


 Please wait while we load your content...
Please wait while we load your content...