Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Energetic and spectroscopic properties of the low-lying C7H2 isomers: a high-level ab initio perspective†
Venkatesan S.
Thimmakondu
 *a and
Amir
Karton
*b
*a and
Amir
Karton
*b
aDepartment of Chemistry, Birla Institute of Technology and Science, Pilani, K K Birla Goa Campus, Goa, 403 726, India. E-mail: venkateshtsv@gmail.com
bSchool of Molecular Sciences, The University of Western Australia, Perth, Western Australia 6009, Australia. E-mail: amir.karton@uwa.edu.au
First published on 23rd June 2017
Abstract
We use high-level ab initio CCSD(T) and CCSDT(Q) methods to investigate the energetic and spectroscopic properties of nine low-lying isomers of C7H2, which lie within 1 eV. Among these, heptatriynylidene (1), 1-(buta-1,3-diynyl)cyclopropenylidene (2) and heptahexaenylidene (9) have been detected experimentally. The other six isomers, 1,2-(diethynyl)cyclopropenylidene (3), bicyclo[4.1.0]hepta-1,2,4,5-tetraene-7-ylidene (4), cyclohepta-1,2,3,4-tetraen-6-yne (5), bicyclo[4.1.0]hepta-4,6-diene-2-yne-7-ylidene (6), bicyclo[4.1.0]hepta-1,5-diene-3-yne-7-ylidene (7) and 1-(buta-1,3-diynyl)propadienylidene (8), remain hypothetical to date. Except for 1, all of the isomers are associated with a non-zero dipole moment (μ ≠ 0). Although Fourier-transform microwave spectroscopy had detected 2 and 9, our study reveals that six hypothetical isomers (3–8) are thermodynamically sandwiched between the experimentally known and astronomically relevant isomers 2 and 9. The structural parameters, dipole moments, rotational and centrifugal distortion constants, harmonic vibrational frequencies, and infra-red intensities presented here may be useful for the laboratory detection of these previously unidentified isomers (3–8) and also all others (2–9) in astronomical sources.
1 Introduction
Identification of molecules in non-terrestrial environments is definitely an open challenge for the scientific community. Paradoxically, in the vast majority of cases, this challenge can only be completely resolved by the synthesis of these new (non-terrestrial) molecules in terrestrial environments.1–12 Although radioastronomers observe rotational transitions in space, we need a match to confirm the presence of the exact same molecule on earth. Needless to say, quite exotic molecules have been detected in the interstellar medium (ISM) along with simple molecules.1,12–23 The challenges associated with the synthesis and identification of these new molecules on earth is one of the primary reasons why only 200 molecules have been confirmed so far in space, instead of a greater number.12 Apart from acetylenic radicals (CnH)24–31 and cyanopolyynes (HCnN),27,32–36 cumulene carbenes (CnH2),16–18,27,37–47 which show a significant increase in dipole moment with respect to increase in the carbon chain length, have also been found in the ISM. Ever since three cumulene carbenes (where, n = 3, 4, and 6) were identified in space16–18 followed by their detection in the laboratory,27,37–39 both experimentalists and theoreticians have started focusing their attention not only on these compounds but also on their isomers and higher homologous series.40–43,48–57Although more than hundreds of structural isomers are theoretically possible for C7H2,58 to date merely six isomers (see Fig. 1 and 2) have been detected experimentally43,59,60–63 and none have been detected in space. Heptatriynylidene (1), whose dipole moment is zero by symmetry, was first detected in a 5 K Ne-matrix59 and later detected by cavity ringdown spectroscopy61,62 as well as charge reversal and neutralization re-ionization mass spectra of the corresponding anion.63 1-(buta-1,3-diynyl)cyclopropenylidene (2)60 and heptahexaenylidene (9)43 were detected with a Fourier-transform microwave (FTM) spectrometer. Bowie and co-workers characterized five isomers (1, 9, 11, 14, and 15) after synthesizing four C7H2 radical anion precursors using charge reversal and neutralization reionization spectra.63 Though laboratory investigations suggested the possibility of other low-lying isomers,1 it is not clear what the structures of these low-lying isomers are on the C7H2 potential-energy surface (PES). On the contrary, earlier theoretical studies either did not mention the bicyclic/seven-membered rings (4–7)63,68 or predicted the wrong relative stability of the isomers (4, 5, and 7).58 The motivation behind the current study not only stems from the electronic structures of these isomers but also from the relative stabilities of these isomers on the C7H2 PES.
The present work is an elaborate theoretical investigation of nine low-lying isomers of C7H2 (1–9; see Fig. 1). Specifically, we obtain the energies of these isomers at the CCSDT(Q)/CBS level by means of the W3lite-F12 composite method.64 This method approximates the coupled cluster energy with single, double, triple, and quasiperturbative quadruple excitations (CCSDT(Q)) at the complete basis-set (CBS) limit.65,66 We also calculate the equilibrium geometries and a range of spectroscopic constants for these molecules at the CCSD(T) level. The high energy isomers (10–15; see Fig. 2), which lie above the cumulene carbene isomer (9), are not considered here in detail.67
Aoki and Ikuta have carried out geometry optimizations at the MP2/D95** level of theory for eight different isomers of C7H2 (1–3, 8–11 and also a bent carbene, whose structure is not given here),68 but their study did not include any bicyclic or seven-membered ring structures. While seven of the structures reported by them are given in Fig. 1, a bent geometry (4′ as per their labelling) reported as a minimum by them was not found to be a minimum in our earlier study at the CCSD(T)/cc-pVTZ level of theory.69 We found that the bent geometry becomes linear at higher levels of theory.69 Also, their study predicted that 2 is the most stable isomer.68 Isomers 3 and 1 were predicted to lie above 2 with a relative energy difference of 1.8 and 4.9 kcal mol−1, respectively. Aromatic stability (Hückel's (4n + 2)π electrons rule with n = 0 here) due to the presence of 2π electrons inside the three-membered ring was justified as the reason why 2 and 3 were more stable than the triplet ground electronic state of 1.68 Nevertheless, density functional theory (DFT) calculations done by two different groups showed that 1 is the most stable isomer.58,63 Our earlier and present work also confirm this result.69 Apart from their experimental work, Bowie and co-workers have carried out DFT calculations at the B3LYP/aug-cc-pVDZ//B3LYP/6-31G(d) level of theory for ten different isomers of C7H2 (1–3, 8–12, and 14–15).63,70 Once again, no bicyclic or seven-membered ring structures were included in their study. Unequivocally, the latter are not only structurally intriguing but also energetically low-lying as we found in the present work (see Table 1).
| Level | Protocol | ΔErel | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| SCF | W2-F12 | −17.79 | −16.06 | 15.63 | 18.75 | 15.29 | 19.32 | 2.86 | 13.08 |
| CCSD | W2-F12 | 18.01 | 18.08 | −3.46 | −5.45 | 2.56 | −1.35 | 15.09 | 11.31 |
| (T) | W2-F12 | 2.75 | 3.17 | −6.60 | −7.29 | −3.40 | −3.12 | 0.61 | −4.33 |
| Inner-shell | W2-F12 | 0.61 | 0.65 | 1.29 | 1.04 | 1.17 | 1.15 | 0.87 | 0.89 |
| Scalar relativistic | W2-F12 | −0.06 | −0.07 | −0.08 | −0.05 | −0.05 | −0.03 | −0.12 | −0.11 |
| T–(T) | W3.2lite | −0.39 | −0.40 | 0.78 | 0.66 | 0.26 | 0.39 | −0.60 | −0.63 |
| (Q) | W3.2lite | 0.65 | 0.76 | −0.94 | −1.32 | −0.29 | −0.03 | 0.23 | −0.83 |
| E e[CCSD(T)/CBS] | W2-F12 | 3.52 | 5.77 | 6.78 | 7.01 | 15.57 | 15.97 | 19.32 | 20.85 |
| E e[CCSDT(Q)/CBS] | W3.2lite-F12 | 3.77 | 6.13 | 6.62 | 6.35 | 15.54 | 16.33 | 18.94 | 19.38 |
| ZPVE | CCSD(T)/cc-pVTZ | 1.79 | 1.51 | 3.73 | 3.88 | 3.65 | 3.87 | 1.32 | 0.96 |
| E 0[CCSDT(Q)/CBS] | W3.2lite-F12 | 5.56 | 7.64 | 10.35 | 10.23 | 19.19 | 20.20 | 20.26 | 20.34 |
Thaddeus and co-workers stated that there may be other isomers of C7H2 within 1 eV apart from what Aoki and Ikuta predicted in their theoretical work.1,68 The present study supports this comment made by experimentalists several years ago. Three bicyclic rings (bicyclo[4.1.0]hepta-1,2,4,5-tetraene-7-ylidene (4), bicyclo[4.1.0]hepta-4,6-diene-2-yne-7-ylidene (6), and bicyclo[4.1.0]hepta-1,5-diene-3-yne-7-ylidene (7)) and one seven-membered ring (cyclohepta-1,2,3,4-tetraen-6-yne (5)) are indeed within 1 eV at the CCSDT(Q)/CBS level of theory, and surprisingly all of them (4–7) are energetically below the experimentally detected cumulene carbene isomer of C7H2 (9) (see Table 1). Though this paper is not an exhaustive survey of all isomers, it is quite clear that the experimentalists’ remark is true. We believe that our study will inspire experimental investigations for detecting these elusive molecules. Although thermodynamically more stable than 9, isomers 3–8 are yet to be found in the laboratory. We note, however, that the kinetic stability of these isomers still remains to be investigated. From the structural point of view, 4 and 5 clearly exhibit a biradical character and therefore trapping these two molecules might certainly present some challenges to experimentalists. However, we believe that these can be trapped in low-temperature environments using matrix-isolation techniques.
Sun et al. calculated the relative energies of 113 isomers of C7H2 at the CCSD(T)/cc-pVTZ//UB3LYP/6-311G(d,p) level of theory.58 However, their relative energies are in disagreement with the more accurate energies reported here at the CCSDT(Q)/CBS//CCSD(T)/cc-pVTZ level of theory. These differences are partly attributed to the differences between the B3LYP and CCSD(T) structures and the post-CCSD(T) energetic contributions, which are considered in the present work. Moreover, optimization for some of the isomers (4, 5, 7, and 9) reported by them had been done at a lower symmetry. Though the correct point group symmetry is C2v for the later isomers, optimizations were done with a Cs symmetry point group. This could also be one of the contributing factors for the differences in relative energies between our results and theirs. Moreover, we note that isomer 6 was not among the 113 isomers that were part of their study.
In this paper, our focus is largely on six isomers (2–4 and 6–8). Our earlier work focused on isomers 1, 5, and 9.69 Therefore, an elaborate discussion of these isomers is avoided here and interested readers are referred to our earlier work.69 However, for the purpose of relative energy comparison and completeness, we have taken values from our earlier work and also have done calculations at higher levels of theory to get the thermodynamic stability of the low-lying isomers of C7H2. While isomers 2 and 9 have already been found in the laboratory by FTM spectroscopy,43,60 it is worth noting that the dipole moments of 3–8 are also non-zero (see Table 2).71 Both 2 and 3 can be considered as derivatives of cyclopropenylidene (c-C3H2). The latter molecule and also its doubly deuterated derivative (c-C3D2) were not only found in the laboratory but also in space.15,19 Also, 8 can be considered as a derivative of propadienylidene (the shortest member of cumulene carbene), which has also been found in the laboratory and in space.16,37 On the contrary, the bicyclic rings (4, 6, and 7) and the seven-membered ring isomer (5) thermodynamically fall between these two important astronomically relevant derivatives, which has not been discussed thus far in the literature. We hope that the rotational constants, centrifugal distortion constants, optimal geometry parameters, dipole moments and harmonic vibrational frequencies of 12C and 13C isotopes, and infrared intensities of these hypothetical isomers of C7H2 (3–8) will encourage experimentalists to find some of these isomers in the laboratory. Perhaps without finding these molecules in the laboratory, it would be a herculean task to confirm the presence of these molecules in space.
| Constanta | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| a Centrifugal distortion constants for isomer 9 is from an S-reduced Hamiltonian, whereas for all other isomers they are from the A-reduced Hamiltonian. b From ref. 69. See the discussions therein for detail. c From ref. 60. d From ref. 43. | |||||||||
| A e | — | 34343.21 (34722.14)c | 7716.69 | 6210.57 | 6117.11 | 6941.35 | 5613.16 | 18527.26 | 289548.07 (276259.00)d |
| B e | 832.63 | 1034.45 (1045.21)c | 1539.34 | 3851.13 | 3745.45 | 3603.21 | 4194.48 | 992.86 | 842.85 (851.91)d |
| C e | — | 1004.20 (1014.26)c | 1283.34 | 2377.10 | 2323.06 | 2371.95 | 2400.61 | 942.36 | 840.40 (849.29)d |
| Δ J | 0.8546 × 10−5 | 0.1689 × 10−4 | 0.3985 × 10−3 | 0.3330 × 10−3 | 0.2028 × 10−3 | 0.2360 × 10−3 | 0.3912 × 10−3 | 0.1501 × 10−3 | 0.8562 × 10−5 |
| Δ K | 0.8546 × 10−5 | 0.127735 | 0.109249 | 0.3272 × 10−2 | −0.1822 × 10−3 | 0.2216 × 10−2 | 0.6910 × 10−3 | 2.59533 | 22.1709 |
| Δ JK | −0.1709 × 10−4 | 0.6908 × 10−2 | −0.1116 × 10−1 | −0.2984 × 10−3 | 0.2185 × 10−2 | 0.3640 × 10−3 | 0.1218 × 10−4 | −0.3223 × 10−1 | 0.1098 × 10−1 |
| δ J | — | 0.5514 × 10−6 | 0.1455 × 10−3 | 0.1301 × 10−3 | 0.7111 × 10−4 | 0.8193 × 10−4 | 0.1577 × 10−3 | 0.3137 × 10−4 | −0.2595 × 10−7 |
| δ K | — | 0.3622 × 10−2 | 0.8015 × 10−3 | 0.4231 × 10−3 | 0.1345 × 10−2 | 0.5942 × 10−3 | 0.5253 × 10−3 | 0.2501 × 10−2 | −0.6170 × 10−8 |
| μ a | — | 2.306 | 3.774 | −3.357 | −1.878 | 2.311 | 3.068 | 4.659 | −7.336 |
| μ b | — | 2.855 | −1.855 | 2.413 | |||||
| |μ| | — | 3.670 | 3.774 | 3.357 | 1.878 | 2.964 | 3.068 | 5.247 | 7.336 |
2 Ab initio calculations
The geometries of all isomers of C7H2 reported in this study were optimized using both second-order Møller–Plesset perturbation theory72 and coupled-cluster (CC) methods. The considered CC methods are CC with single and double excitations (CCSD)73,74 and CCSD with quasiperturbative triple excitations (CCSD(T)).75–77 These calculations were carried out with the correlation-consistent cc-pVnZ basis sets of Dunning78 (n = D and T), which consist of 108 and 238 basis functions, respectively, for the C7H2 isomers. The frozen-core approximation is utilized in the geometry optimizations. To speed up the geometry optimization, the force constant matrix obtained at a lower level is used successively at a higher level. For all the stationary points obtained, harmonic vibrational frequencies were determined by analytic calculation of second derivatives.79 These electronic structure calculations were done with the CFOUR program package.80In order to obtain reliable relative energies for isomers 1–9, high-level benchmark data have been obtained using the W3lite-F12 theory.64,66 The W3lite-F12 theory (and its earlier version, the W3.2lite theory)81–83 represents layered extrapolations to the relativistic, all-electron CCSDT(Q)/CBS limit and can achieve near-benchmark accuracy for atomization reactions (i.e., they are associated with root-mean-square deviations, RMSDs, from accurate atomization energies of about 1 kJ mol−1 = 0.24 kcal mol−1).64 For example, the related W3-F12 theory is associated with an RMSD of 0.27 kcal mol−1 for a set of 140 very accurate atomization energies obtained at the full configuration interaction (FCI) infinite basis-set limit.64,66,81,83 The W3lite-F12 theory combines F12 methods84 with basis-set extrapolations in order to reproduce the CCSDT(Q)/CBS energy. In W3lite-F12, the CCSD(T)/CBS energy is obtained from the W2-F12 theory,64 and the post-CCSD(T) contributions are obtained from the W3.2lite theory.82 In brief, the Hartree–Fock (HF) component is calculated with the cc-pVQZ-F12 basis set of Peterson et al., which was developed for explicitly correlated calculations.85,86 Note that the complementary auxiliary basis (CABS) singles correction is included in the self-consistent field (SCF) energy.87–89 The valence CCSD-F12 correlation energy is extrapolated from the cc-VTZ-F12 and cc-VQZ-F12 basis sets, using the E(L) = E∞ + A/Lα two-point extrapolation formula, with α = 5.94. In all of the explicitly-correlated CC calculations, the diagonal, fixed-amplitude 3C(FIX) ansatz88,90,91 and the CCSD-F12b approximation89,92 are employed. The quasiperturbative triples, (T), corrections are calculated with the cc-pVTZ-F12 basis set and scaled by the factor f = 0.987 × EMP2–F12/EMP2. This approach has been shown to accelerate the basis-set convergence.64,92 The post-CCSD(T) corrections are obtained from standard CC calculations (i.e., without inclusion of F12 terms). Specifically, the higher-order connected triples (CCSDT--CCSD(T)) valence correlation contribution is calculated using the cc-pVDZ and cc-pVTZ(nof1d) basis sets, where cc-pVTZ(nof1d) indicates the combination of the sp part of the cc-pVTZ basis set combined with the d function from the cc-pVDZ basis set on heavy atoms and the s part of the cc-pVTZ basis set combined with the p function from the cc-pVDZ basis set on hydrogen.82 The parenthetical connected quadruples contribution (CCSDT(Q)–CCSDT) is calculated with the cc-pVDZ basis set. The CCSD inner-shell contribution is calculated with the core-valence weighted correlation-consistent aug-cc-pwCVTZ basis set of Peterson and Dunning,93 whilst the (T) inner-shell contribution is calculated with the cc-pwCVTZ basis set without the f functions.64
The W3lite-F12 single-point energy calculations were carried out using our CCSD(T)/cc-pVTZ equilibrium geometries. Zero-point vibrational energies (ZPVEs) are calculated at the same level of theory and scaled by a scaling factor of 0.9868 as recommended in ref. 94. All the CCSD(T) energy calculations involved in the W3lite-F12 energies were done with the Molpro program package,95,96 whilst the post-CCSD(T) calculations were carried our with the MRCC program.97,98
3 Results and discussion
The component breakdown of the W3lite-F12 relative energies for the C7H2 isomers is given in Table 1. Rotational and centrifugal distortion constants, and inertial axis dipole moment components calculated from the CCSD(T)/cc-pVTZ equilibrium geometries are given in Table 2. The optimal geometry parameters of isomers 2, 3, 4, 6, 7, and 8 at different levels along with other theoretical work (wherever available) are documented in Tables 3, 4, 5, 6, 7, and 8, respectively. The harmonic vibrational frequencies of isomers 1–3, 4–6, and 7–9 calculated at the CCSD(T)/cc-pVTZ level of theory are documented in Tables 9, 10, and 11, respectively. For brevity, the isotopic shifts obtained from the harmonic vibrational frequencies (12C–13C) for isomers 1–3, 4–6, and 7–9 calculated at the same level of theory are documented in the ESI,† in Tables S18, S19, and S20, respectively. The atom numbering scheme we have adopted for isomers 1–9 is given in Fig. 3, which is relevant for calculating the isotopic shifts. In the matrix-isolation of C3H2 isomers,40,47 such values were proven to be very useful in assigning the infrared spectra rather than the absolute harmonic vibrational frequencies. Cartesian coordinates, total electronic energies, ZPVEs, ZPVE corrected total electronic energies, and dipole moments corresponding to the optimized geometries of 1–9 at different levels are also given in the ESI.† We also note that the electronic structure of isomers 1, 5, and 9 had been discussed in detail in an earlier work by us and therefore these details are not repeated here.69| Parameter | cc-pVDZ | cc-pVTZ | Other work | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MP2 | CCSD | CCSD(T) | MP2 | CCSD | CCSD(T) | Ref. 68 | Ref. 63 | Ref. 58 | |
| a At the MP2/D95** level of theory. b At the B3LYP/6-31G(d) level of theory. c At the UB3LYP/6-311G(d,p) level of theory. | |||||||||
| R(C1C2) | 1.4008 | 1.4133 | 1.4105 | 1.3865 | 1.3988 | 1.3957 | 1.4000 | 1.3780 | 1.2980 |
| R(C2C3) | 1.2431 | 1.2291 | 1.2386 | 1.2266 | 1.2107 | 1.2208 | 1.2380 | 1.2240 | 1.2620 |
| R(C3C4) | 1.3767 | 1.3924 | 1.3885 | 1.3614 | 1.3769 | 1.3725 | 1.3750 | 1.3590 | 1.3260 |
| R(C4C5) | 1.2385 | 1.2264 | 1.2347 | 1.2209 | 1.2073 | 1.2160 | 1.2340 | 1.2150 | 1.2250 |
| R(C5H6) | 1.0767 | 1.0781 | 1.0797 | 1.0624 | 1.0622 | 1.0641 | 1.0680 | 1.0670 | 1.0620 |
| R(C1C7) | 1.3559 | 1.3465 | 1.3543 | 1.3406 | 1.3300 | 1.3383 | 1.3510 | 1.3420 | 1.5190 |
| R(C1C8) | 1.4631 | 1.4570 | 1.4682 | 1.4412 | 1.4332 | 1.4448 | 1.4530 | 1.4580 | 1.4540 |
| R(C7H9) | 1.0917 | 1.0917 | 1.0940 | 1.0769 | 1.0758 | 1.0783 | 1.0810 | 1.0830 | 1.0740 |
| θ(C1C2C3) | 178.72 | 179.01 | 178.73 | 179.11 | 179.37 | 179.16 | 178.40 | 179.90 | 178.60 |
| θ(C2C3C4) | 179.39 | 179.52 | 179.34 | 179.66 | 179.79 | 179.67 | 179.40 | 179.60 | 179.60 |
| θ(C3C4C5) | 179.87 | 179.90 | 179.84 | 179.81 | 179.86 | 179.82 | 179.90 | 179.90 | 179.90 |
| θ(C4C5H6) | 179.98 | 179.99 | 179.96 | 179.95 | 179.99 | 179.96 | 180.00 | 179.60 | 179.90 |
| θ(C1C7H9) | 147.84 | 147.84 | 147.43 | 148.26 | 148.36 | 147.99 | 148.40 | 146.50 | 138.10 |
| θ(C7C1C2) | 149.96 | 149.77 | 149.72 | 150.75 | 150.45 | 150.47 | 149.90 | 150.80 | 146.30 |
| θ(C8C1C2) | 148.88 | 148.71 | 148.94 | 148.27 | 148.17 | 148.34 | 148.80 | 149.00 | 161.80 |
| Parameter | cc-pVDZ | cc-pVTZ | Other work | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MP2 | CCSD | CCSD(T) | MP2 | CCSD | CCSD(T) | Ref. 68 | Ref. 63 | Ref. 58 | |
| a At the MP2/D95** level of theory. b At the B3LYP/6-31G(d) level of theory. c At the UB3LYP/6-311G(d,p) level of theory. | |||||||||
| R(C1C2, C4C5) | 1.4068 | 1.4191 | 1.4176 | 1.3919 | 1.4037 | 1.4018 | 1.4050 | 1.3910 | 1.3410 |
| R(C2C3, C3C4) | 1.4525 | 1.4482 | 1.4572 | 1.4299 | 1.4242 | 1.4336 | 1.4450 | 1.4340 | 1.3500 |
| R(C1C7, C5C6) | 1.2354 | 1.2247 | 1.2322 | 1.2177 | 1.2058 | 1.2138 | 1.2310 | 1.2120 | 1.2220 |
| R(C7H9, C6H8) | 1.0769 | 1.0784 | 1.0799 | 1.0625 | 1.0626 | 1.0644 | 1.0680 | 1.0670 | 1.0630 |
| R(C2C4) | 1.3634 | 1.3506 | 1.3594 | 1.3479 | 1.3339 | 1.3433 | 1.3570 | 1.3500 | 1.6390 |
| θ(C1C2C3, C5C4C3) | 149.54 | 149.27 | 149.52 | 149.03 | 148.76 | 148.97 | 149.60 | 149.60 | 167.50 |
| θ(C2C3C4) | 55.98 | 55.59 | 55.61 | 56.23 | 55.85 | 55.87 | — | — | — |
| θ(C2C1C7, C4C5C6) | 179.84 | 179.65 | 179.55 | 179.91 | 179.95 | 179.93 | 179.90 | 179.80 | 178.30 |
| θ(C1C7H9, C5C6H8) | 179.78 | 179.90 | 179.97 | 179.48 | 179.61 | 179.57 | 180.00 | 179.50 | 179.30 |
| θ(C1C2C4, C5C4C2) | 148.45 | 148.53 | 148.29 | 149.08 | 149.17 | 148.96 | 148.40 | 148.50 | 139.90 |
| Parameter | cc-pVDZ | cc-pVTZ | Other work | ||||
|---|---|---|---|---|---|---|---|
| MP2 | CCSD | CCSD(T) | MP2 | CCSD | CCSD(T) | Ref. 58 | |
| a At the UB3LYP/6-311G(d,p) level of theory. | |||||||
| R(C1C2, C1C3) | 1.4209 | 1.3997 | 1.4082 | 1.3996 | 1.3797 | 1.3870 | 1.4100 |
| R(C2C4, C3C5) | 1.3444 | 1.2931 | 1.3166 | 1.3258 | 1.2737 | 1.2951 | 1.3620 |
| R(C4C6, C5C7) | 1.3827 | 1.4018 | 1.3980 | 1.3686 | 1.3879 | 1.3848 | 1.3840 |
| R(C6H8, C7H9) | 1.0976 | 1.0981 | 1.1001 | 1.0836 | 1.0829 | 1.0852 | 1.0850 |
| R(C2C3) | 1.5012 | 1.7624 | 1.6697 | 1.5013 | 1.7630 | 1.6858 | 1.4350 |
| R(C6C7) | 1.4802 | 1.4401 | 1.4600 | 1.4680 | 1.4258 | 1.4448 | 1.4200 |
| θ(C1C2C4, C1C3C5) | 178.74 | 166.39 | 171.21 | 177.75 | 165.22 | 169.36 | — |
| θ(C2C4C6, C3C5C7) | 119.19 | 123.88 | 121.71 | 119.87 | 124.51 | 122.66 | 121.00 |
| θ(C4C6H8, C5C7H9) | 123.82 | 122.27 | 122.53 | 123.95 | 122.34 | 122.60 | 121.30 |
| θ(C1C2C3, C1C3C2) | 58.12 | 50.98 | 53.64 | 57.57 | 50.29 | 52.58 | 59.40 |
| θ(C2C1C3) | 63.77 | 78.04 | 72.72 | 64.87 | 79.42 | 74.84 | 61.20 |
| Parameter | cc-pVDZ | cc-pVTZ | ||||
|---|---|---|---|---|---|---|
| MP2 | CCSD | CCSD(T) | MP2 | CCSD | CCSD(T) | |
| R(C1C2) | 1.4246 | 1.4281 | 1.4322 | 1.4185 | 1.4157 | 1.4243 |
| R(C2C3) | 1.4018 | 1.4229 | 1.4218 | 1.3863 | 1.4090 | 1.4070 |
| R(C3C4) | 1.4343 | 1.4047 | 1.4172 | 1.4242 | 1.3902 | 1.4036 |
| R(C4C5) | 1.4126 | 1.4596 | 1.4512 | 1.4005 | 1.4484 | 1.4410 |
| R(C5C6) | 1.2855 | 1.2825 | 1.2892 | 1.2666 | 1.2662 | 1.2736 |
| R(C2C7) | 1.4825 | 1.4273 | 1.4484 | 1.4568 | 1.4038 | 1.4223 |
| R(C1C7) | 1.4344 | 1.4681 | 1.4664 | 1.4154 | 1.4464 | 1.4459 |
| R(C1C6) | 1.3844 | 1.3525 | 1.3650 | 1.3615 | 1.3327 | 1.3421 |
| R(C3H8) | 1.0948 | 1.0939 | 1.0961 | 1.0807 | 1.0790 | 1.0814 |
| R(C4H9) | 1.0956 | 1.0975 | 1.0992 | 1.0816 | 1.0821 | 1.0842 |
| θ(C1C2C3) | 122.95 | 125.15 | 124.36 | 122.95 | 125.20 | 124.42 |
| θ(C2C3C4) | 118.82 | 118.22 | 118.49 | 118.92 | 118.09 | 118.45 |
| θ(C3C4C5) | 115.15 | 119.27 | 118.42 | 115.36 | 119.31 | 118.65 |
| θ(C4C5C6) | 124.19 | 110.56 | 114.00 | 122.72 | 110.08 | 112.69 |
| θ(C3C2C7) | 177.96 | 172.97 | 174.44 | 178.09 | 173.07 | 174.53 |
| θ(C2C3H8) | 123.78 | 121.58 | 121.95 | 123.65 | 121.59 | 121.85 |
| θ(C3C4H9) | 119.16 | 118.85 | 118.79 | 119.00 | 118.96 | 118.80 |
| Parameter | cc-pVDZ | cc-pVTZ | Other work | ||||
|---|---|---|---|---|---|---|---|
| MP2 | CCSD | CCSD(T) | MP2 | CCSD | CCSD(T) | Ref. 58 | |
| a At the UB3LYP/6-311G(d,p) level of theory. | |||||||
| R(C1C2, C1C3) | 1.4790 | 1.4712 | 1.4817 | 1.4587 | 1.4490 | 1.4600 | 1.3760 |
| R(C2C3) | 1.3750 | 1.3617 | 1.3691 | 1.3596 | 1.3447 | 1.3527 | 1.4940 |
| R(C2C4, C3C5) | 1.4431 | 1.4570 | 1.4581 | 1.4315 | 1.4453 | 1.4463 | 1.3930 |
| R(C4C6, C5C7) | 1.3899 | 1.3777 | 1.3867 | 1.3756 | 1.3615 | 1.3708 | 1.4040 |
| R(C4H8, C5H9) | 1.0934 | 1.0927 | 1.0949 | 1.0791 | 1.0773 | 1.0797 | 1.0810 |
| R(C6C7) | 1.2958 | 1.2937 | 1.3045 | 1.2782 | 1.2747 | 1.2853 | 1.2820 |
| θ(C2C1C3) | 55.40 | 55.13 | 55.03 | 55.56 | 55.29 | 55.20 | 65.70 |
| θ(C3C2C4, C2C3C5) | 125.95 | 125.64 | 125.66 | 125.99 | 125.69 | 125.73 | 123.80 |
| θ(C2C4C6, C3C5C7) | 104.41 | 104.50 | 104.83 | 104.14 | 104.14 | 104.43 | 106.50 |
| θ(C2C4H8, C3C5H9) | 126.65 | 126.48 | 126.47 | 126.70 | 126.53 | 126.53 | 124.50 |
| Parameter | cc-pVDZ | cc-pVTZ | Other work | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MP2 | CCSD | CCSD(T) | MP2 | CCSD | CCSD(T) | Ref. 68 | Ref. 63 | Ref. 58 | |
| a At the MP2/D95** level of theory. b At the B3LYP/6-31G(d) level of theory. c At the UB3LYP/6-311G(d,p) level of theory. | |||||||||
| R(C1C2) | 1.3630 | 1.3508 | 1.3622 | 1.3492 | 1.3350 | 1.3466 | 1.3620 | 1.3460 | 1.3890 |
| R(C1C3) | 1.4160 | 1.4328 | 1.4293 | 1.4015 | 1.4181 | 1.4144 | 1.4150 | 1.4010 | 1.3750 |
| R(C1H4) | 1.1027 | 1.1011 | 1.1034 | 1.0889 | 1.0861 | 1.0887 | 1.0920 | 1.0950 | 1.0870 |
| R(C2C5) | 1.3020 | 1.3074 | 1.3121 | 1.2831 | 1.2869 | 1.2920 | 1.2980 | 1.2850 | 1.2260 |
| R(C3C6) | 1.2466 | 1.2301 | 1.2403 | 1.2298 | 1.2115 | 1.2222 | 1.2410 | 1.2240 | 1.2270 |
| R(C6C7) | 1.3738 | 1.3923 | 1.3875 | 1.3583 | 1.3762 | 1.3714 | 1.3720 | 1.3580 | 1.3500 |
| R(C7C8) | 1.2393 | 1.2264 | 1.2348 | 1.2216 | 1.2072 | 1.2161 | 1.2350 | 1.2150 | 1.2130 |
| R(C8H9) | 1.0773 | 1.0784 | 1.0800 | 1.0629 | 1.0625 | 1.0645 | 1.0680 | 1.0670 | 1.0620 |
| θ(C3C1C2) | 123.23 | 123.69 | 123.45 | 123.36 | 123.89 | 123.64 | 122.90 | 124.30 | 122.80 |
| θ(H4C1C2) | 119.87 | 119.83 | 119.96 | 119.72 | 119.67 | 119.80 | 120.40 | 119.70 | 118.20 |
| θ(C1C2C5) | 178.15 | 178.31 | 178.23 | 177.85 | 177.98 | 177.93 | 177.00 | 179.50 | 171.90 |
| θ(C1C3C6) | 178.57 | 177.76 | 177.72 | 179.00 | 178.24 | 178.39 | 179.00 | 178.70 | 178.90 |
| θ(C3C6C7) | 179.20 | 178.98 | 178.78 | 179.46 | 179.37 | 179.31 | 179.20 | 179.60 | 179.90 |
| θ(C6C7C8) | 179.87 | 179.76 | 179.67 | 179.95 | 179.86 | 179.85 | 179.90 | 180.00 | 180.00 |
| θ(C7C8H9) | 179.76 | 179.72 | 179.62 | 179.75 | 179.74 | 179.70 | 179.90 | 179.90 | 180.00 |
| Mode | Isomer 1a | Isomer 2 | Isomer 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Symmetry | Frequency | Intensity | Symmetry | Frequency | Intensity | Symmetry | Frequency | Intensity | |
| a At the ROCCSD(T)/cc-pVTZ level of theory since the ground electronic state is a triplet. | |||||||||
| 1 | πu | 71 | 3 | a′ | 94 | 4 | a 1 | 96 | 2 |
| 2 | πg | 170 | — | a′′ | 97 | 0 | b 1 | 179 | 1 |
| 3 | πu | 367 | 0 | a′ | 234 | 5 | b 2 | 227 | 0 |
| 4 | πu | 398 | 3 | a′′ | 261 | 3 | a 2 | 230 | — |
| 5 | πg | 417 | — | a′ | 459 | 1 | a 1 | 418 | 3 |
| 6 | πg | 517 | — | a′′ | 495 | 0 | b 1 | 461 | 18 |
| 7 | πu | 518 | 84 | a′ | 502 | 1 | b 2 | 540 | 2 |
| 8 | σ+g | 543 | — | a′ | 519 | 2 | a 1 | 606 | 66 |
| 9 | σ−u | 1041 | 7 | a′′ | 540 | 1 | b 2 | 607 | 24 |
| 10 | σ+g | 1644 | — | a′ | 613 | 43 | a 2 | 633 | — |
| 11 | σ−u | 1870 | 0 | a′′ | 659 | 36 | a 1 | 678 | 7 |
| 12 | σ+g | 2008 | — | a′′ | 887 | 15 | a 2 | 703 | — |
| 13 | σ−u | 2343 | 17 | a′ | 910 | 2 | b 1 | 704 | 60 |
| 14 | σ−u | 3441 | 251 | a′ | 1006 | 3 | b 2 | 777 | 5 |
| 15 | σ+g | 3447 | — | a′ | 1175 | 4 | b 2 | 1224 | 36 |
| 16 | a′ | 1263 | 49 | a 1 | 1237 | 33 | |||
| 17 | a′ | 1715 | 8 | a 1 | 1768 | 0 | |||
| 18 | a′ | 2112 | 3 | b 2 | 2159 | 26 | |||
| 19 | a′ | 2267 | 27 | a 1 | 2169 | 4 | |||
| 20 | a′ | 3260 | 1 | b 2 | 3450 | 122 | |||
| 21 | a′ | 3456 | 101 | a 1 | 3455 | 32 | |||
| Mode | Isomer 4 | Isomer 5 | Isomer 6 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Symmetry | Frequency | Intensity | Symmetry | Frequency | Intensity | Symmetry | Frequency | Intensity | |
| 1 | b 1 | 274 | 1 | b 1 | 254 | 0 | a′′ | 184 | 17 |
| 2 | a 1 | 379 | 3 | a 2 | 330 | — | a′ | 325 | 135 |
| 3 | a 2 | 396 | — | b 2 | 349 | 48 | a′′ | 405 | 6 |
| 4 | b 2 | 477 | 81 | b 2 | 507 | 171 | a′′ | 465 | 0 |
| 5 | a 1 | 499 | 53 | a 2 | 545 | — | a′ | 465 | 27 |
| 6 | b 1 | 505 | 11 | b 1 | 557 | 10 | a′′ | 616 | 1 |
| 7 | a 2 | 599 | — | a 1 | 561 | 10 | a′ | 636 | 13 |
| 8 | b 2 | 731 | 117 | a 1 | 641 | 9 | a′ | 763 | 8 |
| 9 | b 1 | 872 | 15 | a 2 | 893 | — | a′′ | 817 | 35 |
| 10 | b 2 | 873 | 14 | b 1 | 896 | 8 | a′ | 925 | 15 |
| 11 | a 1 | 959 | 2 | a 1 | 916 | 72 | a′′ | 977 | 0 |
| 12 | a 2 | 988 | — | a 1 | 965 | 3 | a′ | 1022 | 28 |
| 13 | a 1 | 1067 | 2 | b 2 | 1148 | 0 | a′ | 1082 | 15 |
| 14 | b 2 | 1258 | 18 | b 2 | 1288 | 13 | a′ | 1159 | 8 |
| 15 | a 1 | 1321 | 2 | a 1 | 1312 | 3 | a′ | 1340 | 18 |
| 16 | a 1 | 1346 | 33 | b 2 | 1344 | 7 | a′ | 1391 | 10 |
| 17 | b 2 | 1447 | 3 | a 1 | 1398 | 8 | a′ | 1422 | 8 |
| 18 | b 2 | 1768 | 42 | b 2 | 1780 | 292 | a′ | 1611 | 15 |
| 19 | a 1 | 1790 | 54 | a 1 | 1902 | 31 | a′ | 1831 | 231 |
| 20 | b 2 | 3166 | 2 | b 2 | 3206 | 11 | a′ | 3184 | 13 |
| 21 | a 1 | 3183 | 21 | a 1 | 3207 | 1 | a′ | 3221 | 2 |
| Mode | Isomer 7 | Isomer 8 | Isomer 9 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Symmetry | Frequency | Intensity | Symmetry | Frequency | Intensity | Symmetry | Frequency | Intensity | |
| 1 | b 1 | 148 | 17 | a′ | 73 | 4 | b 1 | 73 | 1 |
| 2 | b 1 | 328 | 6 | a′′ | 138 | 10 | b 2 | 76 | 0 |
| 3 | b 2 | 402 | 6 | a′ | 177 | 4 | b 1 | 170 | 11 |
| 4 | b 2 | 490 | 44 | a′′ | 197 | 3 | b 2 | 189 | 8 |
| 5 | a 2 | 517 | — | a′ | 290 | 1 | b 2 | 268 | 1 |
| 6 | a 1 | 607 | 1 | a′′ | 318 | 0 | b 1 | 285 | 0 |
| 7 | a 2 | 677 | — | a′ | 467 | 0 | b 2 | 403 | 1 |
| 8 | b 1 | 809 | 32 | a′ | 505 | 2 | b 2 | 455 | 1 |
| 9 | a 1 | 845 | 0 | a′′ | 506 | 2 | b 1 | 518 | 1 |
| 10 | a 2 | 892 | — | a′ | 631 | 41 | a 1 | 554 | 0 |
| 11 | b 2 | 895 | 29 | a′′ | 659 | 37 | b 1 | 556 | 2 |
| 12 | a 1 | 1054 | 37 | a′ | 752 | 49 | b 1 | 909 | 36 |
| 13 | b 2 | 1186 | 7 | a′′ | 870 | 5 | b 2 | 1020 | 0 |
| 14 | a 1 | 1211 | 82 | a′ | 1068 | 15 | a 1 | 1048 | 2 |
| 15 | b 2 | 1295 | 44 | a′ | 1217 | 23 | a 1 | 1424 | 12 |
| 16 | a 1 | 1322 | 21 | a′ | 1396 | 20 | a 1 | 1537 | 21 |
| 17 | b 2 | 1419 | 1 | a′ | 1982 | 891 | a 1 | 1891 | 316 |
| 18 | a 1 | 1598 | 3 | a′ | 2103 | 21 | a 1 | 2093 | 304 |
| 19 | a 1 | 1833 | 3 | a′ | 2253 | 212 | a 1 | 2118 | 1358 |
| 20 | b 2 | 3234 | 4 | a′ | 3129 | 1 | a 1 | 3130 | 0 |
| 21 | a 1 | 3235 | 0 | a′ | 3453 | 99 | b 2 | 3219 | 0 |
3.1 Benchmark CCSDT(Q)/CBS energies
Table 1 gathers the component breakdown of the W3lite-F12 relative energies for the isomers 1–9. An inspection of Table 1 reveals that the correlation effects are very important for describing the relative energies of these isomers. At the HF/CBS level of theory, isomers 2 and 3 are energetically more stable than isomer 1 by as much as 17.79 and 16.06 kcal mol−1, respectively. It is also worth noting that for these two isomers the SCF and CCSD components nearly cancel each other out (see Table 1). At the CCSD/CBS level of theory, isomers 1 and 2 are nearly isoenergetic and the energy gap between them is merely 0.22 kcal mol−1. The valence (T) correlation contributions can be quite large, reaching up to 7.29 kcal mol−1 for isomer 5. The higher-order triple excitations, T–(T), are still chemically significant, reaching up to 0.78 kcal mol−1 (in absolute value) for isomer 4. The quasiperturbative quadruple excitations, (Q), tend to have an opposite sign to the T–(T) component, and can reach up to 0.94 kcal mol−1 (in absolute value) for isomer 4. The core–valence correlation contributions systematically increase the relative energies of the isomers by chemically significant amounts. In particular, they range between 0.61 (isomer 2) and 1.29 (isomer 4) kcal mol−1. The scalar relativistic corrections, on the other hand, systematically decrease the relative energies of the isomers, but are fairly small; that is, they range between 0.05 (isomers 5 and 6) to 0.12 (isomer 8) kcal mol−1.Overall, we obtain the following relative energies at the relativistic, all-electron CCSDT(Q)/CBS level of theory: 3.77 (2), 6.13 (3), 6.62 (4), 6.35 (5), 15.54 (6), 16.33 (7), 18.94 (8), and 19.38 (9) kcal mol−1. Inclusion of the ZPVE component, calculated at the CCSD(T)/cc-pVTZ level of theory, results in the following relative energies at 0 K: 5.56 (2), 7.64 (3), 10.35 (4), 10.23 (5), 19.19 (6), 20.20 (7), 20.26 (8), and 20.34 (9) kcal mol−1. The very small energy separations between many of the isomers (e.g., between isomers 4 and 5, and between isomers 6–9) demonstrates that one has to use highly accurate theoretical methods in order to capture these energy separations accurately.
3.2 1-(buta-1,3-diynyl)cyclopropenylidene (2)
McCarthy and co-workers had detected 2 by FTM spectroscopy nearly two decades ago.60 It is noteworthy that the parent molecule, cyclopropenylidene (c-C3H2), had also been detected in the same laboratory, which was crucial in the identification of several previously detected lines (for example, 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 338 and 18
338 and 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 343 MHz) in astronomical sources.15 A few years before, the doubly deuterated cyclopropenylidene (c-C3D2) was also detected in the ISM.19 In comparison with the parent molecule, we believe that the derivative (2) is also astronomically relevant as it can be obtained by replacing a H atom with the butadiynyl (C4H) group. The dipole moment of 2 is 3.67 Debye estimated at the CCSD(T)/cc-pVTZ level of theory (see Table 2), which is comparable to the parent molecule, whose dipole moment is 3.43 Debye.99 Energetically, 2 lies 5.56 kcal mol−1 above 1 (see Table 1). Aoki and Ikuta had initially predicted that isomer 2 is the most stable isomer of C7H2 based on geometry optimizations at the MP2/D95** level of theory.68 Bowie and co-workers had estimated this energy gap to be 13.65 kcal mol−1 at the B3LYP/aug-cc-pVDZ//B3LYP/6-31G(d) level of theory.63 They also stated that both isomers 2 and 3 are low-lying due to aromatic stability because of the presence of 2π electrons inside the three-membered ring (Hückel's (4n + 2)π electrons rule with n = 0). The relative energies obtained by us rather support this result much better than the relative energies estimated by them.70 On the contrary, Sun and co-workers thermodynamically place 2 above 1 at 49.45 kcal mol−1 estimated at the UB3LYP/6-311G(d,p) level of theory.58 The CCSD(T)/cc-pVTZ single point energy computed by them using the optimized geometry at the same level decreases this gap to be 46.26 kcal mol−1.58 As we mentioned earlier, we are in disagreement with relative energies estimated by them to a large extent.
343 MHz) in astronomical sources.15 A few years before, the doubly deuterated cyclopropenylidene (c-C3D2) was also detected in the ISM.19 In comparison with the parent molecule, we believe that the derivative (2) is also astronomically relevant as it can be obtained by replacing a H atom with the butadiynyl (C4H) group. The dipole moment of 2 is 3.67 Debye estimated at the CCSD(T)/cc-pVTZ level of theory (see Table 2), which is comparable to the parent molecule, whose dipole moment is 3.43 Debye.99 Energetically, 2 lies 5.56 kcal mol−1 above 1 (see Table 1). Aoki and Ikuta had initially predicted that isomer 2 is the most stable isomer of C7H2 based on geometry optimizations at the MP2/D95** level of theory.68 Bowie and co-workers had estimated this energy gap to be 13.65 kcal mol−1 at the B3LYP/aug-cc-pVDZ//B3LYP/6-31G(d) level of theory.63 They also stated that both isomers 2 and 3 are low-lying due to aromatic stability because of the presence of 2π electrons inside the three-membered ring (Hückel's (4n + 2)π electrons rule with n = 0). The relative energies obtained by us rather support this result much better than the relative energies estimated by them.70 On the contrary, Sun and co-workers thermodynamically place 2 above 1 at 49.45 kcal mol−1 estimated at the UB3LYP/6-311G(d,p) level of theory.58 The CCSD(T)/cc-pVTZ single point energy computed by them using the optimized geometry at the same level decreases this gap to be 46.26 kcal mol−1.58 As we mentioned earlier, we are in disagreement with relative energies estimated by them to a large extent.
The rotational and centrifugal distortion constants estimated by us at the CCSD(T)/cc-pVTZ level of theory are in good agreement with the measured values (see Table 2). We also infer from the values of rotational constants that all isomers except 1 are asymmetric tops. However, considering the small difference between Be and Ce, experimentalists do address isomers 2 and 9 as nearly prolate symmetric tops.43,60 As far as bond lengths are concerned (see Table 3), they are systematically overestimated at the MP2, CCSD, and CCSD(T) levels in conjunction with the cc-pVDZ basis set. These results are consistent with previous observations, which are largely due to the lack of higher angular momentum polarization functions.100–105 Considering the shorter bond lengths of C2C3 and C4C5, and also the double bond distance of C1C7, and also the longer bond lengths of C3C4, C1C2 and C1C8 at all levels estimated by us, the scope for multiple valence structures for 2 rather seem to be slim. Our bond lengths are largely in agreement with the previous theoretical studies except with the C1C7 distance estimated by Sun and co-workers.58 As far as bond angles are concerned, four of them (C1C2C3, C2C3C4, C3C4C5, and C4C5H6) are nearly 180 degrees, which rather confirms that the butadiynyl chain is linear. Once again, our values are in disagreement with Sun and co-workers’ values for two of the angles (C1C7H9 and C8C1C2) but in agreement with the other previous theoretical studies. The strongest vibrational mode turns out to be the C–H stretch of a′ symmetry whose frequency is 3456 cm−1 at the CCSD(T)/cc-pVTZ level of theory (see Table 9). The isotopic shifts (12C−13C) in frequencies (see Table S18, ESI†) should serve as a guide in identifying other high frequency vibrational modes. We also note that all the carbon atoms are environmentally different for this isomer and that's the reason the isotopic shifts in the frequencies are calculated for all the carbon atoms.
3.3 1,2-(diethynyl)cyclopropenylidene (3)
This isomer can also be considered as a derivative of cyclopropenylidene by replacing both the H atoms with the ethynyl (C2H) group. The dipole moment of this molecule is 3.77 Debye, which is comparable to 2 (see Table 2). Energetically, it lies 7.64 kcal mol−1 above 1 and just 2.08 kcal mol−1 above 2 at our best estimate. Nevertheless, it is yet to be identified in the laboratory to date. For 2, the inertial axis dipole moment components are in two directions whereas for 3 it is in only one direction (see Table 2). This means that there is only one type of rotational transition possible for 3, which in part explains why it would be somewhat difficult to identify this molecule in comparison with 2, where both a-type and b-type rotational transitions are possible.60 In fact, while analyzing the isoelectronic HC6N species,35 McCarthy and co-workers had pointed out that only b-type rotational transitions are possible for 3. On the contrary, the a-type transitions appear to be less sensitive to the angle of the chains with respect to the ring and therefore they can be predicted with greater accuracy than the b-type transitions.35 Moreover, we also speculate that 3 can undergo Bergman cyclization106 and would become 4.The shorter bond lengths of C1C7 and C5C6 (see Table 4), and longer bond lengths of C1C2, C4C5, C2C3, and C3C4, and the double bond distance of C2C4 at all levels rather tell us that the valence structure given in Fig. 1 for 3 rather seems to be dominant. Theoretical studies on the optimal geometry of 3 were done by others58,63,68 and our geometrical parameters reported along with previous studies are in good agreement. Four nearly 180 degree bond angles (C2C1C7, C1C7H9, C4C5C6, and C5C6H8) obtained at all levels of theory indicate that the ethynyl chain is linear. Aoki and Ikuta had predicted that 3 is the second most stable isomer of C7H2 based on geometry optimizations obtained at the MP2/D95** level of theory.68 Bowie and co-workers had estimated the energy gap between 1 and 3 to be 17.51 kcal mol−1 at the B3LYP/aug-cc-pVDZ//B3LYP/6-31G(d) level of theory,63 which is ∼10 kcal mol−1 higher than the value we have estimated. Sun and co-workers estimate this energy gap to be 54.12 kcal mol−1 at the UB3LYP/6-311G(d,p) level of theory,58 which is again inconsistent with our high-level ab initio results. The C–H asymmetric stretching vibrational mode of b2 symmetry with 122 km mol−1 intensity should rather be easily seen in the IR spectra (see Table 9) between 3400 and 3500 cm−1. There are four different carbon atoms for 3 whose isotopic shifts are given in Table S18, ESI.† We believe that this data would be helpful in identifying this hypothetical molecule both in the laboratory and also in space.
3.4 Bicyclic rings (4, 6 and 7)
The bicyclic rings definitely seem to reserve a special place on the C7H2 PES. In comparison with the other bicyclic rings (6 and 7) reported in this study, 4 lies 8.84 and 9.85 kcal mol−1, respectively, below the other ortho-form (6) and the para-form (7). We note that the meta-form (13) is 32.69 kcal mol−1 above 4 at the CCSD(T)/cc-pVTZ level of theory with ZPVE correction. As we mentioned in the introduction, calculations at a further higher level of theory have not been done for 13. It is worth noting here that among the C6H2 isomers, the meta-form of tetradehydrobenzene is the second most stable isomer42,57 and the presence of an additional carbon atom here had reversed the story as far as energetics are concerned. The resonance structures for 4, 6, and 7 are given in Fig. 4. One of the reasons why 4 is more stable than the other two forms could be due to the fact that three reasonable resonance structures can be drawn for this form whereas for 6 and 7 (and also for 13), we can possibly draw two resonance structures. Although both 4 and 13 exhibit a biradical character, for the former the ground electronic state is a singlet but for the latter the ground electronic state is a triplet. For 6 and 7, valence structures can be drawn with a clear triple bond for each, whereas the same is not true for 4 and 13. The dipole moments of 4, 6, and 7 are 3.36, 2.96, and 3.07 Debye, respectively.71 Unlike tetradehydrobenzenes (C6H2), where the dipole moment of the para-form is zero by symmetry and for the ortho and meta forms it is 1.15 and 1.43 Debye (at the CCSD(T)/cc-pVTZ level of theory), respectively, the dipole moments of the bicyclic rings in C7H2 are quite high and comparable to isomer 2, which has already been detected in the laboratory.603.5 Bicyclo[4.1.0]hepta-1,2,4,5-tetraene-7-ylidene (4)
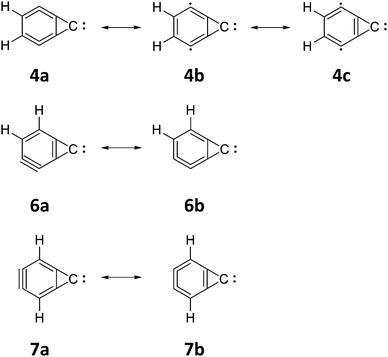
While 4 is clearly more stable than the experimentally known cumulene carbene isomer (9) by 9.99 kcal mol−1, it lies 10.35 kcal mol−1 above 1. The seven-membered ring isomer, 5, lies just 0.12 kcal mol−1 below 4. Also, another most remarkable thing about isomer 4 is the C2C3 bond length (see Table 5). At the CCSD(T)/cc-pVTZ level of theory, we obtained a bond length of 1.6858 Å, which is quite long for a C–C single bond. Perhaps, this is the longest C–C single bond we have seen among the C7H2 isomers we have studied thus far. Nevertheless, we note that such ultralong C–C single bonds were studied in detail in the past in 11,11-dimethyl-1,6-methano[10]annulene.107 It is worth pointing out that at the UB3LYP/6-311G(d,p) level of theory Sun et al.58 obtained a much shorter bond length of 1.4350 Å, indicating that this level of theory is inappropriate for this system.When the basis set is increased within a particular method, normally bond lengths get contracted. That is the trend we had seen throughout for all isomers of C7H2, but the C2C3 bond length reported in this paper for isomer 4 is an exception to this. The C2C4 and C3C5 bond lengths obtained at all levels are intermediate between a triple bond and a double bond. On the contrary, the C6C7 bond length rather seems to be an intermediate between a double bond and a single bond. Taking into account the C1C2, C1C3, C4C6, and C5C7 bond lengths (and also other distances obtained at all levels), it is evident that two valence structures (4a and 4b; see Fig. 4) are in competition for this isomer and 4c rather seems to be less dominant.
The harmonic vibrational frequencies are documented in Table 10. The in-plane ring puckering motion of b2 symmetry should rather be seen between 700 and 800 cm−1 as the intensity of this mode alone is high compared to other vibrational modes. The isotopic shifts of four different carbon atoms are given in Table S19 (ESI†).
3.6 Bicyclo[4.1.0]hepta-4,6-diene-2-yne-7-ylidene (6)
To our knowledge, previous theoretical studies haven't been reported for this isomer. Though Sun and co-workers considered 113 isomers,58 this isomer hasn't been considered. 6 lies 19.19 kcal mol−1 above 1 and just 1.15 kcal mol−1 below 9 (see Table 1). While it is 8.84 kcal mol−1 above the other ortho-form (4), it is rather competitively close to the para-form (7), which lies ∼1 kcal mol−1 above 6. Notably, for this isomer, the inertial axis dipole moment components are in two directions (see Table 2). This means that both a-type and b-type rotational transitions are possible for this isomer like 2 and 8. Therefore, identification of this isomer should be easier compared to other unidentified ring structures (3–5, and 7). Although a well-balanced Lewis structure is given in Fig. 1, another dominant valence structure is certainly possible for this isomer. The optimal geometry obtained by us is collected in Table 6, which rather suggests such an endeavor.Two possible valence structures (6a and 6b) are given in Fig. 4. The bond lengths of C5C6 rather seem to be intermediate between a triple bond and a double bond. However, the bond lengths of C2C3 and C3C4 appear to be an intermediate between a double bond and a single bond. The bond lengths of C1C7, C4C5, C1C2, and C2C7 rather look like a single bond. Nevertheless, only between C5 and C6, a triple bond can be drawn and anywhere else in the ring, the structure would be unreasonable. It is clear that both the valence structures are competing with each other. However, our bond lengths suggest that valence structure 6a is slightly dominant compared to 6b.
The high-frequency C–C stretching mode (involving the movement of C5–C6 as per Fig. 3) of a′ symmetry should readily be seen between 1800 and 1900 cm−1 in the IR spectra as the intensity for this mode alone is in excess of 200 km mol−1 (see Table 10). We also note that compared to the other ortho and para-form (4 and 7), for 6, all seven carbon atoms are environmentally different and therefore seven different isotopic shifts were being calculated in Table S19 (ESI†).
3.7 Bicyclo[4.1.0]hepta-1,5-diene-3-yne-7-ylidene (7)
This isomer lies 20.20 kcal mol−1 above 1. On the contrary, it is competitively close to three other isomers (6, 8, and 9) of C7H2. This para-form lies 1.01 kcal mol−1 above the unsymmetrical ortho-form (6). However, 8 and 9 are just 0.06 and 0.14 kcal mol−1, respectively, above 7. Sun and co-workers had optimized this molecule with a C2 symmetry instead of C2v symmetry.58 The relative energy gap they found relative to 1 is 62.90 kcal mol−1 at the UB3LYP/6-311G(d,p) level of theory. The single point energy computed by them at the CCSD(T)/cc-pVTZ//UB3LYP/6-311G(d,p) level of theory reduces this gap to 43.48 kcal mol−1. Nevertheless, these values are 42.70 and 23.28 kcal mol−1, respectively, higher than our best estimate.The C6C7 bond length obtained at the considered levels of theory (see Table 7) indicates that the distance is intermediate between a triple bond and a double bond. This bond length alone tells us that both the valence structures (7a and 7b) are competing with each other. The longer bond lengths of C1C2 and C1C3 could be attributed to the fact that in both the valence structures, they remain as a single bond. By considering the intermediate distance of a double bond and a single bond of C4C6 and C5C7 in comparison to C2C4 and C3C5, which are close to single bond lengths, it is clear that the valence structure 7b is slightly dominant in comparison to 7a.
Unlike the para-form of the tetradehydrobenzene (C6H2) where the dipole moment is zero by symmetry,42 the dipole moment of 7 is 3.07 Debye, which is comparable to the detected isomers of C7H2 such as, 2. The harmonic vibrational frequencies are listed in Table 11. The highest intensity mode of a1 symmetry, which represents the three-membered ring elongation via the carbene carbon atom (C1), should rather be seen in the IR spectra around 1150–1250 cm−1. The second highest intensity mode of b2 symmetry, which is close-by (1295 cm−1), represents in-plane ring distortion of the six-membered ring. For this molecule, none of the high frequency vibrational modes have shown appreciable intensity in the IR spectra. The isotopic shifts of four different carbon atoms are given in Table S20 (ESI†).
3.8 1-(buta-1,3-diynyl)propadienylidene (8)
This molecule is a butadiynyl derivative of the smallest cumulene carbene (:CCCH2). The latter is not only found in the laboratory but also in ISM.16,37 The dipole moment of 8 at the CCSD(T)/cc-pVTZ level of theory is 5.25 Debye, which is less than the cumulene carbene isomer (9) but higher than isomers 2–7. Also, it lies just 0.08 kcal mol−1 below 9 at the highest level of theory estimated here. In addition, we note that synthesizing this isomer may be easier compared to the other isomers discussed in this paper.Like 2 and 9, the values of Be and Ce for 8 have a small difference and therefore can be considered as a nearly prolate symmetric top. Moreover, the inertial axis dipole moment components are in two directions and therefore both a-type and b-type rotational transitions are possible. The bond lengths of C3C6 and C7C8 obtained at all levels (see Table 8) certainly show the triple bond character. The C2C5 bond length is rather intermediate between a triple bond and a double bond. The presence of a lone pair of electrons on the C5 carbon is evidently seen in the bond distances as the C2C5 length is somewhat shorter than the C1C2 distance at all levels. Taking into account the other bond lengths, it is quite clear that the valence structure given in Fig. 1 for 8 is dominant. Four of the bond angles are nearly 180 degrees at all levels (C1C3C6, C3C6C7, C6C7C8, and C7C8H9) like in isomer 2, which confirms that the butadiynyl chain is linear. Two high frequency modes of C–C stretching type could readily be seen between 1950 and 2300 cm−1 as the intensities of these two modes are quite high. Modes 17 and 19 show predominant stretching of C2–C5 and C3–C6 bonds, respectively. Also, mode 17 shows an isotopic shift of 50 cm−1 when C(2) is isotopically substituted (see Table S20, ESI†), which is the second largest difference we had observed in the isotopic shifts.
4 Summary
Nine low-lying isomers of C7H2 (1–9) whose relative energies are within 1 eV were theoretically investigated. Except 1, all other isomers are potential candidates for detection in the FTM spectrometer as their dipole moments are non-zero. Nevertheless, isomers 2 and 9 have already been detected and we found that the rest of the isomers (3–8) thermodynamically lie between these two detected molecules. In earlier theoretical studies, the relative stability of the bicyclic isomers (4, 6, and 7) and the seven-membered ring isomer (5) either has not been discussed in detail or the energy gaps are overestimated. However, we show here that these molecules are energetically positioned well within 1 eV from the lowest-lying isomer (1). Potential rearrangement of 3 to 4via Bergman cyclization is something that needs to be explored in detail further, which we will undertake in a forthcoming study. The transition states connecting other hypothetical isomers (5–8) of C7H2 especially on the low-energy side of the PES (within 1 eV) also need to be investigated further, which would suggest whether the undetected isomers could easily be detected or interconvert to already detected isomers. The biradical nature of 4 and 5 might certainly render some challenges in the detection of these two isomers. The thermodynamic competitiveness among isomers 6–9 is quite remarkable as we found that these four isomers lie within 1.15 kcal mol−1. Nevertheless, the detection of 9 indicates that the cumulene carbene isomer of C7H2 may also be kinetically stable. Whether new precursors would be of any help in detecting these hypothetical molecules (3–8) remains an open question that may be answered experimentally. In addition, the kinetic stability of the undetected isomers can be answered provided the complete PES of C7H2 can be explored at sufficiently high levels of theory. Finally we note that though thermodynamically not so stable, the cumulene carbene isomer of C7H2 (9) appears to be a kinetically stable molecule as it was found in two different experiments. Without exploring the complete PES of C7H2, it is impossible to comment on the stability of other isomers.Acknowledgements
This work is supported by a research grant (Project No: YSS/2015/000099) from the Science and Engineering Research Board, Department of Science and Technology (DST), New Delhi, Government of India (to VST). Additional computational facilities provided (for VST) through the DST – Fund for Improvement of Science and Technology Infrastructure program (Project No: SR/FST/PSI-142/2009) are also gratefully acknowledged. We gratefully acknowledge the generous allocation of computing time from the National Computational Infrastructure (NCI) National Facility, and system administration support provided by the Faculty of Science at the University of Western Australia (UWA) to the Linux cluster of the Karton group. VST also thanks Dr Mayavan Viji (Chungbuk National University, South Korea) for designing the schemes with chemdraw.References
- P. Thaddeus, M. C. McCarthy, M. J. Travers, C. A. Gottlieb and W. Chen, Faraday Discuss., 1998, 109, 121–135 RSC , and references therein.
- P. Ehrenfreund and S. B. Charnley, Annu. Rev. Astron. Astrophys., 2000, 38, 427–483 CrossRef CAS , and references therein.
- P. Thaddeus and M. C. McCarthy, Spectrochim. Acta, Part A, 2001, 57, 757–774 CrossRef CAS , and references therein.
- E. Herbst, Chem. Soc. Rev., 2001, 30, 168–176 RSC , and references therein.
- R. I. Kaiser, Chem. Rev., 2002, 102, 1309–1358 CrossRef CAS PubMed , and references therein.
- P. J. Sarre, J. Mol. Spectrosc., 2006, 238, 1–10 CrossRef CAS , and references therein.
- E. Herbst and E. F. van Dishoeck, Annu. Rev. Astron. Astrophys., 2009, 47, 427–480 CrossRef CAS , and references therein.
- L. N. Zack and J. P. Maier, Chem. Soc. Rev., 2014, 43, 4602–4614 RSC , and references therein.
- C. P. Endres, S. Schlemmer, P. Schilke, J. Stutzki and H. S. P. Müller, J. Mol. Spectrosc., 2016, 327, 95–104 CrossRef CAS.
- H. S. P. Müller, F. Schlöder, J. Stutzki and G. Winnewisser, J. Mol. Struct., 2005, 742, 215–227 CrossRef.
- H. S. P. Müller, S. Thorwirth, D. A. Roth and G. Winnewisser, Astron. Astrophys., 2001, 370, L49–L52 CrossRef.
- The Cologne Database for Molecular Spectroscopy. For more details, see http://www.astro.uni-koeln.de/cdms/molecules.
- P. Thaddeus, S. E. Cummins and R. A. Linke, Astrophys. J., 1984, 283, L45–L48 CrossRef CAS.
- H. S. P. Müller, J. Cernicharo, M. Agúndez, L. Decin, P. Encrenaz, J. C. Pearson, D. Teyssier and L. B. F. M. Waters, J. Mol. Spectrosc., 2012, 271, 50–55 CrossRef.
- P. Thaddeus, J. M. Vrtilek and C. A. Gottlieb, Astrophys. J., 1985, 299, L63–L66 CrossRef CAS.
- J. Cernicharo, C. A. Gottlieb, M. Guelin, T. C. Killian, G. Paubert, P. Thaddeus and J. M. Vrtilek, Astrophys. J., Lett., 1991, 398, L39–L41 CrossRef.
- J. Cernicharo, C. A. Gottlieb, M. Guelin, T. C. Killian, P. Thaddeus and J. M. Vrtilek, Astrophys. J., 1991, 368, L43–L45 CrossRef CAS.
- W. D. Langer, T. Velusamy, T. B. H. Kuiper, R. Peng, M. C. McCarthy, M. J. Travers, A. Kovács, C. A. Gottlieb and P. Thaddeus, Astrophys. J., 1997, 480, L63–L66 CrossRef CAS.
- S. Spezzano, S. Brünken, P. Schilke, P. Caselli, K. M. Menten, M. C. McCarthy, L. Bizzocchi, S. P. Trevinõ-Morales, Y. Aikawa and S. Schlemmer, Astrophys. J. Lett., 2013, 769, L19 CrossRef.
- J. Cernicharo, M. C. McCarthy, C. A. Gottlieb, M. Agúndez, L. Velilla Prieto, J. H. Baraban, P. B. Changala, M. Guélin, C. Kahane, M. A. Martin-Drumel, N. A. Patel, N. J. Reilly, J. F. Stanton, G. Quintana-Lacaci, S. Thorwirth and K. H. Young, Astrophys. J., Lett., 2015, 806, L3 CrossRef PubMed.
- M. Agúndez, J. Cernicharo and M. Guélin, Astron. Astrophys., 2015, 577, L5 CrossRef PubMed.
- J. M. Hollis, A. J. Remijan, P. R. Jewell and F. J. Lovas, Astrophys. J., 2006, 642, 933–939 CrossRef CAS.
- R. L. Hudson, P. A. Gerakines and M. J. Loeffler, Phys. Chem. Chem. Phys., 2015, 17, 12545–12552 RSC.
- M. Guélin, S. Green and P. Thaddeus, Astrophys. J., 1978, 224, L27–L30 CrossRef.
- P. Thaddeus, C. A. Gottlieb, Å. Hjalmarson, L. E. B. Johansson, W. M. Irvine, P. Friberg and R. A. Linke, Astrophys. J., 1985, 294, L49–L53 CrossRef CAS.
- H. Suzuki, M. Ohishi, N. Kaifu, S.-I. Ishikawa and T. Kasuga, Publ. Astron. Soc. Jpn., 1986, 38, 911–917 CAS.
- M. C. McCarthy, M. J. Travers, A. Kovács, C. A. Gottlieb and P. Thaddeus, Astrophys. J., Suppl. Ser., 1997, 113, 105–120 CrossRef CAS.
- M. B. Bell, P. A. Feldman, J. K. G. Watson, M. C. McCarthy, M. J. Travers, C. A. Gottlieb and P. Thaddeus, Astrophys. J., 1999, 518, 740–747 CrossRef CAS.
- B. E. Turner, E. Herbst and R. Terzieva, Astrophys. J., Suppl. Ser., 2000, 126, 427–460 CrossRef CAS.
- J. Takahashi, Publ. Astron. Soc. Jpn., 2000, 52, 401–407 CrossRef CAS.
- T. D. Crawford, J. F. Stanton, J. C. Saeh and H. F. Schaefer, III, J. Am. Chem. Soc., 1999, 121, 1902–1911 CrossRef CAS.
- H. W. Kroto, C. Kirby, D. R. M. Walton, L. W. Avery, N. W. Broten, J. M. MacLeod and T. Oka, Astrophys. J., 1978, 219, L133–L137 CrossRef CAS.
- G. Winnewisser and C. M. Walmsley, Astron. Astrophys., 1978, 70, L37–L39 CAS.
- N. W. Broten, T. Oka, L. W. Avery, J. M. MacLeod and H. W. Kroto, Astrophys. J., 1978, 223, L105–L107 CrossRef CAS.
- M. C. McCarthy, J.-U. Grabow, M. J. Travers, W. Chen, C. A. Gottlieb and P. Thaddeus, Astrophys. J., 1999, 513, 305–310 CrossRef CAS.
- S. Ikuta, T. Tsuboi and K. Aoki, J. Mol. Struct.: THEOCHEM, 2000, 528, 297–305 CrossRef CAS.
- J. M. Vrtilek, C. A. Gottlieb, E. W. Gottlieb, T. C. Killian and P. Thaddeus, Astrophys. J., Lett., 1990, 364, L53–L56 CrossRef CAS.
- T. C. Killian, J. M. Vrtilek, C. A. Gottlieb, E. W. Gottlieb and P. Thaddeus, Astrophys. J., 1990, 365, L89–L92 CrossRef CAS.
- M. C. McCarthy, M. J. Travers, A. Kovács, W. Chen, S. E. Novick, C. A. Gottlieb and P. Thaddeus, Science, 1997, 275, 518–520 CrossRef CAS PubMed.
- R. A. Seburg, E. V. Patterson, J. F. Stanton and R. J. McMahon, J. Am. Chem. Soc., 1997, 119, 5847–5856 CrossRef CAS.
- R. A. Seburg, R. J. McMahon, J. F. Stanton and J. Gauss, J. Am. Chem. Soc., 1997, 119, 10838–10845 CrossRef CAS.
- K. W. Sattelmeyer and J. F. Stanton, J. Am. Chem. Soc., 2000, 122, 8220–8227 CrossRef CAS.
- A. P. Apponi, M. C. McCarthy, C. A. Gottlieb and P. Thaddeus, Astrophys. J., 2000, 530, 357–361 CrossRef CAS.
- J. F. Stanton, E. Garand, J. Kim, T. I. Yacovitch, C. Hock, A. S. Case, E. M. Miller, Y.-J. Lu, K. M. Vogelhuber, S. W. Wren, T. Ichino, J. P. Maier, R. J. McMahon, D. L. Osborn, D. M. Neumark and W. C. Lineberger, J. Chem. Phys., 2012, 136, 134312 CrossRef PubMed.
- S. N. Reddy and S. Mahapatra, Chem. Phys., 2012, 403, 1–11 CrossRef CAS.
- M. Steglich, J. Fulara, S. Maity, A. Nagy and J. P. Maier, J. Chem. Phys., 2015, 142, 244311 CrossRef PubMed.
- R. A. Seburg and R. J. McMahon, Angew. Chem., Int. Ed., 1995, 34, 2009–2012 CrossRef CAS.
- G. Maier, H. P. Reisenauer, W. Schwab, P. Čársky, B. A. Hess Jr. and L. J. Schaad, J. Am. Chem. Soc., 1987, 109, 5183–5188 CrossRef CAS.
- G. Maier, H. P. Reisenauer, W. Schwab, P. Čársky, V. Špirko, B. A. Hess Jr. and L. J. Schaad, J. Chem. Phys., 1989, 91, 4763–4773 CrossRef CAS.
- D. L. Osborn, K. M. Vogelhuber, S. W. Wren, E. M. Miller, Y.-J. Lu, A. S. Case, L. Sheps, R. J. McMahon, J. F. Stanton, L. B. Harding, B. Ruscic and W. C. Lineberger, J. Am. Chem. Soc., 2014, 136, 10361–10372 CrossRef CAS PubMed.
- S. Spezzano, H. Gupta, S. Brünken, C. A. Gottlieb, P. Caselli, K. M. Menten, H. S. P. Müller, L. Bizzocchi, P. Schilke, M. C. McCarthy and S. Schlemmer, Astron. Astrophys., 2016, 575, A110 CrossRef.
- S. Chandra, P. G. Musrif and R. M. Dharmkare, New Astron., 2005, 10, 385–391 CrossRef CAS.
- S. Chandra, C. Chang, P. G. Musrif, A. B. C. Patzer, W. H. Kegel and E. Sedlmayr, Rom. J. Phys., 2007, 52, 459–466 CAS.
- L. Horný, N. D. K. Petraco and H. F. Schaefer III, J. Am. Chem. Soc., 2002, 124, 14716–14720 CrossRef.
- I. Alkorta and J. Elguero, Struc. Chem., 2005, 16, 77–79 CrossRef CAS.
- R. A. Seburg, J. T. DePinto, E. V. Patterson and R. J. McMahon, J. Am. Chem. Soc., 1995, 117, 835–836 CrossRef CAS.
- H. F. Bettinger, P. v. R. Schleyer and H. F. Schaefer, III, J. Am. Chem. Soc., 1999, 121, 2829–2835 CrossRef CAS.
- B. J. Sun, C. H. Huang, M. F. Tsai, H. L. Sun, L. G. Gao, Y. S. Wang, Y. Y. Yeh, Y. H. Shih, Z. F. Sia, P. H. Chen, R. I. Kaiser and A. H. H. Chang, J. Chem. Phys., 2009, 131, 104305 CrossRef.
- J. Fulara, P. Freivogel, D. Forney and J. P. Maier, J. Chem. Phys., 1995, 103, 8805–8810 CrossRef CAS.
- M. C. McCarthy, M. J. Travers, C. A. Gottlieb and P. Thaddeus, Astrophys. J., 1997, 483, L139–L142 CrossRef CAS.
- C. D. Ball, M. C. McCarthy and P. Thaddeus, Astrophys. J., 1999, 523, L89–L91 CrossRef CAS.
- C. D. Ball, M. C. McCarthy and P. Thaddeus, J. Chem. Phys., 2000, 112, 10149–10155 CrossRef CAS.
- S. Dua, S. J. Blanksby and J. H. Bowie, J. Phys. Chem. A, 2000, 104, 77–85 CrossRef CAS.
- A. Karton and J. M. L. Martin, J. Chem. Phys., 2012, 136, 124114 CrossRef PubMed.
- K. A. Peterson, D. Feller and D. A. Dixon, Theor. Chem. Acc., 2012, 131, 1079 CrossRef.
- A. Karton, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2016, 6, 292–310 CrossRef CAS.
- At CCSD(T)/cc-pVTZ level of theory, isomers 10, 11, 12, 13, and 14 are 22.00, 25.23, 32.84, 44.07, and 80.53 kcal mol−1, respectively, above 1. As far as isomer 15 is concerned, our calculations are not converged. We also note that calculations for 1 and 13 have been done using an ROHF wavefunction (as their ground electronic states were triplets) at the same level of theory. All relative energies mentioned above are ZPVE corrected and scaled.
- K. Aoki and S. Ikuta, THEOCHEM, 1994, 310, 229–238 CrossRef.
- V. S. Thimmakondu, Comput. Theor. Chem., 2016, 1079, 1–10 CrossRef CAS.
- At the B3LYP/aug-cc-pVDZ//B3LYP/6-31G(d) level of theory, isomers 2, 3, 8, 9, 10, 11, 12, 14, and 15 are 13.65, 17.51, 26.65, 22.17, 26.36, 35.11, 33.99, 85.89, and 106.13 kcal mol−1, respectively, above 1.
- The dipole moments of isomers 10, 11, 12, 13, and 14 are 6.26, 5.16, 11.41, 3.29, and 2.26 Debye, respectively, at the CCSD(T)/cc-pVTZ level of theory.
- C. Møller and M. S. Plesset, Phys. Rev., 1934, 46, 618–622 CrossRef.
- G. D. Purvis III and R. J. Bartlett, J. Chem. Phys., 1982, 76, 1910–1918 CrossRef.
- J. F. Stanton, J. Gauss, J. D. Watts and R. J. Bartlett, J. Chem. Phys., 1991, 94, 4334–4345 CrossRef CAS.
- K. Raghavachari, G. W. Trucks, J. A. Pople and M. Head-Gordon, Chem. Phys. Lett., 1989, 157, 479–483 CrossRef CAS.
- R. J. Bartlett, J. D. Watts, S. A. Kucharski and J. Noga, Chem. Phys. Lett., 1990, 165, 513–522 CrossRef CAS.
- J. F. Stanton, Chem. Phys. Lett., 1997, 281, 130–134 CrossRef CAS.
- T. H. Dunning, Jr., J. Chem. Phys., 1989, 90, 1007–1023 CrossRef.
- J. Gauss and J. F. Stanton, Chem. Phys. Lett., 1997, 276, 70–77 CrossRef CAS.
- J. F. Stanton, J. Gauss, M. E. Harding and P. G. Szalay with contributions from A. A. Auer, R. J. Bartlett, U. Benedikt, C. Berger, D. E. Bernholdt, Y. J. Bomble, L. Cheng, O. Christiansen, M. Heckert, O. Heun, C. Huber, T.-C. Jagau, D. Jonsson, J. Jusélius, K. Klein, W. J. Lauderdale, D. A. Matthews, T. Metzroth, D. P. O'Neill, D. R. Price, E. Prochnow, K. Ruud, F. Schiffmann, W. Schwalbach, S. Stopkowicz, A. Tajti, J. Vázquez, F. Wang and J. D. Watts and the integral packages MOLECULE (J. Almlöf and P. R. Taylor), PROPS (P. R. Taylor), ABACUS (T. Helgaker, H. J. A. Jensen, P. Jø rgensen and J. Olsen), and ECP routines by A. V. Mitin and C. van Wüllen. For the current version, see http://www.cfour.de.
- A. Karton, E. Rabinovich, J. M. L. Martin and B. Ruscic, J. Chem. Phys., 2006, 125, 144108 CrossRef PubMed.
- A. Karton, I. Kaminker and J. M. L. Martin, J. Chem. Phys., 2009, 113, 7610–7620 CrossRef CAS PubMed.
- N. Sylvetsky, K. A. Peterson, A. Karton and J. M. L. Martin, J. Chem. Phys., 2016, 144, 214101 CrossRef PubMed.
- S. Ten-no and J. J. Noga, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 114–125 CrossRef CAS.
- K. A. Peterson, T. B. Adler and H.-J. Werner, J. Chem. Phys., 2008, 128, 084102 CrossRef PubMed.
- K. E. Yousaf and K. A. Peterson, J. Chem. Phys., 2008, 129, 184108 CrossRef PubMed.
- K. E. Yousaf and K. A. Peterson, Chem. Phys. Lett., 2009, 476, 303–307 CrossRef CAS.
- G. Knizia and H.-J. Werner, J. Chem. Phys., 2008, 128, 154103 CrossRef PubMed.
- T. B. Adler, G. Knizia and H.-J. Werner, J. Chem. Phys., 2007, 127, 221106 CrossRef PubMed.
- S. Ten-no, Chem. Phys. Lett., 2004, 398, 56–61 CrossRef CAS.
- H.-J. Werner, T. B. Adler and F. R. Manby, J. Chem. Phys., 2007, 126, 164102 CrossRef PubMed.
- G. Knizia, T. B. Adler and H.-J. Werner, J. Chem. Phys., 2009, 130, 054104 CrossRef PubMed.
- K. A. Peterson and T. H. Dunning, J. Chem. Phys., 2002, 117, 10548–10560 CrossRef CAS.
- M. K. Kesharwani, B. Brauer and J. M. L. Martin, J. Phys. Chem. A, 2015, 119, 1701–1714 CrossRef CAS PubMed.
- MOLPRO is a package of ab initio programs written by H.-J. Werneret al., MOLPRO 2012.1; University College Cardiff Consultants Limited: Cardiff, UK, 2012. See also: http://www.molpro.net.
- H.-J. Werner, P. J. Knowles, G. Knizia, F. R. Manby and M. Schütz, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 242–253 CrossRef CAS.
- MRCC, a quantum chemical program suite written by M. Kállayet al., See also: http://www.mrcc.hu.
- Z. Rolik, L. Szegedy, I. Ladjanszki, B. Ladoczki and M. Kállay, J. Chem. Phys., 2013, 139, 094105 CrossRef PubMed.
- H. Kanata, S. Yamamoto and S. Saito, Chem. Phys. Lett., 1987, 140, 221–224 CrossRef CAS.
- P. R. Spackman, D. Jayatilaka and A. Karton, J. Chem. Phys., 2016, 145, 104101 CrossRef PubMed.
- D. Feller and K. A. Peterson, J. Chem. Phys., 2007, 126, 114105 CrossRef PubMed.
- S. Wang and H. F. Schaefer III, J. Chem. Phys., 2006, 124, 044303 CrossRef PubMed.
- K. L. Bak, J. Gauss, P. Jørgensen, J. Olsen, T. Helgaker and J. F. Stanton, J. Chem. Phys., 2001, 114, 6548 CrossRef CAS.
- J. M. L. Martin and P. R. Taylor, J. Phys. Chem., 1996, 100, 6047–6056 CrossRef CAS.
- Y. Xie, G. E. Scuseria, B. F. Yates, Y. Yamaguchi and H. F. Schaefer, J. Am. Chem. Soc., 1989, 111, 5181–5185 CrossRef CAS.
- R. J. McMahon, R. J. Halter, R. L. Fimmen, R. J. Wilson, S. A. Peebles, R. L. Kuczkowski and J. F. Stanton, J. Am. Chem. Soc., 2000, 122, 939–949 CrossRef CAS.
- A. Humason, W. Zou and D. Cremer, J. Phys. Chem. A, 2015, 119, 1666–1682 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Cartesian coordinates, total electronic energies, ZPVEs, and dipole moments corresponding to the optimized geometries of 1–9 at different levels of theory are given. The isotopic shifts (12C-mono-substituted-13C) in harmonic vibrational frequencies are also given. See DOI: 10.1039/c7cp02848b |
| This journal is © the Owner Societies 2017 |