What are the key factors governing the nucleation of CO2 hydrate?†
Abstract
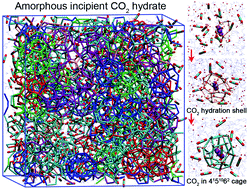
Microsecond molecular dynamics simulations were performed to provide molecular insights into the nucleation of CO2 hydrate. The adsorption of sufficient CO2 molecules around CO2 hydration shells is revealed to be crucial to effectively stabilize the hydrogen bonds formed therein, catalyzing the hydration shells into hydrate cages and inducing the nucleation. Moreover, a high aqueous CO2 concentration is found to be another key factor governing the nucleation of CO2 hydrate, and only above a critical concentration can the nucleation of CO2 hydrate occur. The 4151062 cages, with size similar to the CO2 hydration shell and an elliptical space closely matching a linear CO2 molecule, play a dominant role in initiating the nucleation and remain the most abundant. The incipient CO2 hydrate is rather amorphous due to the abundance of metastable cages (mostly 4151062).


 Please wait while we load your content...
Please wait while we load your content...