CO adsorption, oxidation and carbonate formation mechanisms on Fe3O4 surfaces†
Abstract
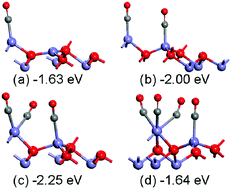
By means of density functional theory calculations that account for the on-site Coulomb interaction via a Hubbard term (DFT+U), we systematically investigated CO adsorption on Fe3O4 surfaces at different coverages. It has been found that more than one CO can coadsorb on one surface iron atom on both Fetet1 and Feoct2 terminations of Fe3O4(111). The uncapped oxygen atom is the active site for CO oxidation on both Fetet1 and Feoct2 terminations of Fe3O4(111). For Fe3O4(110), two CO molecules prefer to coadsorb on one surface iron atom on the A layer; CO prefers to adsorb at the bridge site of the surface octahedral iron atoms at low coverage, while CO prefers to adsorb at the surface tetrahedral iron atom at high coverage on the B layer. It has been found that the surface oxygen atom which is not coordinated to the tetrahedral iron atom is the active site for CO oxidation on the B termination of Fe3O4(001). On the Fe3O4 surfaces, the formation of carbonate has been found to be very stable thermodynamically, which agrees well with experiments. The adsorption mechanism has been analyzed on the basis of projected density of states (PDOS).


 Please wait while we load your content...
Please wait while we load your content...