Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Electron-triggered chemistry in HNO3/H2O complexes†
Jozef
Lengyel
 *ab,
Milan
Ončák
b,
Juraj
Fedor
a,
Jaroslav
Kočišek
a,
Andriy
Pysanenko
a,
Martin K.
Beyer
*ab,
Milan
Ončák
b,
Juraj
Fedor
a,
Jaroslav
Kočišek
a,
Andriy
Pysanenko
a,
Martin K.
Beyer
 b and
Michal
Fárník
b and
Michal
Fárník
 *a
*a
aJ. Heyrovský Institute of Physical Chemistry v.v.i., Czech Academy of Sciences, Dolejškova 3, 18223 Prague, Czech Republic. E-mail: michal.farnik@jh-inst.cas.cz
bInstitut für Ionenphysik und Angewandte Physik, Leopold-Franzens-Universität Innsbruck, Technikerstraße 25, 6020 Innsbruck, Austria. E-mail: jozef.lengyel@uibk.ac.at
First published on 24th March 2017
Abstract
Polar stratospheric clouds, which consist mainly of nitric acid containing ice particles, play a pivotal role in stratospheric chemistry. We investigate mixed nitric acid–water clusters (HNO3)m(H2O)n, m ≈ 1–6, n ≈ 1–15, in a laboratory molecular beam experiment using electron attachment and mass spectrometry and interpret our experiments using DFT calculations. The reactions are triggered by the attachment of free electrons (0–14 eV) which leads to subsequent intracluster ion–molecule reactions. In these reactions, the nitrate anion NO3− turns out to play the central role. This contradicts the electron attachment to the gas-phase HNO3 molecule, which leads almost exclusively to NO2−. The nitrate containing clusters are formed through at least three different reaction pathways and represent terminal product ions in the reaction cascade initiated by the electron attachment. Besides, the complex reaction pathways represent a new hitherto unrecognized source of atmospherically important OH and HONO molecules.
Introduction
Heterogeneous chemistry involving ice particles, cloud droplets and aerosols plays an important, albeit not yet fully understood, role in the atmospheric chemistry.1 For example, polar stratospheric clouds (PSCs) provide an environment for heterogeneous reactions of chlorine activation on their surface, which lead to more than 90% of the ozone depletion.2 From the chemical perspective, PSCs mostly consist of nitric acid/ice particles.2–4 Under the specific conditions of the upper atmosphere, chemical reactions are often initiated either by photons or electrons. Free electrons originate from, e.g., photoionization, solar and magnetospheric particles or cosmic rays and the corresponding secondary ionization processes. Typically, the electrons are effectively thermalized to low energies (<1 eV) via multiple inelastic collisions.5 At these low energies, they can attach to molecules and clusters in the atmosphere.The gas-phase reactions between free electrons and HNO3 molecules have been studied only using flowing afterglow techniques,6–8 and we are not aware of any crossed-beam electron attachment data. It has been initially concluded6 that the dissociative electron attachment (DEA) in HNO3 leads only to the NO2− product ion which is the most exothermic channel with a reaction enthalpy of ΔrH298K = −0.13 eV. Recently, Shuman et al.8 reported a weak endothermic channel producing OH− (ΔrH298K = 0.31 eV). The formation of NO3− is rather endothermic (ΔrH298K = 0.45 eV) and has not been observed experimentally so far. In complexes with ice nanoparticles, the outcome of the electron-induced reactions can be significantly affected by the environment and by acidic dissociation of HNO3.
In the present experiment, we produce mixed (HNO3)m(H2O)n clusters by supersonic expansions of HNO3/H2O vapor mixtures at different temperatures and buffer gas pressures. We analyzed the composition of the clusters generated under these conditions in our previous investigations with electron-impact ionization and sodium doping.9,10 These clusters then interact with the electron beam and the negative ion production is monitored by a mass spectrometer. In order to provide a reference gas-phase spectrum, we have also performed electron beam attachment experiments to gas-phase HNO3. Employing quantum chemical methods, we characterize the small neutral complexes of (HNO3)m(H2O)n, calculate the energetics of the initial dissociation step, i.e. the generation of NO2−, NO3− or OH− from HNO3, and sample the potential energy surface to locate the energetically most stable isomers and intracluster rearrangements.
Experimental and theoretical methods
Experimental part
The experiments were carried out on the CLUster Beam (CLUB) apparatus in Prague.11–13 The mixed HNO3/H2O clusters were generated in a home-build source via continuous supersonic expansion of the HNO3 solution (70%, Sigma-Aldrich) and helium (99.996%, Messer) as a buffer gas through a divergent conical nozzle of 100 μm diameter, 2 mm long, and approximately 30° full opening angle into the vacuum. The clustering conditions were controlled by heating the HNO3 solution in the reservoir and by the stagnation pressure of the buffer gas. Reservoir temperatures between 60 and 80 °C and buffer gas pressures between 1 and 2 bar were employed. The buffer gas carried the HNO3/H2O vapor to the nozzle heated independently to a higher temperature (usually by 5–10 °C) to avoid any condensation. However, changing the source conditions within the present range had little effect on the mass spectra. We have analyzed the composition of the neutral clusters previously from the positive mass spectra.9,10 From this analysis, the present neutral clusters have a mixed composition of (HNO3)m(H2O)n, with m ≈ 1–6 and n ≈ 1–15.The cluster beam was skimmed and passed through three differentially pumped chambers before entering the ion source of the perpendicularly mounted reflectron time-of-flight mass spectrometer. The voltages on the extraction plates were set to detect negative ions. The (HNO3)m(H2O)n clusters were ionized using free electrons (0–14 eV) from a pulsed electron gun at 10 kHz frequency during 2 μs in the extraction region of the spectrometer. After 0.5 μs delay to exclude the effects of any free electrons, a 2 μs extraction pulse was applied to extract the negative ions, which were detected on the Photonics MCP detector in the Chevron configuration. The electron attachment experiment was described in our recent paper,12 typical electron current at the present experiment was 0.2 μA at 5 eV. The electron-energy scale was calibrated using the 2.2 eV resonance in the O− production from N2O. The individual mass spectra recorded with an electron energy step of 0.25 eV in the range 0–14 eV allow for the evaluation of the electron energy dependence for each individual peak in the mass spectra, however, a detailed analysis of the electron attachment efficiency dependence is beyond the scope of the present study and will be the subject of future work.
The HNO3 gas-phase data were recorded on another apparatus: dissociative electron attachment spectrometer.14 The electron beam is produced in a trochoidal electron monochromator and passes through a collision chamber filled with the studied gas. The electron current is monitored by a Faraday cup located behind the collision cell. The entire experiment is pulsed: the electrons pass the target chamber during 200 ns while it is field-free and after additional 200 ns (when the electrons leave the chamber) a negative voltage of −300 V is pulsed across the chamber which pushes the anions formed in the cell towards the ion time-of-flight mass analyzer in the direction perpendicular to the electron beam. The anions are detected by a microchannel plate, counted, and their arrival times are analyzed.
The target chamber is filled with the vapor of concentrated nitric acid solution in water (70%). The vapor thus consists of a mixture of gas-phase HNO3 and H2O molecules. The presence of water vapor in the sample does not interfere with the results shown in Fig. 1: the DEA to H2O leads to H− and O− production,15 none of the fragments reported form HNO3 (NO2−, OH−, NO3−) originate from water. The spectra recorded after a longer time (tens of minutes) after the pump–freeze–thaw purification of the sample contained a weak O− signal. The electron-energy dependence of this signal was identical to that of O− originating from DEA to NO2.16 Since HNO3 is known to release NO2, we conclude that the detected O− does not originate from DEA to HNO3.
Computational details
Interpretation of the experimental results was supported by DFT calculations. We included the following clusters: (HNO3)m(H2O)n, m = 1–3, n = 0–5, (HNO3)m(H2O)nNO2−, m = 0–2, n = 0–5, (HNO3)m(H2O)nNO3−, m = 0–2, n = 0–5 (also n = 6 for m = 0, 1), (H2O)nOH−, n = 0–5. For all clusters with more than two composing molecules, molecular dynamics was run to find the most stable isomers, using the BLYP/6-31+g* method, with a time step of 40 a.u. (∼1 fs). First, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 steps were performed at 400 K, then another 10
000 steps were performed at 400 K, then another 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 steps at 200 K starting from the last geometry of the first molecular dynamics run. The temperature was maintained by a Nosé–Hoover chain of 4 thermostats with a relaxation time of 0.0015 a.u. The structures were re-optimized at the M06-2X/aug-cc-pVDZ level after every 1000 steps; the M06-2X functional was chosen due to its performance for flexible anionic clusters.17 Only the most stable isomers were considered, the respective structures are shown in Fig. S2 (ESI†). For HNO3(H2O)n, n = 4, 5, ion pair structures were also included, based on the structures presented elsewhere.18 Localized structures are in good agreement with those found by other studies.18–21 All reported energies were calculated at the M06-2X/aug-cc-pVDZ level and include zero-point-energy corrections (all structures represent local minima). Electronic structure calculations were performed using the Gaussian09 program,22 the ABIN code was used for molecular dynamics.23 Further details and benchmark calculations can be found in the ESI.†
000 steps at 200 K starting from the last geometry of the first molecular dynamics run. The temperature was maintained by a Nosé–Hoover chain of 4 thermostats with a relaxation time of 0.0015 a.u. The structures were re-optimized at the M06-2X/aug-cc-pVDZ level after every 1000 steps; the M06-2X functional was chosen due to its performance for flexible anionic clusters.17 Only the most stable isomers were considered, the respective structures are shown in Fig. S2 (ESI†). For HNO3(H2O)n, n = 4, 5, ion pair structures were also included, based on the structures presented elsewhere.18 Localized structures are in good agreement with those found by other studies.18–21 All reported energies were calculated at the M06-2X/aug-cc-pVDZ level and include zero-point-energy corrections (all structures represent local minima). Electronic structure calculations were performed using the Gaussian09 program,22 the ABIN code was used for molecular dynamics.23 Further details and benchmark calculations can be found in the ESI.†
Results and discussion
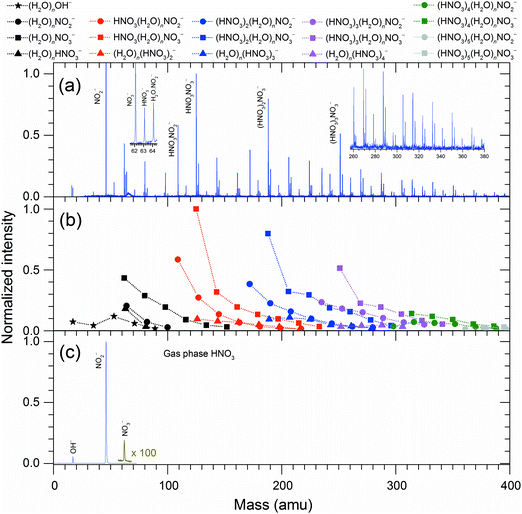
Fig. 1 summarizes the experimental measurements of the DEA to HNO3/H2O complexes and gas-phase HNO3. The gas-phase spectrum of HNO3 (Fig. 1(c)) is dominated by a NO2− fragment (as outlined above, the NO2− + OH dissociation channel is the only exothermic one), a weak OH− fragment and, for the first time detected, a NO3− fragment. The NO2−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OH−
OH−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NO3− intensity ratio is 96.5
NO3− intensity ratio is 96.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.4
3.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03. The ratio of the first two fragments is in very good agreement with that observed in mass spectrometry of flowing afterglow plasma,8 the electron energies in that type of experiment, however, were probably too low to reach the threshold for NO3− production.
0.03. The ratio of the first two fragments is in very good agreement with that observed in mass spectrometry of flowing afterglow plasma,8 the electron energies in that type of experiment, however, were probably too low to reach the threshold for NO3− production.
The cluster beam spectrum (Fig. 1(a and b)) is also dominated by an intense peak of NO2−. This is not surprising as a part of the present signal originates from HNO3 monomers in the beam. However, among the complex anions (with the mass higher than the HNO3 monomer), the dominant species are ions containing NO3−. The total intensity ratio of peaks from complexes containing NO3−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NO2−
NO2−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HNO3−
HNO3−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OH− is 57
OH− is 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The dramatic increase in the NO3− anion signal with respect to the spectrum of gas-phase HNO3 indicates rich intracluster chemistry. In the gas-phase reactions, the NO3− anion is often seen as the terminal product of ion–molecule reactions involving HNO3.6,24–28 In order to elucidate the pathways leading to its production in the present species, Fig. 2 shows energies for various intracluster reactions following several initial dissociation steps and summarizes the suggested pathways (1)–(9).
2. The dramatic increase in the NO3− anion signal with respect to the spectrum of gas-phase HNO3 indicates rich intracluster chemistry. In the gas-phase reactions, the NO3− anion is often seen as the terminal product of ion–molecule reactions involving HNO3.6,24–28 In order to elucidate the pathways leading to its production in the present species, Fig. 2 shows energies for various intracluster reactions following several initial dissociation steps and summarizes the suggested pathways (1)–(9).
 | ||
| Fig. 2 Reaction energies of various dissociation channels after electron attachment to (HNO3)m(H2O)n clusters, m = 1–3, n = 0–5, calculated at the M06-2X/aug-cc-pVDZ level of theory. | ||
It should be noted that the efficiency of DEA channels does not primarily depend on asymptotic energetics.29,30 Electron attachment is a vertical process and the decisive factor for breaking specific bonds, in case of a prompt dissociation, is often the direction of the gradient of the anionic potential energy surface at the initial structure. However, in systems with sufficient degrees of freedom such as clusters, the excitation energy may be redistributed over the vibrational degrees of freedom, with the decay proceeding statistically. In this case, the product distribution is dominated by the energetics of the dissociation channels. We identify three mechanisms leading to NO3− production, one proceeding in the first described way, i.e. kinematically driven, the other two driven by the asymptotic energetics.
Pathways leading to NO3−-containing complexes
| (H2O)n(HNO3)m → H3O+(H2O)n−1(HNO3)m−1NO3− | (10a) |
Collision of the neutral cluster containing an H3O+/NO3− ion pair with a free electron will most probably lead to recombination, forming a metastable H3O radical that subsequently dissociates, yielding a hydrogen atom and water.35 The energy released upon recombination will lead to the evaporation of the hydrogen atom, which is only weakly bound:
| H3O+(H2O)n(HNO3)m−1NO3− + e− → (H2O)n+1(HNO3)m−1NO3− + H | (10b) |
Since the mass of (HNO3)m(H2O)nNO2− is equal to that of HONO(HNO3)m−1(H2O)nNO3−, these ions cannot be distinguished in the mass spectrometry experiment. However, our molecular dynamics calculations indicate that the HONO formation proceeds irreversibly. The gas-phase reaction HNO3 + NO2− → NO3− + HONO has an energy of −0.74 eV (calculated at the M06-2X/aug-cc-pVDZ level of theory). The considerably larger acid strength of HNO3 compared to that of HONO suggests that the proton transfer reaction from HNO3 to NO2− can be expected in small mixed clusters (see Table S3 in the ESI†). The final cluster anion composition will also depend on whether HONO stays adsorbed on the cluster or evaporates:
| HONO(HNO3)m−2(H2O)nNO3− → (HNO3)m−2(H2O)nNO3− + HONO | (11) |
The calculated evaporation energies of H2O, HONO and HNO3 from the anionic complexes are compared in Table 1. It can be seen that the evaporation of a HONO molecule is in all cases more energetically demanding than water evaporation. However, the evaporation energies of HONO do not differ significantly from that of H2O for several cluster sizes. Energetically the most demanding and therefore the least probable is the evaporation of HNO3.
| Ion | Evaporating molecule | n | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | ||
| HONO(H2O)nNO3− | H2O | — | 0.48 | 0.37 | 0.41 | 0.64 | 0.45 |
| HONO | 1.00 | 0.85 | 0.68 | 0.60 | 0.78 | 0.79 | |
| HONO(H2O)nHNO3NO3− | H2O | — | 0.54 | 0.23 | 0.67 | 0.40 | 0.46 |
| HONO | 0.50 | 0.66 | 0.51 | 0.70 | 0.54 | 0.51 | |
| HNO3 | 0.95 | 1.00 | 0.86 | 1.12 | 0.88 | 0.89 | |
Again, such intracluster rearrangement cannot be followed by the mass spectrometry experiment. In our MD simulations, this reaction is seen to proceed spontaneously within 1 ps. It is strongly preferred already in the gas phase, the calculated energy of the corresponding HNO3 + OH− → NO3− + H2O reaction is −3.05 eV. With an increasing degree of hydration, the reaction retains its exothermic character, see ref. 27 and Table S3 in the ESI.†
We cannot determine the relative importance of these three channels leading to NO3− containing complexes as there are factors favoring each of them. As described above, a significant portion of neutral complexes will be acidically dissociated, leading to the first proposed channel. However, it is reasonable to assume that these will yield exclusively NO3−-containing anions, since NO3− is already present in the cluster before the interaction with the free electron takes place, and the electron is attracted by the positive charge center H3O+. The significant abundance of complexes containing NO2− and HNO3− in Fig. 1 suggests that the dissociation via the first channel is not the only process occurring. The fact favoring the second channel is that its first step, DEA to HNO3 yielding NO2−, has a high cross section. The third channel is the most exothermic one, it however requires OH− production as its first step, which has a low probability for the gas-phase HNO3 (Fig. 1(c)).
Pathways leading to NO2−, HNO3− and OH−-containing complexes
The second strongest progression in Fig. 1 are from the clusters containing NO2−. The formation of (H2O)nNO2− clusters is predicted to be the most exothermic channel for n < 2. With increasing degree of hydration, the formation of (H2O)nOH− prevails energetically as discussed above. For complexes with more than one HNO3 molecule, reactions (5) and (8) have an energy of about −1.5 eV and −2.0 eV for 2 and 3 HNO3 molecules, respectively. The formation of NO2− is then followed by an intracluster rearrangement to form HONO and NO3−.Anions of the nominal composition (H2O)n(HNO3)m− represent 8% of the total signal. For small cluster sizes (n ≤ 4), HNO3 is not ionically dissociated in the neutral cluster. Moreover, HNO3 has an appreciable electron affinity of 0.56 ± 0.17 eV.39 HNO3− valence anions are stable, decomposition into NO2− + OH is mildly exothermic. It is therefore entirely plausible that (H2O)n(HNO3)m− clusters contain a valence-bound HNO3− anion, which may decompose upon hydration with a small number of water molecules. In the hydrogen bonded network, it is also possible that NO2− + OH dissociation products remain in the cluster if the dissociation is not exothermic enough to cause product evaporation.
The (H2O)nOH− cluster series represents about 2% of the total signal. Its presence can be explained by (H2O)n(HNO3)m− dissociation into OH− + NO2, with NO2 not forming strong hydrogen bonds. This would be in line with our recent work,38 where OH− formation is observed in binary collisions of hydrated electrons with HNO3 in the gas phase. Electron attachment to a neutral (H2O)nHNO3 cluster would form the same initial state, a hydrated valence-bound HNO3, which quickly undergoes O–N bond cleavage to form (H2O)nOH−.
Atmospheric relevance
The present experiment shows that low energy free electrons are efficiently trapped in the mixed HNO3/H2O clusters. The electrons immediately initiate reactions with HNO3 through different reaction channels. Fig. 3 summarizes the processes occurring after DEA to mixed HNO3/H2O clusters. For clusters with one HNO3 molecule, three different fragment series (with OH−, NO2− or NO3− anions) can be produced. For clusters with more than one HNO3 molecule, clusters containing a NO3− core anion play the central role and represent the terminal product for all considered dissociation channels. | ||
| Fig. 3 Schematic illustration of reaction pathways observed in electron attachment to mixed (HNO3)m(H2O)n clusters. | ||
There is a close analogy between the composition of the studied clusters and PSCs. Therefore, our results can have important implications for the impact of HNO3 on heterogeneous chemistry. In particular, we suggest that DEA to molecules in PSC particles results in the formation of NO3− ions, which are among the most abundant anions in the stratosphere. As an example, (HNO3)2NO3− dominates among all negative stratospheric ions in the PSC region at heights below 30 km.40–42 We show that although DEA to HNO3/H2O particles leads to a cascade of reactions yielding reactive OH and HONO molecules, it is terminated by relatively stable NO3−. It is unlikely that this ion takes part in further reactions, and it rather promotes nucleation of new particles.43 However, the OH and HONO molecules released from the mixed nitric acid–water clusters upon the electron attachment play a pivotal role in the atmospheric chemistry. The atmospheric models do not account for the total yield of HONO in the atmosphere.44 It was indicated that a further, hitherto unrecognized, source of HONO must exist in the atmosphere and heterogeneous chemistry involving atmospheric aerosol particles was suspected to represent such a source. The electron attachment to the nitric acid–water clusters and our suggested reaction pathways represent one of the possible HONO sources.
Conclusions
In order to mimic electron-triggered reactions in PSC particles we investigated electron attachment to mixed nitric acid–water clusters (HNO3)m(H2O)n, m = 1–6, n = 1–15, in a crossed beam experiment. The products were analyzed using negative ion mass spectrometry and the results were interpreted by DFT calculations. The reactions induced by the electron attachment in the range of 0–14 eV yielded predominantly NO3− ion containing species through different reaction pathways. This is in contrast to both the electron attachment to gas-phase HNO3, yielding primarily NO2−, and the reaction of gas-phase HNO3 with a hydrated electron, which leads to the formation of hydrated OH− clusters. The initial driving force in the present DEA to HNO3/H2O complexes is the proton transfer to form the H3O+/NO3− ion pair where the electron recombines with the proton, yielding the hydrogen atom and NO3−. The NO3− anion represents the terminal product ion also in the intracluster reaction pathways driven by the energetics, which yield also OH and HONO molecules.Acknowledgements
This work was supported by the Czech Science Foundation project No. 15-12386S. J. L. and M. O. acknowledge the support through the Lise Meitner Programme of the Austrian Science Fund (FWF): projects No. M1983-N34 and M2001-NBL. The computational results presented have been achieved using the HPC infrastructure LEO of the University of Innsbruck.References
- A. R. Ravishankara, Science, 1997, 276, 1058 CrossRef CAS.
- O. Kirner, R. Müller, R. Ruhnke and H. Fischer, Atmos. Chem. Phys., 2015, 15, 2019 CrossRef CAS.
- T. Peter, Annu. Rev. Phys. Chem., 1997, 48, 785 CrossRef CAS PubMed.
- S. T. Martin, Chem. Rev., 2000, 100, 3403 CrossRef CAS PubMed.
- L. Campbell and M. J. Brunger, Int. Rev. Phys. Chem., 2016, 35, 297 CrossRef CAS.
- F. C. Fehsenfeld, C. J. Howard and A. L. Schmeltekopf, J. Chem. Phys., 1975, 63, 2835 CrossRef CAS.
- N. G. Adams, D. Smith, A. A. Viggiano, J. F. Paulson and M. J. Henchman, J. Chem. Phys., 1986, 84, 6728 CrossRef CAS.
- N. S. Shuman, T. M. Miller and A. A. Viggiano, J. Chem. Phys., 2012, 136, 124307 CrossRef PubMed.
- J. Lengyel, A. Pysanenko, J. Kočišek, V. Poterya, C. C. Pradzynski, T. Zeuch, P. Slavíček and M. Fárník, J. Phys. Chem. Lett., 2012, 3, 3096 CrossRef CAS PubMed.
- J. Lengyel, A. Pysanenko, P. Rubovič and M. Fárník, Eur. Phys. J. D, 2015, 69, 269 CrossRef.
- M. Fárník and V. Poterya, Front. Chem., 2014, 2, 4 Search PubMed.
- J. Lengyel, J. Kočišek, M. Fárník and J. Fedor, J. Phys. Chem. C, 2016, 120, 7397 CAS.
- J. Kočišek, K. Grygoryeva, J. Lengyel, M. Fárník and J. Fedor, Eur. Phys. J. D, 2016, 70, 98 CrossRef.
- O. May, J. Fedor and M. Allan, Phys. Rev. A, 2009, 80, 12706 CrossRef.
- J. Fedor, P. Cicman, B. Coupier, S. Feil, M. Winkler, K. Głuch, J. Husarik, D. Jaksch, B. Farizon, N. J. Mason, P. Scheier and T. D. Märk, J. Phys. B: At., Mol. Opt. Phys., 2006, 39, 3935 CrossRef CAS.
- S. A. Rangwala, E. Krishnakumar and S. V. K. Kumar, Phys. Rev. A, 2003, 68, 52710 CrossRef.
- M. Walker, A. J. A. Harvey, A. Sen and C. E. H. Dessent, J. Phys. Chem. A, 2013, 117, 12590 CrossRef CAS PubMed.
- J. R. Scott and J. B. Wright, J. Phys. Chem. A, 2004, 108, 10578 CrossRef CAS.
- D. J. Goebbert, E. Garand, T. Wende, R. Bergmann, G. Meijer, K. R. Asmis and D. M. Neumark, J. Phys. Chem. A, 2009, 113, 7584 CrossRef CAS PubMed.
- N. Heine, T. I. Yacovitch, F. Schubert, C. Brieger, D. M. Neumark and K. R. Asmis, J. Phys. Chem. A, 2014, 118, 7613 CrossRef CAS PubMed.
- M. Lalitha and L. Senthilkumar, J. Mol. Graphics Modell., 2014, 54, 148 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci and G. A. Petersson, et al., Gaussian 09, Revision D.01, Gaussian, Inc, Wallingford CT, 2013 Search PubMed.
- P. Slavíček, M. Ončák, D. Hollas and O. Svoboda, ABIN, Version 1.0, http://https://github.com/PHOTOX/ABIN, accessed 05.08.2016 Search PubMed.
- E. E. Ferguson and F. Arnold, Acc. Chem. Res., 1981, 14, 327 CrossRef CAS.
- O. Möhler and F. Arnold, J. Atmos. Chem., 1991, 13, 33 CrossRef.
- L. Huey, Int. J. Mass Spectrom. Ion Processes, 1996, 153, 145 CrossRef.
- H. Wincel, E. Mereand and A. W. Castleman, Jr., J. Phys. Chem., 1996, 100, 7488 CrossRef CAS.
- J. Lengyel, C. van der Linde, A. Akhgarnusch and M. K. Beyer, Int. J. Mass Spectrom., 2016 DOI:10.1016/j.ijms.2016.09.023.
- H. Hotop, M.-W. Ruf, M. Allan and I. I. Fabrikant, Adv. At., Mol., Opt. Phys., 2003, 49, 85 CAS.
- R. Janečková, O. May, A. R. Milosavljević and J. Fedor, Int. J. Mass Spectrom., 2014, 365–366, 163 CrossRef.
- B. D. Kay, V. Hermann and A. W. Castleman Jr., Chem. Phys. Lett., 1981, 80, 469 CrossRef CAS.
- P. R. McCurdy, W. P. Hess and S. S. Xantheas, J. Phys. Chem. A, 2002, 106, 7628 CrossRef CAS.
- K. R. Leopold, Annu. Rev. Phys. Chem., 2011, 62, 327 CrossRef CAS PubMed.
- S. Riikonen, P. Parkkinen, L. Halonen and R. B. Gerber, J. Phys. Chem. Lett., 2013, 4, 1850 CrossRef CAS PubMed.
- M. Ončák, P. Slavíček, M. Fárník and U. Buck, J. Phys. Chem. A, 2011, 115, 6155 CrossRef PubMed.
- N. Guggemos, P. Slavíček and V. V. Kresin, Phys. Rev. Lett., 2015, 114, 43401 CrossRef PubMed.
- I. I. Fabrikant, J. Phys. B: At., Mol. Opt. Phys., 2016, 49, 222005 CrossRef.
- J. Lengyel, J. Med, P. Slavíček and M. K. Beyer, 2017, submitted.
- J. F. Paulson and F. Dale, J. Chem. Phys., 1982, 77, 4006 CrossRef CAS.
- A. A. Viggiano and F. Arnold, Planet. Space Sci., 1981, 29, 895 CrossRef CAS.
- H. Heitmann and F. Arnold, Nature, 1983, 306, 747 CrossRef.
- A. A. Viggiano and F. Arnold, Ion Chemistry and Composition of the Atmosphere, CRC Press, Inc., Boca Raton, 1995 Search PubMed.
- F. Arnold, Space Sci. Rev., 2008, 137, 225 CrossRef CAS.
- B. J. Finlayson-Pitts and J. N. Pitts, Chemistry of the Upper and Lower Atmosphere: Theory, Experiments and Applications, Academic Press, San Diego, CA, 2000 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Mass spectrum from electron ionization of H2O/HNO3 complexes, benchmark calculations, and the calculated cluster structures. See DOI: 10.1039/c7cp01205e |
| This journal is © the Owner Societies 2017 |