Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
High cross-plane thermoelectric performance of metallo-porphyrin molecular junctions†
Mohammed
Noori‡
ab,
Hatef
Sadeghi‡
 *a,
Qusiy
Al-Galiby‡
ac,
Steven W. D.
Bailey
a and
Colin J.
Lambert‡
*a,
Qusiy
Al-Galiby‡
ac,
Steven W. D.
Bailey
a and
Colin J.
Lambert‡
 *a
*a
aQuantum Technology Centre, Department of Physics, Lancaster University, Lancaster, LA1 4YB, UK. E-mail: h.sadeghi@lancaster.ac.uk; c.lambert@lancaster.ac.uk
bDepartment of Physics, Collage of Science, Thi-Qar University, Thi-Qar, Iraq
cDepartment of Physics, Collage of Education, Al Qadisiyah University, Iraq
First published on 19th June 2017
Abstract
We investigated the thermoelectric properties of flat-stacked 5,15-diphenylporphyrins containing divalent metal ions Ni, Co, Cu or Zn, which are strongly coordinated with the nitrogens of pyridyl coated gold electrodes. Changing metal atom has little effect on the thermal conductance due to the phonons. The room-temperature Seebeck coefficients of these junctions are rather high, ranging from 90 μV K−1 for Cu, Ni and Zn-porphyrins to −16 μV K−1 for Co-porphyrin. These values could be further increased by lowering molecular energy levels relative to the DFT-predicted Fermi energy. In contrast, the phonon contribution to the thermal conductance of these junctions is rather insensitive to the choice of metal atom. The thermopower, thermal conductance and electrical conductance combined to yield the room-temperature values for the thermoelectric figure of merit ZT ranging from 1.6 for Cu porphyrin to ∼0.02 for Ni-porphyrin.
Introduction
Single-molecule thermoelectricity is a rapidly expanding field of fundamental research, which will eventually inform the design and synthesis of high-performance thermoelectric molecular films. Early studies showed that factors governing the sign and magnitude of the Seebeck coefficient S include the length of conjugated molecules and the type of terminal group. For examples thiol-terminated molecules typically exhibit HOMO-dominated transport and a positive S, ranging from 6.8 to 2.4 μV K−1 for n-alkanedithiols, depending on the length n,1,2 from 7 μV K−1 to 16 μV K−1 for n-benzenedithiols, depending on the number n of phenyl rings, ranging from 7 μV K−1 to 16 μV K−1.3–9 Positive Seebeck coefficients up to 24 μV K−1 were measured for 1,4-bis((trimethylstannyl)methyl)-n-phenyl (n = 1, 2, 3, 4), up to 10.4 μV K−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,10,11 for 1,4-n-phenylenediamine (PDA) (n-1, 2, 3) and as high as 14.8 μV K−1 for oligothiophenes on gold.12 On the other hand pyridyl terminal groups promote LUMO-dominated transport leading to negative Seebeck coefficients as high as −9 μV K−1 and −10 μV K−1 respectively11,13 4,4′-for bipyridine and 1,2-di(4-pyridyl)ethylene respectively. Fullerenes also exhibit negative Seebeck coefficients, ranging from −10 to −30 μV K−1 for C6014 to −33 μV K−1 for C60 dimers15 and up to −31.6 μV K−1 C82 endohedral fullerenes.16 The sign of the endohedral fullerene Sc3N@C80 was shown to be sensitive to pressure, ranging from −25 μV K−1 to +25 μV K−1, depending on the orientation of the molecule on a gold substrate.17 Other factors predicted to govern the thermoelectric performance of molecules include the type of electrode. Although most of the above studies used gold electrodes, alternative choices could include graphene,18,19 silicene,20,21 platinum,22 palladium,22 iron23 and nickel,24 which provide a range of Debye frequencies for controlling phonon transport across the electrode–molecule boundary.24
1,10,11 for 1,4-n-phenylenediamine (PDA) (n-1, 2, 3) and as high as 14.8 μV K−1 for oligothiophenes on gold.12 On the other hand pyridyl terminal groups promote LUMO-dominated transport leading to negative Seebeck coefficients as high as −9 μV K−1 and −10 μV K−1 respectively11,13 4,4′-for bipyridine and 1,2-di(4-pyridyl)ethylene respectively. Fullerenes also exhibit negative Seebeck coefficients, ranging from −10 to −30 μV K−1 for C6014 to −33 μV K−1 for C60 dimers15 and up to −31.6 μV K−1 C82 endohedral fullerenes.16 The sign of the endohedral fullerene Sc3N@C80 was shown to be sensitive to pressure, ranging from −25 μV K−1 to +25 μV K−1, depending on the orientation of the molecule on a gold substrate.17 Other factors predicted to govern the thermoelectric performance of molecules include the type of electrode. Although most of the above studies used gold electrodes, alternative choices could include graphene,18,19 silicene,20,21 platinum,22 palladium,22 iron23 and nickel,24 which provide a range of Debye frequencies for controlling phonon transport across the electrode–molecule boundary.24
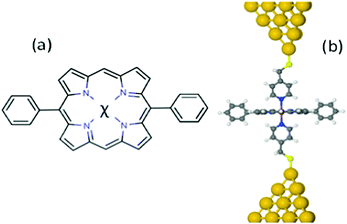
Here, in an effort to further enhance the thermoelectric performance of single-molecule junctions by controlling phonon transport while maintaining good electrical contact, we examine the effect of coating the electrodes with functional units, which form coordination bonds with the molecule of interest. As an example, we examine junctions formed from 5,15-diphenylporphyrins containing divalent metal ions Ni, Co, Cu or Zn, which are strongly coordinated with the nitrogens of pyridyl coated gold electrodes. Porphyrins are attractive as building blocks for molecular-scale devices, because they are conjugated, rigid, chemically stable molecules and their properties could be modified by coordinating a variety of metallic ions (metallo-porphyrins).25–33 In what follows our aim is to explore the potential of the metallo-porphyrin-based molecular junctions shown in Fig. 1a for high-efficiency thermoelectricity. Since the in-plane electrical conductance of porphyrins is rather low, we consider “cross-plane” junctions in which the current flows perpendicular to the porphyrin plane.34 A shown in Fig. 1b, a porphyrin plane is contacted to gold electrodes via pyridyl groups attached to the gold by thiol anchors, with the plane of the porphyrin stacked perpendicular to the direction of charge transport.
From the point of view of optimising thermoelectric properties, junctions formed from these molecules are of interest, because by varying the metal atom (denoted χ in Fig. 1) residing in the core of the organic porphyrin framework, the molecular energy levels can be tuned relative to the Fermi energy EF of the electrodes and if energy levels are close enough to EF, then this should lead to transport resonances, which enhance the thermopower.35 In what follows, we shall demonstrate that this is indeed the case and that large positive and negative thermopowers are achievable. Moreover, our calculations show that although the thermal conductance due to the phonons dominates the total thermal conductance, high values of ZT are obtained.
Recent experiments and theory show that the conductances G of the metallo-porphyrins are enhanced when they are sandwiched between two gold electrodes with pyridine-4-yl-methanethiol anchors (Fig. 1b).34,36 Since large G is desirable, the 5,15-diphenylporphyrins (χ-DPP) studied in this paper contains metal atoms χ = Co, Cu, Ni or Zn, which are strongly coordinated with the nitrogens of pyridyl coated gold electrodes (Fig. 1a). Our aim in this paper is to investigate the thermoelectric properties of these metallo-porphyrins devices when the metal atom is varied.
Methods
To calculate the electronic and vibrational properties of each metallo-porphyrin, we used the spin density functional (DFT) code SIESTA,37 which employs Troullier–Martins pseudopotentials to represent the potentials of the atomic cores,38 and a local atomic-orbital basis set. We used a double-zeta polarized basis set39 for all atoms and the local density functional approximation (LDA-CA) by Ceperley and Adler.40 The Hamiltonian and overlap matrices are calculated on a real-space grid defined by a plane-wave cutoff of 150 Ry. Each molecule was relaxed to the optimum geometry until the forces on the atoms are smaller than 0.02 eV Å−1. The only periodicity is in the electrodes, which are assumed to be composed of a periodic series of principal layers (see Fig. S10, ESI†). In the transverse direction, there is no periodicity and the unit cell is sufficiently large that there is no interaction between the molecule and its image. The mean field Hamiltonian obtained using DFT describes the whole structure including the leads, the interface between the leads and molecule and molecule itself. In this way, all parts of the structure are treated in a holistic manner, at the same level of DFT.Results and discussion
To obtain the optimized geometry (and the associated mean field Hamiltonian) of χ-DPP we employed the SIESTA37 implementation of spin-polarized density functional theory (DFT) and from the converged mean field DFT Hamiltonian, we used our quantum transport code, Gollum41 to compute the electronic transmission coefficient Tσel(E) for electrons with energy E and spin σ = [↑, ↓] passing from one electrode to the other via the χ-DPP. For each χ-DPP in Fig. 1b and 2a–d shows total electronic transmission coefficients as a function of energy for χ = Co, Cu, Zn or Ni. The corresponding spin-dependent transmissions Tσel(E) are shown in the ESI.†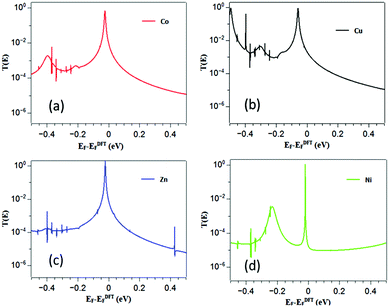 | ||
| Fig. 2 (a–d) show the electronic transmission coefficients T(E) = T↑el(E) + T↓el(E) as a function of energy E for χ-DPP with χ = Co, Cu, Zn and Ni. | ||
Due to the presence of thiol anchors, the DFT-predicted Fermi energy is close to the HOMO transmission resonances of each χ-DPP and the slope of Tσel(EF) is negative. Hence from eqn (S5) of the ESI,† one expects the Seebeck coefficient S to be positive. Using the Landauer formula (see ESI†), the electrical conductance was calculated from the transmission coefficient shown in Fig. 2.
The electrical conductances (Fig. S2 of the ESI†) follow the trend GZn > GCu > GCo > GNi in agreement with the experimental values of Zn-DPP 8.2 × 10−2, Cu-DPP 4.4 × 10−2, Co-DPP 2.2 × 10−2 and Ni-DPP 1.8 × 10−2.33
To calculate the vibrational modes of each structure, we use the harmonic approximation to construct the dynamical matrix D and a Green's function approach to calculate the phononic transmission coefficient Tph(ω) for phonons with energy ℏω passing through the device as explained in ref. 35. Fig. 3 shows the phonon transmission coefficients for each χ-DPP.
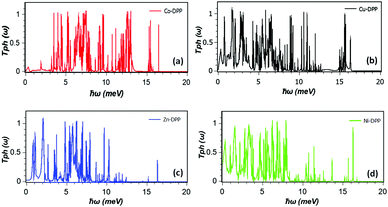 | ||
| Fig. 3 (a–d) Phonon transmission coefficient as a function of ℏω for χ-DPP with χ = Co, Cu, Zn and Ni. | ||
The thermal conductance of the junction (κ = κph + κel) is obtained by summing the contributions from both electrons (κel) and phonons (κph). The electronic (phononic) thermal conductances are calculated from the electronic (phononic) transmission coefficients (as described in ref. 35).
Comparison between Fig. 4a and b shows that in the vicinity of DFT Fermi energy, the main source of the thermal conductance in these junctions is due to phonons, whose room-temperature contribution is relatively insensitive to the choice of metal atom, varying between 6 and 10 pW K−1 depending on the choice. The inset of Fig. 4b shows the cumulative distribution of eigenvalues (integrated density of states) of each molecule and reveals that the lower frequency modes of the Co porphyrin in the region 0 to 1.5 meV are pushed to higher frequencies, leading to a low-frequency transmission gap below 3 meV. This reflects the higher binding energy of a single pyridyl group to the Co metal atom compared with the other. (These follow the trend Co = −1.2 eV, Zn = −1.2 eV, Cu = −0.4 eV and Ni = −0.17 eV.) (These follow the trend Co = −1.2 eV, Zn = −1.2 eV, Cu = −0.4 eV and Ni = −0.17 eV.)
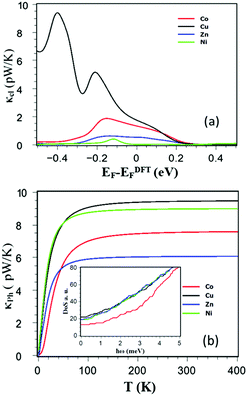 | ||
| Fig. 4 (a) Show electronic contribution of the thermal conductance κel and (b) show phononic contribution of the thermal conductance κph for DPP with Co, Cu, Zn and Ni. | ||
Consequently the low-frequency cumulative distribution of Co and the low-temperature thermal conductance initially lies below that of the others and then rises with a steeper slope. Fig. 3c shows that Zn also has a low-frequency transmission gap below 1 meV, which suppresses the low-temperature phonon thermal conductance.
Fig. 5 shows the Seebeck coefficient S and total figure of merit ZT over a range of Fermi energies at room temperature for each metallo-porphyrin. Fig. 5a demonstrates that both the magnitude and sign of S are sensitive to the metal atom at the centre of the DPP, which determine the location of transport resonances relative to the Fermi energy. Since the Fermi energy from DFT is not necessarily reliable, we have presented results for a range of Fermi energies (Fig. 5a).
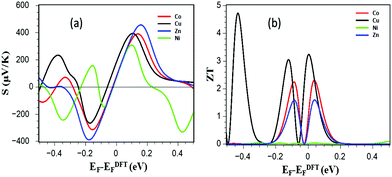 | ||
| Fig. 5 Show (a) Seebeck coefficient S (thermopower) and (b) total figure of merit ZT over a range of Fermi energies at room temperature for each metallo-porphyrin. | ||
For the junctions discussed in this paper, the conductances have been measured experimentally36 so we have estimated the Fermi energy by comparing these measurements with our calculated conductances. This yields a value of EF = −0.03 eV, compared with the DFT-predicted Fermi energy. Table 1 shows the Seebeck coefficients of porphyrins in the presence of Cu, and then Co, Zn and Ni at EF = −0.03 eV. The predicted trend is SCu > SNi > SZn > SCo and when combined with the higher Cu CPP the yield room-temperature values of ZT range from 1.66 for Cu porphyrin to 0.07 for Zn porphyrin, as shown in Fig. 5b.
| Metallic | S (μV K−1) | ZT | ||
|---|---|---|---|---|
| E F = −0.03 eV | E F = 0.0 eV | E F = −0.03 eV | E F = 0.0 eV | |
| Cu | +90 | +182 | 1.66 | 3.22 |
| Co | −16 | +77 | 0.05 | 0.92 |
| Zn | −23 | +72 | 0.07 | 0.56 |
| Ni | −32 | +66 | 0.018 | 0.05 |
Conclusion
The room-temperature Seebeck coefficients of these junctions are rather high, ranging from 90 μV K−1 for Cu-porphyrin to −16 μV K−1 for Co-porphyrin. The corresponding room-temperature values of ZT range from 1.66 for Cu porphyrin to ∼0.02 for Ni porphyrin. These values could be further increased by shifting molecular energy levels relative to the Fermi, either by doping or gating, which moves the Fermi energy closer to the regions of higher thermopower in Fig. 5. These results demonstrate that metallo-porphyrins are attractive building blocks for molecular-scale thermoelectricity and by passing thermal and charge currents perpendicular to the plane of the porphyrins, large values of the Seebeck coefficient and figure of merit are possible.Acknowledgements
This work is supported by UK EPSRC grants EP N017188/1, EP/M014452/1, the European Union Marie-Curie Network MOLESCO 606728 and the Ministry of Higher Education and Scientific Research, Thi-Qar University, IRAQ.References
- J. A. Malen, et al. , Nano Lett., 2009, 9, 1164 CrossRef CAS PubMed.
- S. Y. Guo, G. Zhou and N. J. Tao, Nano Lett., 2013, 13, 4326 CrossRef CAS PubMed.
- A. Tan, S. Sadat and P. Reddy, Appl. Phys. Lett., 2010, 96, 013110 CrossRef.
- A. Tan, et al. , J. Am. Chem. Soc., 2011, 133, 8838 CrossRef CAS PubMed.
- S. K. Lee, et al. , Nano Lett., 2014, 14, 5276 CrossRef CAS PubMed.
- Y. Kim, et al. , Appl. Phys. Lett., 2016, 109, 033102 CrossRef.
- M. Tsutsui, M. Taniguchi and T. Kawai, Nano Lett., 2008, 8, 3293 CrossRef CAS PubMed.
- T. Morikawa, et al. , Nanoscale, 2014, 6, 8235 RSC.
- S. Kaneko, et al. , Appl. Phys. Express, 2015, 8, 095503 CrossRef.
- D. Kim, P. S. Yoo and T. Kim, J. Korean Phys. Soc., 2015, 66, 602 CrossRef CAS.
- T. Kim, et al. , Nano Lett., 2014, 14, 794 CrossRef CAS PubMed.
- W. B. Chang, et al. , Chem. Mater., 2014, 26, 7229 CrossRef CAS.
- J. R. Widawsky, et al. , Nano Lett., 2012, 12, 354 CrossRef CAS PubMed.
- S. K. Yee, et al. , Nano Lett., 2011, 11, 4089 CrossRef CAS PubMed.
- C. Evangeli, et al. , Nano Lett., 2013, 13, 2141 CrossRef CAS PubMed.
- S. K. Lee, et al. , Nanoscale, 2015, 7, 20497 RSC.
- L. Rincon-Garcia, et al. , Nat. Mater., 2016, 15, 289 CrossRef CAS PubMed.
- H. Sadeghi, J. A. Mol, C. S. Lau, G. A. D. Briggs, J. Warner and C. J. Lambert, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 2658–2663 CrossRef CAS PubMed.
- C. S. Lau, et al. , Nano Lett., 2016, 16, 170–176 CrossRef CAS PubMed.
- H. Sadeghi, S. Bailey and C. J. Lambert, Appl. Phys. Lett., 2014, 104(10), 103104 CrossRef.
- H. Sadeghi, S. Sangtarash and C. J. Lambert, Sci. Rep., 2015, 5, 9514 CrossRef CAS PubMed.
- V. M. García-Suárez, A. R. Rocha, S. W. Bailey, C. J. Lambert, S. Sanvito and J. Ferrer, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 045437 CrossRef.
- V. M. García-Suárez, C. M. Newman, C. J. Lambert, J. M. Pruneda and J. Ferrer, J. Phys.: Condens. Matter, 2004, 16, 5453 CrossRef.
- H. Sadeghi, S. Sangtarash and C. J. Lambert, Nano Lett., 2015, 15, 7467–7472 CrossRef CAS PubMed.
- G. Dorough, J. Miller and F. M. Huennekens, J. Am. Chem. Soc., 1951, 73, 4315–4320 CrossRef CAS.
- G. Sedghi, V. M. García-Suárez, L. J. Esdaile, H. L. Anderson, C. J. Lambert, S. Martín, D. Bethell, S. J. Higgins, M. Elliott and N. Bennett, Nat. Nanotechnol., 2011, 6, 517–523 CrossRef CAS PubMed.
- D. Gust and J. D. Roberts, J. Am. Chem. Soc., 1977, 99, 3637–3640 CrossRef CAS PubMed.
- Z. Li, M. Smeu, M. A. Ratner and E. Borguet, J. Phys. Chem. C, 2013, 117, 14890–14898 CAS.
- Z. Li and E. Borguet, J. Am. Chem. Soc., 2011, 134, 63–66 CrossRef PubMed.
- Z.-F. Liu, S. Wei, H. Yoon, O. Adak, I. Ponce, Y. Jiang, W.-D. Jang, L. M. Campos, L. Venkataraman and J. B. Neaton, Nano Lett., 2014, 14, 5365–5370 CrossRef CAS PubMed.
- Y. Li, J. Yao, S. Zhong and Z. Zou, Curr. Appl. Phys., 2011, 11, 1349–1353 CrossRef.
- R. Ferradás, V. M. García-Suárez and J. Ferrer, 2012, arXiv preprint arXiv:1208.3157.
- I. Beletskaya, V. S. Tyurin, A. Y. Tsivadze, R. Guilard and C. Stern, Chem. Rev., 2009, 109, 1659–1713 CrossRef CAS PubMed.
- A. C. Aragonès, N. Darwish, W. J. Saletra, L. Pérez-García, F. Sanz, J. Puigmartí-Luis, D. B. Amabilino and I. Díez-Pérez, Nano Lett., 2014, 14, 4751–4756 CrossRef PubMed.
- H. Sadeghi, S. Sangtarash and C. J. Lambert, Nano Lett., 2015, 15, 7467–7472 CrossRef CAS PubMed.
- M. Noori, A. C. Aragonès, G. Di Palma, N. Darwish, S. W. Bailey, Q. Al-Galiby, I. Grace, D. B. Amabilino, A. González-Campo and I. Díez-Pérez, Sci. Rep., 2016, 6, 37352 CrossRef CAS PubMed.
- J. M. Soler, E. Artacho, J. D. Gale, A. García, J. Junquera, P. Ordejón and D. Sánchez-Portal, J. Phys.: Condens. Matter, 2002, 14, 2745 CrossRef CAS.
- N. Troullier and J. L. Martins, Phys. Rev. B: Condens. Matter Mater. Phys., 1991, 43, 1993 CrossRef CAS.
- T. Ozaki, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 67, 155108 CrossRef.
- D. M. Ceperley and B. Alder, Phys. Rev. Lett., 1980, 45, 566 CrossRef CAS.
- J. Ferrer, C. J. Lambert, V. M. García-Suárez, D. Z. Manrique, D. Visontai, L. Oroszlany, R. Rodríguez-Ferradás, I. Grace, S. Bailey and K. Gillemot, New J. Phys., 2014, 16, 093029 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7cp02229h |
| ‡ These author contributed equally to this work. |
| This journal is © the Owner Societies 2017 |