Single-molecule force spectroscopy of fast reversible bonds
Abstract
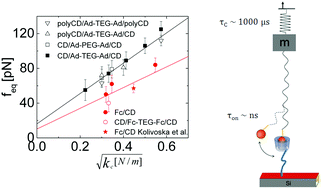
In single-molecule force spectroscopy, the unbinding force is often used to quantify the interaction strength of single molecular bonds. We analyze force spectroscopy of fast reversible bonds probed in thermodynamic equilibrium by considering the dynamics of force probe and molecular linker. The effect of cantilever and linker dynamics is systematically addressed by measuring the unbinding force of single cyclodextrin inclusion complexes by atomic force spectroscopy for a variety of molecular linkers and varying force probe stiffness. The unbinding force of individual bonds probed in thermodynamic equilibrium is not unique for the molecular system but scales with 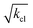 , the square root of the force probe stiffness, and is largely independent of the molecular linker stiffness. The observations are explained by an effective potential resulting from fast linker fluctuations and fast rebinding kinetics which is probed by an AFM cantilever. The slow cantilever dynamics in the kHz range act as mechanical low pass filter, allowing for fast rebinding kinetics of the molecular complex in the order of 106 kHz. The binding energy of the complex can be estimated from the unbinding force as a function of cantilever stiffness, however with some uncertainty arising from lack of a model in three dimensions.
, the square root of the force probe stiffness, and is largely independent of the molecular linker stiffness. The observations are explained by an effective potential resulting from fast linker fluctuations and fast rebinding kinetics which is probed by an AFM cantilever. The slow cantilever dynamics in the kHz range act as mechanical low pass filter, allowing for fast rebinding kinetics of the molecular complex in the order of 106 kHz. The binding energy of the complex can be estimated from the unbinding force as a function of cantilever stiffness, however with some uncertainty arising from lack of a model in three dimensions.


 Please wait while we load your content...
Please wait while we load your content...