Change of the isoelectric point of hemoglobin at the air/water interface probed by the orientational flip-flop of water molecules†
Abstract
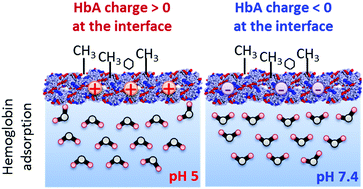
Elucidation of the molecular mechanisms of protein adsorption is of essential importance for further development of biotechnology. Here, we use interface-selective nonlinear vibrational spectroscopy to investigate protein charge at the air/water interface by probing the orientation of interfacial water molecules. We measured the Im χ(2) spectra of hemoglobin, myoglobin, serum albumin and lysozyme at the air/water interface in the CH and OH stretching regions using heterodyne-detected vibrational sum frequency generation (HD-VSFG) spectroscopy, and we deduced the isoelectric point of the protein by monitoring the orientational flip-flop of water molecules at the interface. Strikingly, our measurements indicate that the isoelectric point of hemoglobin is significantly lowered (by about one pH unit) at the air/water interface compared to that in the bulk. This can be predominantly attributed to the modifications of the protein structure at the air/water interface. Our results also suggest that a similar mechanism accounts for the modification of myoglobin charge at the air/water interface. This effect has not been reported for other model proteins at interfaces probed by conventional VSFG techniques, and it emphasizes the importance of the structural modifications of proteins at the interface, which can drastically affect their charge profiles in a protein-specific manner. The direct experimental approach using HD-VSFG can unveil the changes of the isoelectric point of adsorbed proteins at various interfaces, which is of major relevance to many biological applications and sheds new light on the effect of interfaces on protein charge.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...