Open Access Article
Open Access ArticleNon-zeolitic properties of the dipeptide L-leucyl–L-leucine as a result of the specific nanostructure formation†
Marat A.
Ziganshin
 *a,
Aisylu S.
Safiullina
*a,
Aisylu S.
Safiullina
 a,
Sufia A.
Ziganshina
a,
Sufia A.
Ziganshina
 b,
Alexander V.
Gerasimov
b,
Alexander V.
Gerasimov
 a and
Valery V.
Gorbatchuk
a and
Valery V.
Gorbatchuk
 a
a
aA. M. Butlerov Institute of Chemistry, Kazan Federal University, Kremlevskaya ul. 18, Kazan, 420008, Russia. E-mail: Marat.Ziganshin@kpfu.ru
bKazan Zavoisky Physical-Technical Institute of the Kazan Scientific Center of the Russian Academy of Sciences, Sibirskii trakt 10/7, Kazan, 420029, Russia
First published on 3rd May 2017
Abstract
The non-zeolitic behavior of L-leucyl–L-leucine and its self-organization in solid state and from solutions with the formation of different nanostructures are reported. This dipeptide forms porous crystals, but does not exhibit molecular sieve effects typical of classical zeolites and biozeolites. The specific sorption properties of L-leucyl–L-leucine result from a change in its crystal packing from channel-type to layered-type, when binding strong proton acceptors or proton donors of molecular size greater than 18–20 cm3 mol−1. The high sorption capacity of L-leucyl–L-leucine toward dichloromethane results from the self-organization of the dipeptide, by forming nanofibers or web-like structures. The low thermal stability of clathrates of the dipeptide containing large guest molecules and the selectivity of L-leucyl–L-leucine toward alcohols over nitriles can be used to separate organic mixtures such as methanol/n-butanol and methanol/acetonitrile.
Introduction
Short-chain oligopeptides along with well-studied non-peptide oligomers which are capable of self-organizing in solutions and in solid phase are attractive building blocks for the design of new nanostructured materials with complex, hierarchical architectures.1,2 Oligopeptide based materials can have specific properties, such as piezoelectric activity, specific electrochemical behavior, high optical nonlinearity and nanoscale wettability,3 as well as magnetic susceptibility4 and luminescence.5 So, oligopeptide materials are of great interest for a variety of technologies3,5 and in medicine.6,7Based on oligopeptides, stable physical hydrogels8 and hydrogel nanoparticles,9 high-performance catalysts,10 biomedical materials with piezoelectric properties,11 flexible organic–inorganic hybrid systems for nanotechnology and biomedical science,12 biodegradable hybrid materials for electronics13 and materials for optoelectronic devices14 may be synthesized. Oligopeptides are also successfully used for the fabrication of biosensors15 and 2D-ordered films,16 and as lithographic masks.17
The type of oligopeptide nanomaterial depends on the sequence18,19 and number20,21 of amino acid residues, types of protecting groups in the oligopeptide molecule,22 the type of solvent or solvent ratios from which the oligopeptide is crystallized,18,23,24 whether the oligopeptide is prepared at high25 or low26 temperature, exposure to ultraviolet irradiation,27 the type of surface on which the self-organization of the oligopeptide occurs,28,29 and the presence of water30 or other trace species31 within the solvent. These factors complicate the prediction of nanostructures formed under different conditions, but also allow for a huge variety of nanostructures with wide ranging properties.
Some short-chain oligopeptides can form porous crystals32 with hydrophobic or hydrophilic layers or channels.33 As a result, such crystals exhibit zeolite-like properties,34,35 and can selectively bind some gases36,37 or separate mixtures of gases.38,39
The weak interactions between the monomer units in oligopeptide crystals leads to a complex dependence of the oligopeptides sorption properties on the molecular size of the sorbate (i.e. guest).19 This may cause changes in the packing of the oligopeptide,40–42 and can even destroy the crystal43 during the binding of vapor-phase guests. The sorption capacity of oligopeptide crystals generally decreases with increasing molecular size of the sorbate in homologous series.19
Some oligopeptide crystals obtained from solutions exhibit unusual sorption capacities. For example, the dipeptide L-leucyl–L-leucine (Leu–Leu) forms isomorphous 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solvates with ethanol, 1-propanol, 2-propanol,44 2-methyl-1-propanol45 and 1
1 solvates with ethanol, 1-propanol, 2-propanol,44 2-methyl-1-propanol45 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (0.5
(0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5) with 1-propanol
0.5) with 1-propanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-propanol mixtures.44 Transitioning from ethanol to propanols constitutes a 1.35 times increase in molecular size, according to molecular refraction (MRD) values. However, the cell parameters of the channel-type crystalline Leu–Leu do not significantly change. Transitioning from ethanol to isobutanol constitutes a 1.7 times increase in molecular size, which does significantly change the crystal parameters of Leu–Leu. In the latter case, layered crystals of Leu–Leu form with isobutanol.45 Layered crystals of the same composition of Leu-Leu also form with dimethyl sulfoxide (DMSO),46 but the crystal cell parameters differ significantly from those of Leu–Leu with 2-methyl-1-propanol, despite the similar sizes of DMSO and 2-methyl-1-propanol. With water, the dipeptide forms crystals with a composition of 0.87 mol of H2O per mol of Leu–Leu.47 In these crystals, solvent molecules form hydrogen bonds with one or two dipeptide molecules. The difficulty in preparing Leu–Leu crystals with organic guests has resulted in the data reported on the interaction of Leu–Leu for only six compounds, three of which are homologues.44–47 This information is insufficient for predicting the sorption properties of the dipeptide in “solid Leu–Leu + vapor” systems.
2-propanol mixtures.44 Transitioning from ethanol to propanols constitutes a 1.35 times increase in molecular size, according to molecular refraction (MRD) values. However, the cell parameters of the channel-type crystalline Leu–Leu do not significantly change. Transitioning from ethanol to isobutanol constitutes a 1.7 times increase in molecular size, which does significantly change the crystal parameters of Leu–Leu. In the latter case, layered crystals of Leu–Leu form with isobutanol.45 Layered crystals of the same composition of Leu-Leu also form with dimethyl sulfoxide (DMSO),46 but the crystal cell parameters differ significantly from those of Leu–Leu with 2-methyl-1-propanol, despite the similar sizes of DMSO and 2-methyl-1-propanol. With water, the dipeptide forms crystals with a composition of 0.87 mol of H2O per mol of Leu–Leu.47 In these crystals, solvent molecules form hydrogen bonds with one or two dipeptide molecules. The difficulty in preparing Leu–Leu crystals with organic guests has resulted in the data reported on the interaction of Leu–Leu for only six compounds, three of which are homologues.44–47 This information is insufficient for predicting the sorption properties of the dipeptide in “solid Leu–Leu + vapor” systems.
The present work is the first reported comprehensive study of the sorption properties of Leu–Leu toward a wide range of organic compounds, including proton donors, proton acceptors and aprotic compounds. The data presented herein reveal the non-zeolite properties of this dipeptide. Different experimental methods are used to investigate and explain the specific sorption properties of the dipeptide. The sorption of organic vapors or water by a dipeptide layer was studied on a quartz crystal microbalance (QCM-sensor). The thermal stabilities of the products of the interaction of Leu–Leu with vapors were studied using thermogravimetric analysis with simultaneous differential scanning calorimetry, and mass-spectrometry detection of the evolved vapors. The self-organization (we use this term rather than self-assembly because of the non-equilibrium conditions of the systems studied48) of the dipeptide in solutions, and the effect of vapors on the surface morphologies of thin dipeptide films were observed by atomic force microscopy. Changes in the crystal packing of Leu–Leu were characterized by X-ray powder diffraction.
Experimental
Materials
The dipeptide L-leucyl–L-leucine (Leu–Leu) (Bachem) was used without additional purification.Purified organic solvents49 had at least 99.5% purity.
QCM study of guest binding
A sensor device with 10 MHz QCM crystals in thickness shear mode (TSM) was used.50 The dipeptide coatings were prepared on the gold surface of quartz crystals.19 These coatings lead to a decrease of ΔF ∼ 800 Hz in the crystal frequency after solvent removal. The thickness value (∼40 nm) was estimated by the layer area, mass and density of Leu–Leu ρ = 1.156 g cm−3, calculated from X-ray single crystal data.47QCM sensor experiment has been carried out as describe elsewhere.19,43 The stoichiometry of clathrates was determined with an error of 10%. The residual water content in these coatings, determined as describe elsewhere,43 does not exceed 1.0 w/w%.
Thermoanalysis by simultaneous TG/DSC/MS
Simultaneous thermogravimetry (TG) and differential scanning calorimetry (DSC) analysis of dipeptide powder with mass spectrometric (MS) evolved gas analysis was performed using an STA 449 C Jupiter (Netzsch) thermoanalyzer coupled with a QMS 403C Aeolos (Netzsch) quadrupolar mass-spectrometer as described elsewhere.50,51X-ray powder diffraction (XRPD)
XRPD studies of the dipeptide were performed using a MiniFlex 600 diffractometer (Rigaku) equipped with a D/teX Ultra detector.52 In this experiment, Cu Kα radiation (40 kV, 15 mA) was used and data were collected at room temperature in the range of 2θ from 3 to 50 with a step of 0.02 and exposure time at each point of 0.24 s without sample rotation.Atomic force microscopy (AFM).
AFM images were recorded using a Titanium (NT-MDT, Russia) atomic force microscope. Measurements were performed in air using a tapping mode.19,43 The revolution cartridge of cantilevers CNG (NT-MDT, Russia) was used. For AFM experiments, dipeptide films with a diameter of 3 mm were prepared from different solutions on the surfaces of highly oriented pyrolytic graphite (HOPG) plates (1 × 1 cm) using the same technique as for the QCM study.19 HOPG was freshly cleaved before use. To study the effects of vapors on the film morphology, first, an AFM image was obtained for the initial dipeptide film dried from methanol solution. Then, the dipeptide layer was saturated with water or organic vapors. Thereafter, the guest was removed from the dipeptide as for the sensor experiment,53 and the AFM image of the film was obtained.Results and discussion
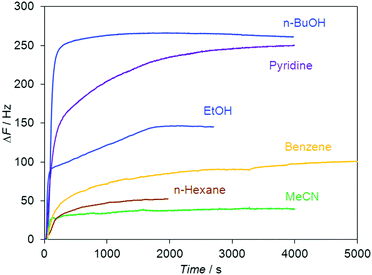
The QCM sensor responses (ΔF) of the quartz crystals coated with Leu–Leu were determined for the vapors of 19 organic guests and water at a relative vapor pressure of P/P0 = 0.85 at 298 K. Typical sensor responses for the guest vapors are given in Fig. 1.The guest/host molar ratio S (Table 1) was calculated using the equation:
| S = (ΔF/ΔFdipeptide) × (Mdipeptide/Mguest), |
| Guest | MRDa (cm3 mol−1) | S (mol guest mol−1 Leu–Leu) |
|---|---|---|
| a Molar refraction MRD = (M/d) × (nD2 − 1)/(nD2 + 2), where M is the molecular weight of the guest, and d and nD are the density and refractive index of the liquid guest, respectively. b X-ray data from ref. 47. c X-ray data from ref. 44. | ||
| H2O | 3.7 | 1.09 (0.87)b |
| CH3OH | 8.3 | 1.02 |
| C2H5OH | 12.9 | 0.97 (1.0)c |
| n-C3H7OH | 17.5 | 1.09 (1.0)c |
| i-C3H7OH | 17.6 | 1.08 (1.0)c |
| n-C4H9OH | 22.1 | 1.10 |
| CH3CN | 11.1 | 0.31 |
| C2H5CN | 16.0 | 0.22 |
| C3H7CN | 20.4 | 0.17 |
| C4H9CN | 25.2 | 0.16 |
| cyclo-C6H12 | 27.7 | 0.20 |
| n-C6H14 | 29.9 | 0.19 |
| n-C7H16 | 34.5 | 0.16 |
| CH2Cl2 | 16.4 | 1.30 |
| CHCl3 | 21.3 | 1.83 |
| CCl4 | 26.4 | 0.37 |
| C6H6 | 26.3 | 0.41 |
| C6H5CH3 | 31.1 | 0.35 |
| C5H5N | 24.2 | 0.97 |
| CH3NO2 | 12.5 | 0.28 |
The observed values of guest content S in Leu–Leu saturated with water, ethanol and propanols are in good agreement with single crystal XRD data for the corresponding inclusion compounds.44,47 Also, the content of n-butanol, calculated from QCM data, corresponds to the content of isobutanol in the crystal of the dipeptide.45
MRD values were used as size parameters for guest molecules,19,51,54 to analyze the effect of guest size on the sorption capacity of Leu–Leu. The correlation between the stoichiometry of the complexes and the MRD values of the guest molecules has been plotted in Fig. 2a.
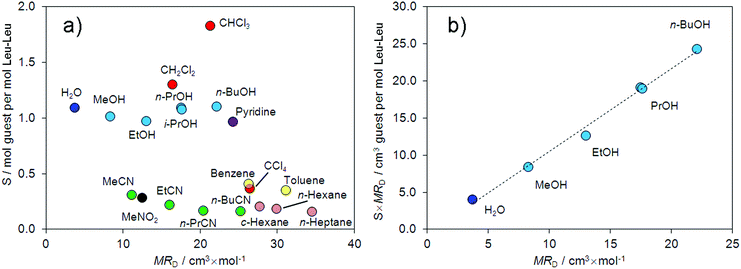 | ||
| Fig. 2 Correlation of (a) the guest content S in Leu–Leu with guest molar refraction MRD and (b) S × MRD parameter with molar refraction of the guest. | ||
From this correlation, the studied guests can be divided into two groups. Guests from the first group form clathrates in which the stoichiometry S is ≥1 mol of guest per mol of dipeptide. Guests from the second group form clathrates in which the stoichiometry S is <0.4 mol of guest per mol of dipeptide. Proton donors (water, alcohols, and chloroform), strong proton acceptors (pyridine), and dichloromethane constituted the first group. The second group consisted of weak proton acceptors (nitriles, and nitromethane), tetrachloromethane, arenes, and alkanes. Similar selectivities toward alcohols and nitriles were previously reported for the tripeptide L-leucyl–L-leucyl–L-leucine (Leu–Leu–Leu).43 The sorption capacities of Leu–Leu–Leu toward water and proton acceptors were similar. The main difference in the sorption properties of the tripeptide and dipeptide is the decrease in the stoichiometry S over the homologous series of alcohols in the case of Leu–Leu–Leu,43 whereas this value does not change for Leu–Leu.
We have proposed to use the product of sorption capacity (stoichiometry) of the dipeptide (S) and molar refraction of the guest (MRD) as a parameter for comparing the sorption properties of the dipeptide with other sorbates, including zeolite-like adsorbents. This S × MRD value represents the real space volume in the dipeptide phase required to accommodate the guest. We reported a correlation between S × MRD and the effect of guest vapors on the morphology of the tripeptide film.43 The dependence of S × MRD on the MRD values for water and the homologous series of alcohols is demonstrated in Fig. 2b. A linear increase of the S × MRD value by 175% was observed in the range from MeOH to n-BuOH.
The observed dependence shows an increase in the accessible volume for the guest in the dipeptide with increasing guest molecular size. This behavior is similar to that of clathrate-forming receptors such as calixarenes55 but not to that of zeolites. For example, for zeolite A the calculated value of S × MRD decreases upon transitioning from water to n-PrOH and from MeOH to n-PrOH by 12 and 5%, respectively.56 Similar decreases in the sorption capacity of 78 and 8% were observed for zeolites NaA and CaA, respectively, upon transitioning from the sorbate MeOH to n-PrOH.57 A largely constant value of S × MRD has also been observed for the sorption of benzene and toluene by faujasite zeolites.56 For human serum albumin,58 the dipeptides valyl-alanine and alanyl-valine,19 and first generation organophosphorus dendrimers,59 the value of S × MRD decreases over the homologous series of alcohols.
The observed specific sorption behavior of Leu–Leu may be due to two reasons. Firstly, hydrogen bonds are likely to form between Leu–Leu and guests such as methanol, n-butanol, chloroform, and pyridine as was similarly demonstrated for water,47 ethanol, and propanols.44 Secondly, a change in the packing of Leu–Leu from channel-type to layered crystals may occur upon sorption of molecules with MRD values larger than 18–20 cm3 mol−1 (i.e. larger than propanols), as is inferred from the crystal packing of Leu–Leu with 2-methyl-1-propanol45 and DMSO.46 As a result, an additional volume appears in the dipeptide phase, in the presence of sorbates capable to forming strong hydrogen bonds. This selectivity of the dipeptide toward alcohols over nitriles may be used to separate mixtures of these compounds, even when their molecular sizes are similar. This assumption was tested in the current study using thermal analysis. We also propose an explanation for the high sorption capacity of Leu–Leu toward dichloromethane, which cannot form hydrogen bonds.
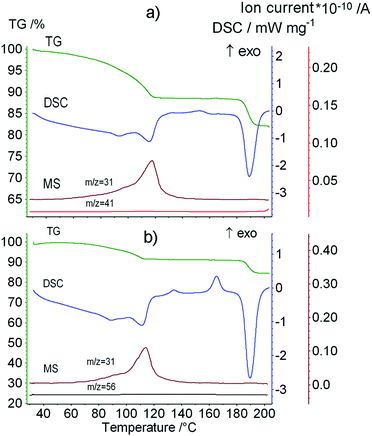
Before studying the thermal decomposition of clathrates of the dipeptide, we investigated the thermal properties of the initial Leu–Leu powder. The data obtained from TG/DSC/MS analysis are shown in Fig. 3. Upon heating, the Leu–Leu powder exhibited weight losses of 4.74 and 7.14% at 124 and 177 °C, respectively. The differential thermogravimetric (DTG) peaks corresponding to these processes were observed at 130 and 180 °C, respectively. Mass spectrometry indicated that these changes in sample weight had m/z ratios of 18, and so were associated with the loss of water (Fig. 3). The first step of weight loss was assumed to result from the evaporation of bound water, and the second to the chemical reaction, involving the formation of amide bonds, as was reported for diphenylalanine.52
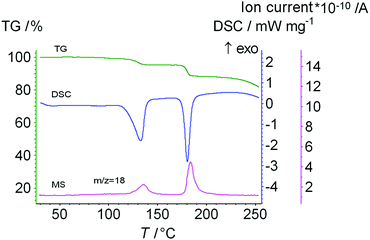 | ||
| Fig. 3 The data from TG/DSC/MS analysis of the initial Leu–Leu sample. The heating rate is 10 K min−1. | ||
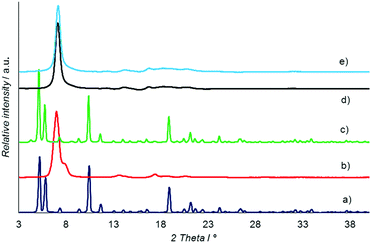
X-ray powder diffraction was then used to test this assumption. XRPD patterns of the initial Leu–Leu powder (Fig. 4a) and samples after heating to 130 (Fig. 4b) and 180 °C (Fig. 4d) were obtained. XRPD patterns of the heated samples after their saturation with water were also obtained. For this purpose, the samples after heating to 130 and 180 °C were kept in contact with saturated water vapor for 3 days (Fig. 4c) and liquid water for 5 days at 25 °C (Fig. 4e).
The XRPD patterns of the initial Leu–Leu powder (Fig. 4a) and powder saturated with water after heating to 130 °C (Fig. 4c) had similar profiles, and were comparable to the XRPD pattern simulated from single crystal X-ray data for Leu–Leu monohydrate,47 ESI.† The XRD pattern of the sample heated to 130 °C (Fig. 4b) contained a strong peak at 2θ of 6.9° with a shoulder at 7.5°, and weaker peaks at 13.4, 17, 18.5, and 20.4°. A similar pattern with an intense peak at 7° and broad weaker peaks at 13.8, 16.5, 18, and 20° was obtained for Leu–Leu after heating to 180 °C (Fig. 4d). The XRPD pattern of this powder sample showed no appreciable change after contact with liquid water for 5 days (Fig. 4e).
The heated samples were also investigated using thermal analysis after saturating with water (ESI†). Recovery of the initial water content was observed for the sample heated to 130 °C, but not for the sample heated to 180 °C.
These experiments show that bound water was lost during the first stage of the TG curve (Fig. 3). Therefore, the mass of dry dipeptide m(Leu–Leudry) was calculated as the difference between the mass of the initial sample m(Leu–Leuini) and the mass loss during the first stage Δm1, according to the equation:
| m(Leu–Leudry) = m(Leu–Leuini) − Δm1. |
The water content in the dipeptide S1 (mol of water per mol of dry dipeptide) was calculated according to the equation:
| S1 = Δm1 × MLeu–Leu/(MH2O × m(Leu–Leudry)) |
The amount of water S2 (mol of water per mol of dry dipeptide) which was lost during the second step of the TG-curve was calculated according to the equation:
| S2 = Δm2 × MLeu–Leu/(MH2O × m(Leu–Leudry)) |
The mass losses during the first Δm1 (desorption) and second Δm2 (chemical reaction) steps were calculated to be 4.74 and 7.14%, respectively, which corresponded to 0.7 and 1.0 mol of water per 1 mol of dipeptide, respectively.
Further, the decomposition of products of the saturation of the dipeptide with water or organic vapors was studied using TG/DSC/MS analysis (Fig. 5 and ESI†).
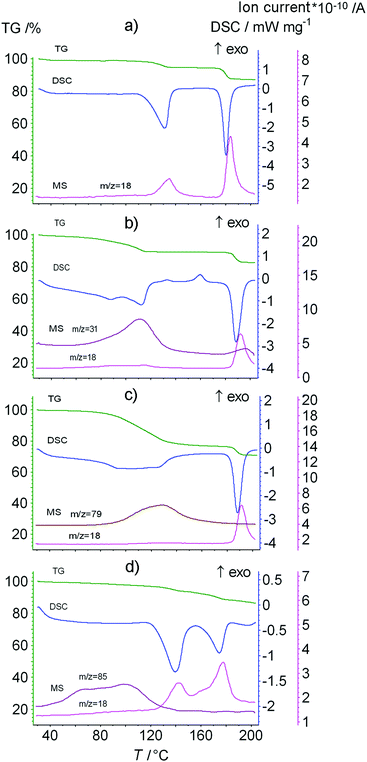 | ||
| Fig. 5 The data from TG/DSC/MS analysis of the Leu–Leu sample saturated with vapors of (a) water, (b) methanol, (c) pyridine and (d) chloroform. The heating rate is 10 K min−1. | ||
For some clathrates of Leu–Leu with organic compounds, elimination of the organic compound in the first stage has been shown to be associated with the release of water (Fig. 5). Therefore, a correction for the amount of water was made when calculating the content of the organic guest. For this, the MS-calibration was performed in the isothermal mode at 130 °C.60 The combined weight of the released water and organic guest was estimated from the ratio of the areas of the m/z = 18 mass spectrometry signals of the initial Leu–Leu sample (containing only water) and the product saturated with the organic sorbate (containing water and the organic sorbate). The content of the organic compound was calculated according to the equation:
| S = (Δm1 − ΔmH2O) × MLeu–Leu/(Mguest × m(Leu–Leudry)) |
The thermoanalysis results are given in Table 2 including the mass loss Δm, clathrate composition STG, temperature of guest elimination Tonset and temperatures of DTG-peaks Tmax.
| Guest | T b (°C) | Δm (%) | S (mol guest mol−1 Leu–Leu) | S 1 (mol H2O mol−1 Leu–Leu) | T onset (°C) | T max (°C) |
|---|---|---|---|---|---|---|
| a Boiling temperature of liquid sorbates. b Water content in the clathrate estimated from the value of ΔmH2O with an error of 0.2 mol water per mol of Leu–Leu. c There is only water in the evolved vapors. d The signal of m/z = 18 is zero. | ||||||
| H2O | 100 | 5.44 | 0.8 | 121 | 130 | |
| MeOH | 64 | 10.6 | 0.8 | —d | 92 | 111 |
| EtOH | 78 | 16.0 | 0.9 | 0.2 | 117 | 126 |
| n-PrOH | 97 | 20 | 1.0 | —d | 117 | 127 |
| i-PrOH | 83 | 19.9 | 0.9 | 0.2 | 116 | 125 |
| n-BuOH | 117 | 21.9 | 0.9 | —d | 99 | 130 |
| Pyridine | 115 | 23.3 | 0.9 | —d | 89 | 122 |
| CH2Cl2 | 40 | 5.37 | —c | 0.8 | 127 | 141 |
| CHCl3 | 61.2 | 7.34 | 0.1 | 0.2 | 125 | 137 |
| CCl4 | 76.7 | 5.19 | —c | 0.7 | 125 | 136 |
| C6H6 | 80 | 5.7 | —c | 0.8 | 125 | 135 |
| CH3CN | 81.6 | 5.31 | —c | 0.8 | 126 | 135 |
The thermal analysis data indicated that Leu–Leu does not form stable clathrates at room temperature with acetonitrile, benzene, tetrachloromethane and dichloromethane. Only water was detected in the evolved vapor during the decomposition of the products of Leu–Leu saturated with these organic compounds, Table 2. The mass loss corresponded to the content of water in the initial Leu–Leu sample. The temperatures of guest elimination Tonset and the DTG-peaks Tmax for these saturated products were higher than the corresponding values for the initial Leu–Leu sample, and for Leu–Leu saturated with water vapor. Leu–Leu saturated with chloroform vapor formed a stable clathrate containing 0.1 mol of chloroform and 0.2 mol of water per mol of Leu–Leu. Anhydrous clathrates were obtained after saturating Leu–Leu powder with vapors of methanol, n-propanol, n-butanol and pyridine. Leu–Leu saturated with vapors of ethanol, isopropanol and chloroform contained small water contents, which did not exceed the error of calculation. The content of alcohols and pyridine in the clathrates of Leu–Leu corresponded to the stoichiometry calculated from the QCM sensor data and X-ray diffraction data, Tables 1 and 2.
The ΔT = Tmax − Tb value can be used as a measure of the thermal stability of the clathrates, where Tb is the boiling point of the guest.51 For the homologous series of alcohols, there was a decrease in thermal stability of the clathrate with increasing guest boiling temperature. This may indicate a weakening of the interaction between the dipeptide and guest with increasing guest size, because of steric restrictions hindering the optimal packing of larger guest molecules in the dipeptide phase.
The selectivity of the dipeptide toward alcohols over nitriles, and the low stabilities of clathrates containing large guests can be used for practical application. In this study, we demonstrated the separation of methanol/acetonitrile and methanol/n-butanol mixtures by Leu–Leu. Two samples were prepared by saturating Leu–Leu powders with vapors of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v% methanol/acetonitrile and methanol/n-butanol mixtures for 3 days. Thermal analysis data of the saturated products are shown in Fig. 6, and only methanol vapor was detected in the evolved gases.
1 v/v% methanol/acetonitrile and methanol/n-butanol mixtures for 3 days. Thermal analysis data of the saturated products are shown in Fig. 6, and only methanol vapor was detected in the evolved gases.
The low content of chloroform in the clathrate of Leu–Leu may have resulted from several reasons. Chloroform has a low boiling point, and forms weak hydrogen bonds with acceptors. Methanol has a closer boiling point to chloroform, Table 2, but forms stronger hydrogen bonds with proton acceptors (e.g. acetone), compared with chloroform.61 For clathrates of Leu–Leu with pyridine and n-butanol, the differences between the temperatures of clathrate decomposition and guest boiling points are 26 and 18 °C, respectively. A similar difference (20–30 °C) could be expected for the clathrate with chloroform, so its decomposition should begin at temperatures near 31–41 °C, Table 2. This accounted for the loss of most of the chloroform from the clathrate during 15–20 min in argon flow prior to thermal analysis. The formation of the clathrate of Leu–Leu with chloroform was confirmed by the decrease of the dipeptide water content to 0.2 mol of H2O per mol of Leu–Leu, Table 2. For non-bonded guests, the water content of the saturated product is the same as that of the initial clathrate. Thus, the decrease in water content was likely caused by the exchange of chloroform. The high sorption capacity of Leu–Leu toward chloroform (Fig. 2 and Table 1) may be explained by the formation of layered crystals, as in the case of clathrates with pyridine and n-butanol because of the similar sizes of these guest molecules.
However, this argument does not account for the high sorption capacity of Leu–Leu toward dichloromethane. Thus, we investigated the self-organization of Leu–Leu from methanol, chloroform, pyridine, and dichloromethane solutions using atomic force microscopy. The effect of ethanol, chloroform and dichloromethane vapors on the morphology of dipeptide films was also studied.
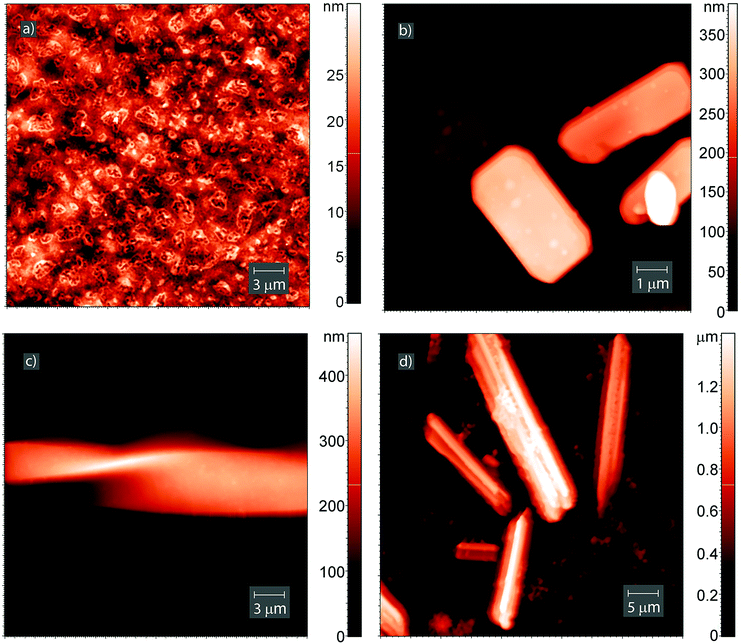
Evaporation of methanol from the dipeptide solution led to the formation of a relatively smooth Leu–Leu film, which contained shapeless objects of varying sizes on the surface of highly oriented pyrolytic graphite. The average height spread on a 30 × 30 μm scan was 30 nm and the mean square roughness of the surface Rq was 5.3 nm (Fig. 7a). Apparently, the same amorphous film forms on the surface of the QCM sensor.
The saturation of the dipeptide film with ethanol vapor yielded relatively small crystals on its surface (ESI†) with height, length and width approximately 20–140 nm, 0.2–0.85 μm and 0.2–0.77 μm, respectively.
Evaporation of pyridine solution leads to the formation of flattened octagonal crystals like cut emeralds (Fig. 7b and ESI†). The height, length and width of a single crystal were approximately 270 nm, 4.6 μm and 2.7 μm, respectively. The side face of the crystal contained steps with heights of 20–60 nm (ESI†).
Exposing the initial Leu–Leu film to saturated chloroform vapor for 2 days yielded elongated crystals on its surface, including a spiral-shaped crystal (Fig. 7c). This crystal has a length more than 50 μm, width of approximately 9 μm, and thickness in the twisted region of approximately 190 nm. When chloroform was used as the solvent, the dipeptide crystallized from solution formed elongated crystals with heights of 90 nm to 1.5 μm (Fig. 7d and ESI†). The lengths of the crystals are in the range from 300 nm to 30 μm. The formation of such large crystals was observed only for the systems with chloroform. One can assume that the crystals formed have a specific packing with a large free internal volume which can accommodate a significant amount of this guest.
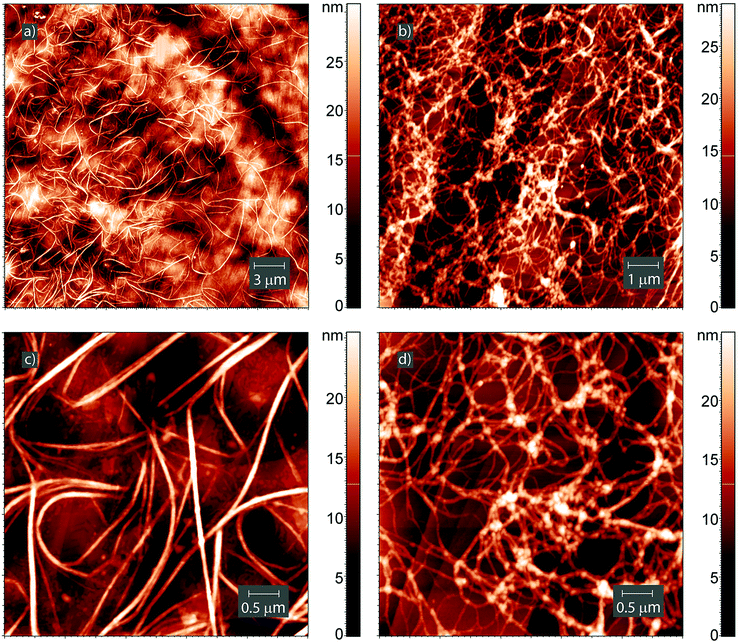
Different results were obtained for the initial Leu–Leu film exposed to dichloromethane (Fig. 8). After saturating the Leu–Leu film with dichloromethane vapor for one day, linear and curved nanofibers with heights of 4–25 nm, lengths of 8–9 μm and base widths of 80–150 nm were observed on the surface (Fig. 8a and c). The fibers thickened where they intersected with each other (Fig. 8c). Also, the SEM images were obtained for the Leu–Leu film saturated with dichloromethane vapor for 200 min (ESI†). The lengths of 8–9 μm and widths of 80–150 nm are in excellent agreement with observations by AFM. Evaporation of dichloromethane from the dipeptide solution yielded a web-like structure, similar to that of dried gels17,62 (Fig. 8b and d). The threads had a necklace-like structure, and were composed of small spherical particles with diameters of 40–80 nm. The distances between nodes of the web are in the range from 200 to 800 nm. The web-like structure on HOPG also was observed using SEM (ESI†). The formation of such nanostructures during the interaction of the dipeptide film with dichloromethane vapor may have caused the high sorption capacity of Leu–Leu, because the gel structure can hold a large amount of solvents (sorbents).63 The gelation of Leu–Leu in dichloromethane vapor or solution may have been due to the presence of water in the dipeptide. This is similar to the gel formation of diphenylalanine in ethanol/CH2Cl2 mixtures containing trace water.31
Conclusions
We have demonstrated the unusual non-zeolitic properties of L-leucyl–L-leucine, which exhibited an increase in the sorption volume of the dipeptide phase with increasing sorbate molecular size. This resulted from a change in the packing of the dipeptide crystals, induced by binding organic molecules larger than 18–20 cm3 mol−1 that were capable of hydrogen bonding with the dipeptide. A decrease in the thermal stability of the clathrates of L-leucyl–L-leucine with increasing guest size was observed. The dipeptide exhibited selectivity toward alcohols over nitriles. L-Leucyl–L-leucine could bind aliphatic alcohols in 3–5 times higher molar ratios than nitriles, despite their similar molecular sizes. This was exploited for separating methanol/n-butanol and methanol/acetonitrile mixtures.The solvent strongly affected the crystallization of L-leucyl–L-leucine. Evaporation from methanol solution yielded an amorphous dipeptide film. Evaporation from chloroform and pyridine yielded elongated and flattened octagonal crystals, respectively. Evaporation from dichloromethane yielded a gel-like structure. Exposing thin dipeptide films to saturated chloroform and dichloromethane vapors yielded spiral crystals and separated nanofibers, respectively.
So, using solvents or vapors with different physicochemical properties may allow us to control the type and shape of nanostructures based on L-leucyl–L-leucine by the same technique as used for the amphiphilic derivatives of the tripeptide.64 Such an opportunity is of great interest for the design of nanoarchitectures, including anisotropic nanostructures.65
We believe that the obtained results may be useful for the practical application of short-chain oligopeptides and for the design of new nanostructured materials.
Competing interest
The authors declare no competing financial interest.Acknowledgements
This study was supported by grant no. 14.Y26.31.0019 from the Ministry of Education and Science of Russian Federation. The authors thank Dr Lyadov Nikolay, Zavoisky Physical-Technical Institute, Kazan, Russia, for SEM experiments.Notes and references
- S. Kim, J. Kim, H. J. S. Lee and C. B. Park, Small, 2015, 11, 3623–3640 CrossRef CAS PubMed.
- S. Yagai, Bull. Chem. Soc. Jpn., 2015, 88, 28–58 CrossRef.
- G. Rosenman, P. Beker, I. Koren, M. Yevnin, B. Bank-Srour, E. Mishina and S. Semin, J. Pept. Sci., 2011, 17, 75–87 CrossRef CAS PubMed.
- R. J. A. Hill, V. L. Sedman, S. Allen, P. M. Williams, M. Paoli, L. Adler-Abramovich, E. Gazit, L. Eaves and S. J. B. Tendler, Adv. Mater., 2007, 19, 4474–4479 CrossRef CAS.
- S. Semin, A. van Etteger, L. Cattaneo, N. Amdursky, L. Kulyuk, S. Lavrov, A. Sigov, E. Mishina, G. Rosenman and T. Rasing, Small, 2015, 11, 1156–1160 CrossRef CAS PubMed.
- Y. Kuang, Y. Gao and B. Xu, Chem. Commun., 2011, 47, 12625–12627 RSC.
- S. Marchesan, A. V. Vargiu and K. E. Styan, Molecules, 2015, 20, 19775–19788 CrossRef CAS PubMed.
- G. Fichman and E. Gazit, Acta Biomater., 2014, 10, 1671–1682 CrossRef CAS PubMed.
- R. Ischakov, L. Adler-Abramovich, L. Buzhansky, T. Shekhter and E. Gazit, Bioorg. Med. Chem., 2013, 21, 3517–3522 CrossRef CAS PubMed.
- K. S. Lee and J. R. Parquette, Chem. Commun., 2015, 51, 15653–15656 RSC.
- K. Ryan, J. G. Beirne, G. Redmond, J. I. Kilpatrick, J. Guyonnet, N.-V. Buchete, A. L. Kholkin and B. J. Rodriguez, ACS Appl. Mater. Interfaces, 2015, 7, 12702–12707 CAS.
- X. Yan, P. Zhu, J. Fei and J. Li, Adv. Mater., 2010, 22, 1283–1287 CrossRef CAS PubMed.
- S. Khanra, T. Cipriano, T. Lam, T. A. White, E. E. Fileti, W. A. Alves and S. Guha, Adv. Mater. Interfaces, 2015, 2, 1500265 CrossRef.
- Y. Li, L. Yan, K. Liu, J. Wang, A. Wang, S. Bai and X. Yan, Small, 2016, 12, 2575–2579 CrossRef CAS PubMed.
- S. Kogikoski Jr, C. P. Sousa, M. S. Liberato, T. Andrade-Filho, T. Prieto, F. F. Ferreira, A. R. Rocha, S. Guhad and W. A. Alves, Phys. Chem. Chem. Phys., 2016, 18, 3223–3233 RSC.
- N. Hendler, N. Sidelman, M. Reches, E. Gazit, Y. Rosenberg and S. Richter, Adv. Mater., 2007, 19, 1485–1488 CrossRef CAS.
- T. H. Han, T. Ok, J. Kim, D. O. Shin, H. Ihee, H.-S. Lee and S. O. Kim, Small, 2010, 6, 945–951 CrossRef CAS PubMed.
- H. Erdogan, E. Babur, M. Yilmaz, E. Candas, M. Gordese, Y. Dede, E. E. Oren, G. B. Demire, M. K. Ozturk, M. S. Yavuz and G. Demire, Langmuir, 2015, 31, 7337–7345 CrossRef CAS PubMed.
- M. A. Ziganshin, N. S. Gubina, A. V. Gerasimov, V. V. Gorbatchuk, S. A. Ziganshina, A. P. Chuklanov and A. A. Bukharaev, Phys. Chem. Chem. Phys., 2015, 17, 20168–20177 RSC.
- P. Tamamis, L. Adler-Abramovich, M. Reches, K. Marshall, P. Sikorski, L. Serpell, E. Gazit and G. Archontis, Biophys. J., 2009, 96, 5020–5029 CrossRef CAS PubMed.
- A. Handelman, N. Kuritz, A. Natan and G. Rosenman, Langmuir, 2016, 32, 2847–2862 CrossRef CAS PubMed.
- E. Mayans, G. Ballano, J. Casanovas, A. Diaz, M. M. Perez-Madrigal, F. Estrany, J. Puiggali, C. Cativiela and C. Aleman, Chem. – Eur. J., 2015, 21, 16895–16905 CrossRef CAS PubMed.
- T. O. Mason, D. Y. Chirgadze, A. Levin, L. Adler-Abramovich, E. Gazit, T. P. J. Knowles and A. K. Buell, ACS Nano, 2014, 8, 1243–1253 CrossRef CAS PubMed.
- R. Huang, W. Qi, R. Su, J. Zhao and Z. He, Soft Matter, 2011, 7, 6418–6421 RSC.
- R. Huang, Y. Wang, W. Qi, R. Su and Z. He, Nanoscale Res. Lett., 2014, 9, 653 CrossRef PubMed.
- X. Wang, Y. C. Chen and B. Li, RSC Adv., 2015, 5, 8022–8027 RSC.
- R. Wei, C.-C. Jin, J. Quan, H.-l. Nie and L.-M. Zhu, Biopolymers, 2013, 101, 272–278 CrossRef PubMed.
- M. A. Ziganshin, I. G. Efimova, A. A. Bikmukhametova, V. V. Gorbachuk, S. A. Ziganshina, A. P. Chuklanov and A. A. Bukharaev, Prot. Met. Phys. Chem. Surf., 2013, 49, 274–279 CrossRef CAS.
- V. V. Korolkov, S. Allen, C. J. Roberts and S. J. B. Tendler, Faraday Discuss., 2013, 166, 257–267 RSC.
- M. A. Ziganshin, A. A. Bikmukhametova, A. V. Gerasimov, V. V. Gorbatchuk, S. A. Ziganshina and A. A. Bukharaev, Prot. Met. Phys. Chem. Surf., 2014, 50, 49–54 CrossRef CAS.
- J. Wang, K. Liu, L. Yan, A. Wang, S. Bai and X. Yan, ACS Nano, 2016, 10, 2138–2143 CrossRef CAS PubMed.
- D. V. Soldatov, I. L. Moudrakovski, E. V. Grachev and J. A. Ripmeester, J. Am. Chem. Soc., 2006, 128, 6737–6744 CrossRef CAS PubMed.
- C. H. Görbitz, Chem. – Eur. J., 2007, 13, 1022–1031 CrossRef PubMed.
- R. Afonso, A. Mendes and L. Gales, J. Mater. Chem., 2012, 22, 1709–1723 RSC.
- R. Anedda, D. V. Soldatov, I. L. Moudrakovski, M. Casu and J. A. Ripmeeste, Chem. Mater., 2008, 20, 2908–2920 CrossRef CAS.
- S. Guha, T. Chakraborty and A. Banerjee, Green Chem., 2009, 11, 1139–1145 RSC.
- A. Comotti, S. Bracco, G. Distefano and P. Sozzani, Chem. Commun., 2009, 284–286 RSC.
- R. V. Afonso, J. Durao, A. Mendes, A. M. Damas and L. Gales, Angew. Chem., 2010, 122, 3098–3100 CrossRef.
- A. Comotti, A. Fraccarollo, S. Bracco, M. Beretta, G. Distefano, M. Cossi, L. Marchese, C. Riccardi and P. Sozzani, CrystEngComm, 2013, 15, 1503–1507 RSC.
- S. Guha, M. G. B. Drew and A. Banerjee, CrystEngComm, 2009, 11, 756–762 RSC.
- C. H. Görbitz, Acta Crystallogr., Sect. B: Struct. Sci., 2002, 58, 849–854 Search PubMed.
- T. J. Burchell, D. V. Soldatov and J. A. Ripmeester, J. Struct. Chem., 2008, 49, 188–191 CrossRef CAS.
- M. A. Ziganshin, I. G. Efimova, V. V. Gorbatchuk, S. A. Ziganshina, A. P. Chuklanov, A. A. Bukharaev and D. V. Soldatov, J. Pept. Sci., 2012, 18, 209–214 CrossRef CAS PubMed.
- C. H. Görbitz, Acta Chem. Scand., 1998, 52, 1343–1349 CrossRef.
- C. H. Görbitz, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1999, 55, 670–672 Search PubMed.
- S. N. Mitra and E. Subramanian, Biopolymers, 1994, 14, 1139–1143 CrossRef PubMed.
- C. H. Gorbitz, Chem. – Eur. J., 2001, 7, 5153–5159 CrossRef CAS.
- J. D. Halley and D. A. Winkler, Complexity, 2008, 14, 10–17 CrossRef.
- W. L. F. Armarego and C. L. L. Chai, Purification of Laboratory Chemicals, Oxford, Butterworth-Heinemann, UK, 2009 Search PubMed.
- L. S. Yakimova, M. A. Ziganshin, V. A. Sidorov, V. V. Kovalev, E. A. Shokova, V. A. Tafeenko and V. V. Gorbatchuk, J. Phys. Chem. B, 2008, 112, 15569–15575 CrossRef CAS PubMed.
- M. A. Ziganshin, A. V. Gerasimov, V. V. Gorbatchuk and A. T. Gubaidullin, J. Therm. Anal. Calorim., 2015, 119, 1811–1816 CrossRef CAS.
- M. A. Ziganshin, A. V. Gerasimov, S. A. Ziganshina, N. S. Gubina, G. R. Abdullina, A. E. Klimovitskii, V. V. Gorbatchuk and A. A. Bukharaev, J. Therm. Anal. Calorim., 2016, 125, 905–912 CrossRef CAS.
- I. G. Efimova, M. A. Ziganshin, V. V. Gorbatchuk, D. V. Soldatov, S. A. Ziganshina, A. P. Chuklanov and A. A. Bukharaev, Prot. Met. Phys. Chem. Surf., 2009, 45, 525–528 CrossRef CAS.
- A. K. Gatiatulin, M. A. Ziganshin, G. F. Yumaeva, A. T. Gubaidullin, K. Suwinska and V. V. Gorbatchuk, RSC Adv., 2016, 6, 61984–61995 RSC.
- M. A. Ziganshin, A. V. Yakimov, G. D. Safina, S. E. Solovieva, I. S. Antipin and V. V. Gorbatchuk, Org. Biomol. Chem., 2007, 5, 1472–1478 CAS.
- R. M. Barrer, J. Inclusion Phenom., 1983, 1, 105–123 CrossRef CAS.
- D. W. Breck, W. G. Eversole, R. M. Miltont, T. B. Reed and T. L. Thomas, J. Am. Chem. Soc., 1956, 78, 5963–5972 CrossRef CAS.
- V. V. Gorbatchuk, M. A. Ziganshin, B. N. Solomonov and M. D. Borisover, J. Phys. Org. Chem., 1997, 10, 901–907 CrossRef CAS.
- A. V. Gerasimov, M. A. Ziganshin, A. E. Vandyukov, V. I. Kovalenko, V. V. Gorbatchuk, A.-M. Caminade and J.-P. Majoral, J. Colloid Interface Sci., 2011, 360, 204–210 CrossRef CAS PubMed.
- V. V. Gorbatchuk, A. K. Gatiatulin, M. A. Ziganshin, A. T. Gubaidullin and L. S. Yakimova, J. Phys. Chem. B, 2013, 117, 14544–14556 CrossRef CAS PubMed.
- Hydrogen Bonding, ed. M. D. Joesten and L. J. Schaad, Marcel Dekker Inc., New York, 1974 Search PubMed.
- X. Yan, Y. Cui, Q. He, K. Wang and J. Li, Chem. Mater., 2008, 20, 1522–1526 CrossRef CAS.
- H. Geng, L. Ye, A.-Y. Zhang, Z. Shao and Z.-G. Feng, J. Colloid Interface Sci., 2017, 490, 665–676 CrossRef CAS PubMed.
- K. Ariga, J. Kikuchi, M. Naito, E. Koyama and N. Yamada, Langmuir, 2000, 16, 4929–4939 CrossRef CAS.
- P. Bairi, K. Minami, J. P. Hill, W. Nakanishi, L. K. Shrestha, C. Liu, K. Harano, E. Nakamura and K. Ariga, ACS Nano, 2016, 10, 8796–8802 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and calculated powder diffractograms; TG/DSC/MS data of the products of L-leucyl-L-leucine saturation with guest vapors; AFM images of the crystals obtained from a pyridine solution on the HOPG surface; Powder diffraction data. See DOI: 10.1039/c7cp01393k |
| This journal is © the Owner Societies 2017 |