Open Access Article
Open Access ArticleIntramolecular dehydration of biomass-derived sugar alcohols in high-temperature water†
Aritomo
Yamaguchi
 *ab,
Natsumi
Muramatsu
a,
Naoki
Mimura
a,
Masayuki
Shirai
ac and
Osamu
Sato
a
*ab,
Natsumi
Muramatsu
a,
Naoki
Mimura
a,
Masayuki
Shirai
ac and
Osamu
Sato
a
aResearch Institute for Chemical Process Technology, National Institute of Advanced Industrial Science and Technology (AIST), 4-2-1 Nigatake, Miyagino, Sendai 983-8551, Japan. E-mail: a.yamaguchi@aist.go.jp; Fax: +81-22-237-5224
bJST, PRESTO, 4-2-1 Nigatake, Miyagino, Sendai 983-8551, Japan
cDepartment of Chemistry and Biological Sciences, Faculty of Science and Engineering, Iwate University, Ueda 4-3-5, Morioka, Iwate 020-8551, Japan
First published on 29th November 2016
Abstract
The intramolecular dehydration of biomass-derived sugar alcohols D-sorbitol, D-mannitol, galactitol, xylitol, ribitol, L-arabitol, erythritol, L-threitol, and DL-threitol was investigated in high-temperature water at 523–573 K without the addition of any acid catalysts. D-Sorbitol and D-mannitol were dehydrated into isosorbide and isomannide, respectively, as dianhydrohexitol products. Galactitol was dehydrated into anhydrogalactitols; however, the anhydrogalactitols could not be dehydrated into dianhydrogalactitol products because of the orientation of the hydroxyl groups at the C-3 and C-6 positions. Pentitols such as xylitol, ribitol, and L-arabitol were dehydrated into anhydropentitols. The dehydration rates of the pentitols containing hydroxyl groups in the trans form, which remained as hydroxyl groups in the product tetrahydrofuran, were larger than those containing hydroxyl groups in the cis form because of the structural hindrance caused by the hydroxyl groups in the cis form during the dehydration process. In the case of the tetritols, the dehydration of erythritol was slower than that of threitol, which could also be explained by the structural hindrance of the hydroxyl groups. The dehydration of L-threitol was faster than that of DL-threitol, which implies that molecular clusters were formed by hydrogen bonding between the sugar alcohols in water, which could be an important factor that affects the dehydration process.
1. Introduction
The production of chemicals from renewable lignocellulosic biomass has attracted much attention because of its potential for creating a sustainable society.1–3 Cellulose and hemicellulose are polysaccharides that are present in lignocellulosic biomass. They can be hydrolyzed into sugars such as glucose and xylose and then hydrogenated into sugar alcohols. Therefore, sugar alcohols such as sorbitol, xylitol, and erythritol are chemicals that can be derived from lignocellulosic biomass.4–6 Sorbitol and xylitol are on the DOE list of top 10 platform chemicals5 and on the list of new top 10 chemicals derived from biomass.2 Fukuoka et al. reported that cellulose could be directly converted into sorbitol by using supported metal catalysts and hydrogen.7 The conversion of cellulose into sorbitol has been further developed by other research groups.8–13 Hemicellulose has also been directly converted into sugar alcohols by using supported metal catalysts and hydrogen through hydrolysis and hydrogenation reactions.14 We reported that cellulose and hemicellulose in lignocellulosic biomass could be directly converted to sugar alcohols such as sorbitol, mannitol, and xylitol without delignification of the biomass.15,16The dehydration of hexitols has attracted attention as a means to provide valuable chemicals from biomass-derived sugar alcohols.17–20 Isosorbide and isomannide, which can be obtained from the dehydration of sorbitol and mannitol, have a rigid structure with two hydroxyl groups; thus, they are promising monomers for the preparation and enhancement of the heat resistance of polyesters and polycarbonates.21 Anhydrohexitol is also a key material for the production of fatty acid esters, which are used as naturally derived surfactants and nontoxic food additives.22,23 Some researchers have reported the dehydration of sorbitol with inorganic acid catalysts such as sulfuric acid and hydrochloric acid at 377–408 K24,25 or with zeolite catalysts.20,26 High-temperature water has attracted much attention as a promising reaction medium for acid-catalyzed reactions.27–29 The self-ionization of water at a high temperature (ca. 523–573 K) is enhanced more than that at ambient temperature, leading to high concentrations of protons and hydroxide ions in high-temperature water.30 We have reported improved green chemistry methods for the dehydration of sorbitol and mannitol in high-temperature water without the addition of any acid catalysts.17,18 It is also important to understand the chemistry of the intramolecular dehydration of other biomass-derived sugar alcohols such as pentitol and tetritol because the resulting products, anhydropentitol and anhydrotetritol, are important chemicals31 for use as surfactants, cosmetics, plastic monomers,32 and raw materials for medicines.33
In this manuscript, we report the intramolecular dehydration of biomass-derived sugar alcohols D-sorbitol, D-mannitol, galactitol, xylitol, ribitol, L-arabitol, erythritol, L-threitol, and DL-threitol in high-temperature water without the addition of any hazardous acid catalysts; furthermore, we investigate the chemistry of this process. We estimate the kinetic parameters of the dehydration reactions and discuss the factors that contribute to the selectivity of the dehydration products. We propose that the conformation of the hydroxyl groups in the tetrahydrofuran ring of the product affects the dehydration rates. The results obtained from the dehydration of L-threitol and DL-threitol indicate that the dehydration rates are also influenced by the formation of clusters of sugar alcohols in water via hydrogen bonding.
2. Experimental
D-Sorbitol, D-mannitol, galactitol, xylitol, ribitol, and erythritol were purchased from Wako Pure Chemical Industries, Ltd; L-arabitol from Tokyo Chemical Industries Co., Ltd; L-threitol from Sigma-Aldrich Co., LLC.; and DL-threitol from Santa Cruz Biotechnology, Inc. All sugar alcohols were used without any further purification.The dehydration of sugar alcohols was performed in a batch reactor (inner volume: 6 cm3) made of a stainless steel 316 tube.17,18 An aqueous solution of the sugar alcohol (3 cm3, 0.5 mol dm−3) was loaded into the reactor and then purged with argon gas to remove the air. For the dehydration of galactitol, 3 cm3 of 0.1 mol dm−3 was used because of its low solubility in water. The reactor was submerged into a molten-salt bath at the desired reaction temperature for a given reaction time and then submerged into a water bath to cool quickly to ambient temperature after the reaction. A mixture of the reactant and products was taken out of the reactor with distilled water, and the solids were separated by filtration.
The quantitative analysis of the unreacted reactant and the liquid products was conducted using high-performance liquid chromatography (HPLC, Shimadzu) equipped with a SUGAR SC1211 column (Shodex) with a refractive index detector (Shimadzu, RID-10A) and a UV-Vis detector (Shimadzu, SPD-20AV). The products were identified by comparing the retention times with those of standard materials: 1,4-anhydro-D-sorbitol (Toronto Research Chemicals, Inc.), 1,5-anhydro-D-sorbitol (Carbosynth, Ltd), 2,5-anhydro-D-sorbitol (Toronto Research Chemicals, Inc.), isosorbide (Alfa Aesar), 1,4-anhydro-D-mannitol (Carbosynth, Ltd), 1,5-anhydro-D-mannitol (Carbosynth Ltd), 2,5-anhydro-D-mannitol (Carbosynth, Ltd), isomannide (Tokyo Chemical Industries Co., Ltd), 1,4-anhydro-D-xylitol (Tokyo Chemical Industries Co., Ltd), and 1,4-anhydro-D-erythritol (Carbosynth, Ltd). The HPLC peaks that could not be identified by comparison with the standard materials were identified by collecting the appropriate fractions using a fraction collector, removing the solvent using a rotary evaporator, and performing 1H and 13C nuclear magnetic resonance (NMR) spectroscopy (Bruker AVANCE III HD 400 MHz) (Fig. S1, ESI†). The products and rate constant for each step are shown in Schemes 1–3 and Schemes S1, S2 in the ESI.† The characteristic properties of the dehydrated product data were previously reported in the literature.24,34–39
3. Results and discussion
3.1. Dehydration of six-carbon sugar alcohols (hexitols)
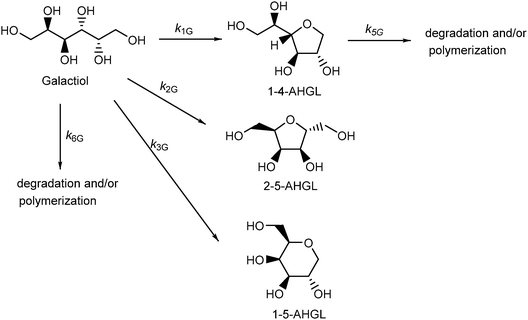
The dehydration reactions of D-sorbitol and D-mannitol in high-temperature water have been described in detail in previous papers.17,18 The reaction schemes are shown in Schemes S1 and S2 (ESI†), and the kinetic parameters for the dehydration reactions are summarized in Tables S1 and S2 (ESI†). Briefly, 1-4-AHSO and 2-5-AHSO were produced by monomolecular dehydration of D-sorbitol in high-temperature water at 523–573 K without any catalysts; the formation rate of 1-4-AHSO was faster than that of the other products formed by monomolecular dehydration. Isosorbide was produced by dehydration of 1-4-AHSO. The major products from the monomolecular dehydration of D-mannitol were 2-5-AHMA and 1-4-AHMA, in contrast to the one major product, 1-4-AHSO, obtained from the monomolecular dehydration of D-sorbitol. Isomannide was produced by the dehydration of 1-4-AHMA; however, the yield of isomannide was lower than that of isosorbide.We have previously reported the intramolecular dehydration of (2R,5R)-(−)-2,5-hexanediol (2R,5R-HDO) into 2,5-dimethyltetrahydrofuran (2,5-DMTHF) in high-temperature water to understand the reaction mechanism of polyalcohol dehydration.29 The cis selectivity of 2,5-DMTHF in the products was high, indicating that the polyalcohol dehydration proceeded mainly via an SN2 substitution process. First, the hydroxyl group at the C-x position is protonated, and then the oxygen atom at the C-y position attacks the C-x carbon and a water molecule is eliminated simultaneously, resulting in the production of a cyclic ether (represented as x-y-AHzz, where zz represents the following two letter codes SO = sorbitol, GL = galactitol, etc.).
The dehydration of galactitol (dulcitol) was performed at 523–573 K in high-temperature water without any acid catalysts. The dehydration of galactitol proceeded in high-temperature water at 523 K to yield 1-4-AHGL and 2-5-AHGL (Scheme 1 and Fig. S2a, ESI†). The possible product 1-5-AHGL was not detected. The formation rates of 1-4-AHGL and 2-5-AHGL increased with increasing reaction temperature (Fig. 1a, b and Fig. S2a, b, ESI†). Further dehydration of 1-4-AHGL did not proceed in high-temperature water at 523–573 K because the hydroxyl groups at the C-3 and C-6 positions were oriented in opposite directions across the tetrahydrofuran ring in the molecular structure of 1-4-AHGL (Fig. S3, ESI†).35,38 In the cases of 1-4-AHSO and 1-4-AHMA, the hydroxyl groups at the C-3 and C-6 positions are oriented in the same direction (Fig. S3, ESI†), thus 1-4-AHSO and 1-4-AHMA could be converted into diols by dehydration between these hydroxyl groups. To understand the kinetics of the galactitol dehydration, the rate constants (k1G, k2G, k3G, k5G, and k6G in Scheme 1) were estimated (Table 1) using linear regression analyses by minimizing the residuals of the data (eqn (S5)–(S8) in the ESI†). The activation energies for the rate constants (k1G, k2G, and k6G) were evaluated from Arrhenius plots (Fig. S4, ESI†) and are shown in Table 1. The rate constant k1G (galactitol to 1-4-AHGL) was the largest, indicating that the dehydration step of galactitol to 1-4-AHGL proceeded faster than the other steps.
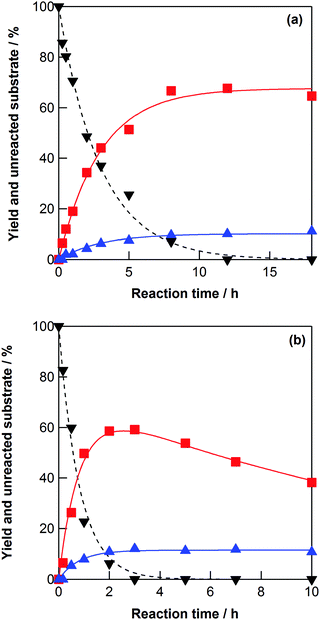 | ||
| Fig. 1 Yields of 1-4-AHGL (■) and 2-5-AHGL (▲), and unreacted galactitol (▼), as a function of elapsed time for the galactitol dehydration reactions at (a) 548 and (b) 573 K in water (initial galactitol concentration: 0.1 mol dm−3). The lines show the best fits of the obtained data to the equations in the ESI† with the kinetic parameters in Table 1. | ||
| Reaction temperature (K) | 523 | 548 | 560 | 573 | Activation energy (kJ mol−1) |
|---|---|---|---|---|---|
| k 3G = 0. | |||||
| k 1G (mol h−1) | 0.049 | 0.23 | 0.50 | 0.84 | 144 |
| k 2G (mol h−1) | 0.0056 | 0.034 | 0.075 | 0.14 | 133 |
| k 5G (mol h−1) | — | — | 0.022 | 0.062 | — |
| k 6G (mol h−1) | 0.017 | 0.075 | 0.13 | 0.24 | 163 |
The selectivities of the 1-4-form and the 2-5-form from the monomolecular dehydration of six-carbon sugar alcohols are represented as 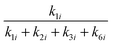 and
and 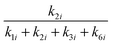 (i = S, M, and G) (Table S3, ESI†), respectively. Table S3 (ESI†) clearly shows that the selectivities of the 1-4-form were much higher than those of the 2-5-form in the case of sorbitol and galactitol dehydration. Conversely, the selectivity of the 2-5-form was higher than that of the 1-4-form in the case of mannitol dehydration. The probable dehydration mechanism is as follows. The hydroxyl groups at the C-2 and C-3 positions in mannitol, which remain as hydroxyl groups in the product tetrahydrofuran ring, are in the cis form during the conversion process from mannitol to 1-4-AHMA; thus, structural hindrance obstructs the reaction pathway to 1-4-AHMA, and the selectivity of 2-5-AHMA was relatively high. Conversely, the hydroxyl groups at the C-2 and C-3 positions in sorbitol and galactitol are in the trans form during the dehydration of sorbitol and galactitol; thus, the major products from monomolecular dehydration were 1-4-AHSO and 1-4-AHGL, indicating that the 1–4-form is the favorable product because of the absence of structural hindrance from the hydroxyl groups at the C-2 and C-3 positions. The selectivities of 2-5-AHSO (0.091–0.19) were slightly higher than those of 2-5-AHGL (0.077–0.11) (Table S3, ESI†). The hydroxyl groups at the C-3 and C-4 positions in galactitol, which remain as hydroxyl groups in the tetrahydrofuran ring of the product, are in the cis form during the conversion process from galactitol to 2-5-AHGL; thus, structural hindrance obstructs the reaction pathway to 2-5-AHGL, and the selectivity of 2-5-AHGL is lower than that of 2-5-AHSO.
(i = S, M, and G) (Table S3, ESI†), respectively. Table S3 (ESI†) clearly shows that the selectivities of the 1-4-form were much higher than those of the 2-5-form in the case of sorbitol and galactitol dehydration. Conversely, the selectivity of the 2-5-form was higher than that of the 1-4-form in the case of mannitol dehydration. The probable dehydration mechanism is as follows. The hydroxyl groups at the C-2 and C-3 positions in mannitol, which remain as hydroxyl groups in the product tetrahydrofuran ring, are in the cis form during the conversion process from mannitol to 1-4-AHMA; thus, structural hindrance obstructs the reaction pathway to 1-4-AHMA, and the selectivity of 2-5-AHMA was relatively high. Conversely, the hydroxyl groups at the C-2 and C-3 positions in sorbitol and galactitol are in the trans form during the dehydration of sorbitol and galactitol; thus, the major products from monomolecular dehydration were 1-4-AHSO and 1-4-AHGL, indicating that the 1–4-form is the favorable product because of the absence of structural hindrance from the hydroxyl groups at the C-2 and C-3 positions. The selectivities of 2-5-AHSO (0.091–0.19) were slightly higher than those of 2-5-AHGL (0.077–0.11) (Table S3, ESI†). The hydroxyl groups at the C-3 and C-4 positions in galactitol, which remain as hydroxyl groups in the tetrahydrofuran ring of the product, are in the cis form during the conversion process from galactitol to 2-5-AHGL; thus, structural hindrance obstructs the reaction pathway to 2-5-AHGL, and the selectivity of 2-5-AHGL is lower than that of 2-5-AHSO.
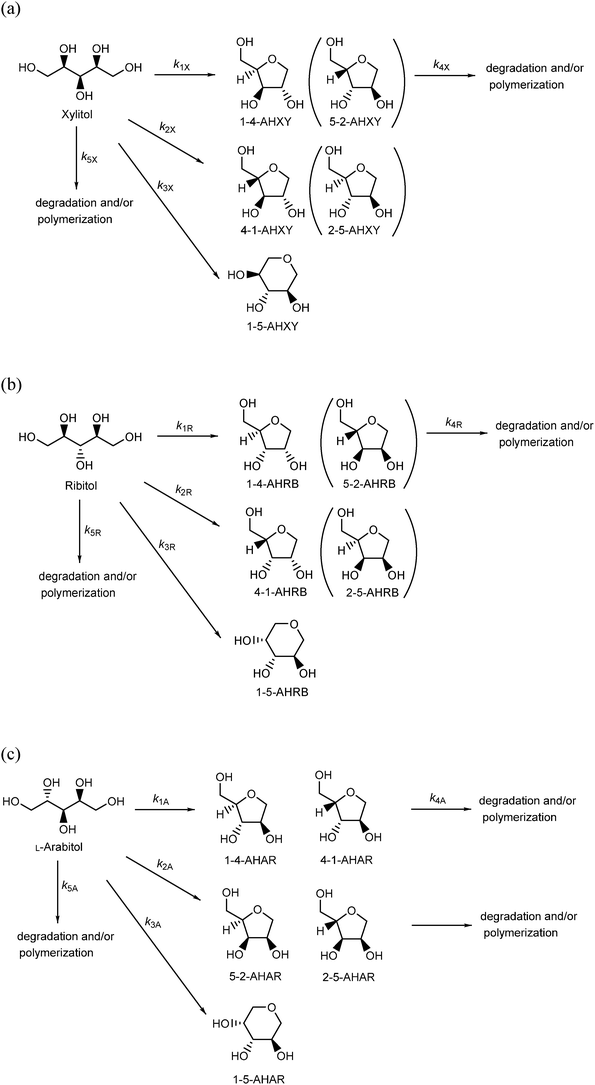
3.2. Dehydration of five-carbon sugar alcohols (pentitols)
The dehydration of xylitol, ribitol, and L-arabitol was investigated in high-temperature water at 523–573 K without any catalysts (Scheme 2). In the case of xylitol dehydration, 1-4-AHXY was the only product (Fig. 2a and Fig. S5, ESI†), indicating that the oxygen atom at the C-4 position attacks the carbon atom (C-1) that has a protonated hydroxyl group. Xylitol is a meso compound (Scheme 2a), therefore 5-2-AHXY can be obtained via an equivalent reaction to form 1-4-AHXY. The measured amount of 1-4-AHXY reported in this manuscript includes 5-2-AHXY, which is an enantiomer of 1-4-AHXY and could not be separated using HPLC. Xylitol was not dehydrated into 4-1-AHXY (or 2-5-AHXY) (Fig. 2a and Fig. S5, ESI†), indicating that the oxygen atom at the terminal carbons (C-1 and C-5) did not attack the carbon atom (C-4 and C-2).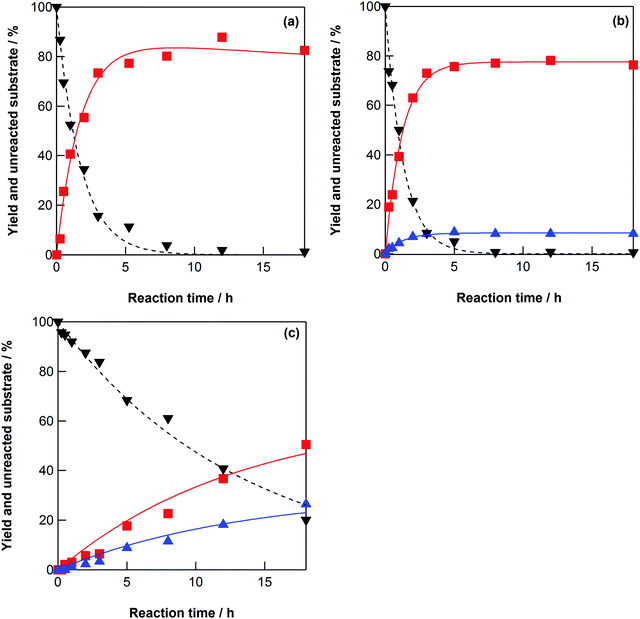 | ||
| Fig. 2 (a) Yield of 1-4-AHXY (■) and unreacted xylitol (▼), (b) yields of 1-4-AHRB (■) and 4-1-AHRB (▲), and unreacted ribitol (▼), and (c) yields of the sum of 1-4-AHAR and 4-1-AHAR (■) and the sum of 2-5-AHAR and 5-2-AHAR (▲), and unreacted L-arabitol (▼) as a function of elapsed time for the xylitol, ribitol, and L-arabitol dehydration reactions at 548 K in water (initial concentration: 0.5 mol dm−3). The lines show the best fits of the obtained data to the equations in the ESI† with the kinetic parameters in Tables 2–4. | ||
Ribitol was dehydrated into 1-4-AHRB (or 5-2-AHRB) and 4-1-AHRB (or 2-5-AHRB) in high-temperature water at 523–573 K without any catalysts (Scheme 2b, Fig. 2b, and Fig. S6, ESI†). Ribitol is also a meso compound and the main dehydration product was 1-4-AHRB, indicating that the oxygen atom at the C-4 or C-2 position attacked the terminal carbon atom (C-1 or C-5, respectively) that has a protonated hydroxyl group. The rate constants for the dehydration of xylitol and ribitol were estimated using the rate constants (Scheme 2a and b) and the rate equations (eqn (S13)–(S16) and (S21)–(S24)) and are summarized in Tables 2 and 3, respectively. The rate constant for the conversion of xylitol into 1-4-AHXY, k1X, was slightly larger than that for the conversion of ribitol into 1-4-AHRB, k1R. The reason for the difference in the rate constants is similar to that for the dehydration of six-carbon sugar alcohols: the hydroxyl groups at the C-2 and C-3 positions in xylitol, which remain as hydroxyl groups in the product tetrahydrofuran, are in the trans form during the conversion process from xylitol to 1-4-AHXY, and the hydroxyl groups at the C-2 and C-3 positions in ribitol are in the cis form during the conversion process from ribitol to 1-4-AHRB; therefore, the structural hindrance during the dehydration of ribitol is larger than that during the dehydration of xylitol.
| Reaction temperature (K) | 523 | 548 | 560 | 573 | Activation energy (kJ mol−1) |
|---|---|---|---|---|---|
| k 2X = 0, k3X = 0. | |||||
| k 1X (mol h−1) | 0.12 | 0.51 | 0.64 | 2.8 | 145 |
| k 4X (mol h−1) | 0.0035 | 0.0041 | 0.008 | 0.077 | 170 |
| k 5X (mol h−1) | 0.015 | 0.077 | 0.12 | 0.56 | 134 |
| Reaction temperature (K) | 523 | 548 | 560 | 573 | Activation energy (kJ mol−1) |
|---|---|---|---|---|---|
| k 3R = 0. | |||||
| k 1R (mol h−1) | 0.12 | 0.61 | 1.3 | 1.9 | 142 |
| k 2R (mol h−1) | 0.010 | 0.065 | 0.15 | 0.20 | 154 |
| k 4R (mol h−1) | 0.00059 | 0.0020 | 0.0073 | 0.022 | 180 |
| k 5R (mol h−1) | 0.017 | 0.096 | 0.24 | 0.25 | 144 |
In the case of the dehydration of L-arabitol, 1-4-AHAR, 4-1-AHAR, 5-2-AHAR, and 2-5-AHAR were produced (Scheme 2c, Fig. 2c, and Fig. S7, ESI†). The molecules of 1-4-AHAR, 4-1-AHAR, 5-2-AHAR, and 2-5-AHAR were the same as 4-1-AHXY, 1-4-AHXY, 4-1-AHRB, and 1-4-AHRB, respectively (Scheme 2). The yields of 1-4-AHAR, 4-1-AHAR, 5-2-AHAR, and 2-5-AHAR from the dehydration of L-arabitol at 548 K are plotted in Fig. S8 (ESI†); 1-4-AHAR was the main product. From Fig. 2c, the sum of the yields of 1-4-AHAR and 4-1-AHAR, in which the hydroxyl groups in the tetrahydrofuran ring of the product were in the trans form, was higher than that of 5-2-AHAR and 2-5-AHAR, in which they were in the cis form. This can be explained by the structural hindrance of the hydroxyl groups and is consistent with the selectivity observed during the dehydration of six-carbon sugar alcohols. The rate constants for the dehydration of L-arabitol were estimated using the rate constants in Scheme 2c and the rate equations (eqn (S29)–(S32)) and are summarized in Table 4. The rate constant for the conversion of L-arabitol into 1-4-AHAR and 4-1-AHAR, k1A, was much lower than those for the dehydration of xylitol and ribitol, k1X and k1R (Tables 2 and 3), which is consistent with the result that the consumption of L-arabitol (Fig. 2c) was much slower than that of xylitol and ribitol (Fig. 2a and b) at 548 K in water. The reason for the difference in the rate constants between L-arabitol, xylitol, and ribitol is still unclear. One possible reason is that molecular clusters of sugar alcohols are formed in water via hydrogen bonding40,41 and affect the dehydration rate. If the hydrogen bonds in the molecular clusters are strong, the dehydration rate is slow because of the energy required to cleave the hydrogen bonds. The formation of clusters with strong hydrogen bonding could explain the lower rate constant of L-arabitol. We discuss the formation of molecular clusters in water in Section 3.3.
| Reaction temperature (K) | 523 | 548 | 560 | 573 | Activation energy (kJ mol−1) |
|---|---|---|---|---|---|
| k 3A = 0, k4A = 0. | |||||
| k 1A (mol h−1) | 0.0084 | 0.047 | 0.078 | 0.15 | 144 |
| k 2A (mol h−1) | 0.0039 | 0.023 | 0.041 | 0.077 | 149 |
| k 5A (mol h−1) | 0.0024 | 0.0039 | 0.0059 | 0.0083 | 61 |
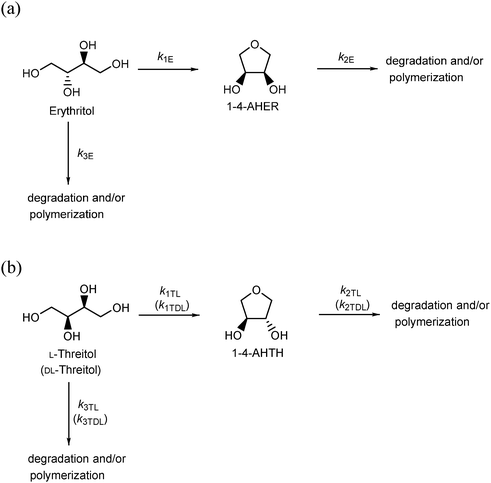
3.3. Dehydration of four-carbon sugar alcohols (tetritols)
The dehydration of four-carbon sugar alcohols, such as erythritol, L-threitol, and DL-threitol, was investigated in high-temperature water at 523–573 K without any catalysts (Scheme 3). Erythritol was dehydrated into 1-4-AHER, which is cis-3,4-tetrahydrofurandiol (Scheme 3a, Fig. 3a, and Fig. S9, ESI†). Both L-threitol and DL-threitol were dehydrated into 1-4-AHTH, which is trans-3,4-tetrahydrofurandiol (Scheme 3b, Fig. 3b, c and Fig. S10, S11, ESI†). The rate constants for the dehydration of erythritol, L-threitol, and DL-threitol were estimated using the rate constants in Scheme 3 and the rate equations (eqn (S35), (S36), (S39), (S40), (S43), and (S44)) and are summarized in Tables 5 and 6, respectively. The order of the rate constants for the dehydration was L-threitol (k1TL) > DL-threitol (k1TDL) > erythritol (k1E). The erythritol dehydration is slower than the threitol dehydration, which can be explained by the structural hindrance of the hydroxyl groups at the C-2 and C-3 positions. This is consistent with the results of the dehydration of six-carbon and five-carbon sugar alcohols. The L-threitol dehydration is faster than the DL-threitol dehydration. The physicochemical properties of enantiomers such as L-threitol and D-threitol should be the same. If the dehydration reaction of L-threitol or D-threitol proceeds without any interaction with the other chiral molecules, the rate constants of L-threitol and DL-threitol should be the same. However, the rate constant of L-threitol was larger than that of DL-threitol, indicating that the dehydration reaction of L-threitol or D-threitol proceeds with an interaction with the other chiral molecule. We can explain this result by considering the formation of molecular clusters between the sugar alcohols in water via hydrogen bonding, which we have discussed in Section 3.2. The DL-threitol dehydration is slower than the L-threitol dehydration, which indicates that D-threitol and L-threitol form clusters with each other with stronger hydrogen bonds than the clusters formed by only D-threitol. In other words, the interaction of the hydrogen bonds between a “right-hand” molecule and a “left-hand” molecule is larger than that between “right-hand” molecules. We do not have any direct evidence for the formation of these molecular clusters by hydrogen bonding; thus, computational simulation of this system should be carried out to evaluate this possibility.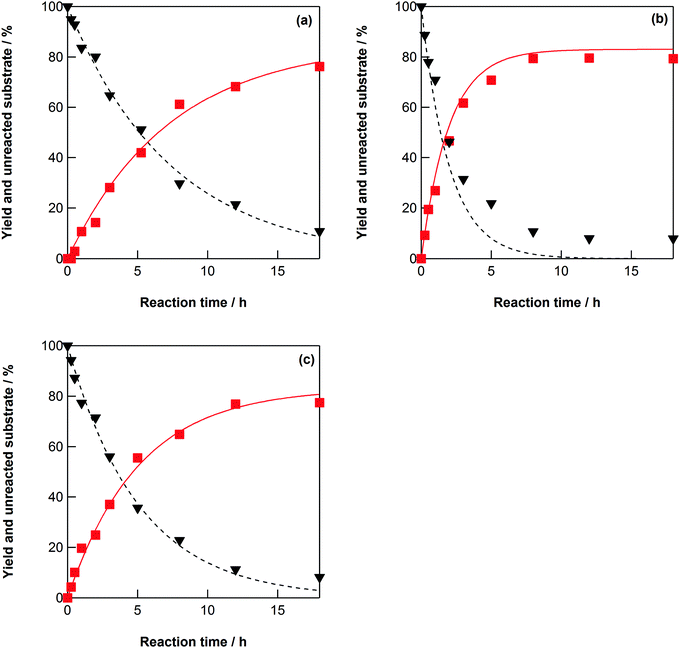 | ||
| Fig. 3 (a) Yield of 1-4-AHER (■) and unreacted erythritol (▼), (b) yield of 1-4-AHTH (■) and unreacted L-threitol (▼), and (c) yield of 1-4-AHTH (■) and unreacted DL-threitol (▼) as a function of elapsed time for the erythritol, L-threitol, and DL-threitol dehydration reactions at 548 K in water (initial concentration: 0.5 mol dm−3). The lines show the best fits of the obtained data to the equations in the ESI† with the kinetic parameters in Tables 5 and 6. | ||
| Reaction temperature (K) | 523 | 548 | 560 | 573 | Activation energy (kJ mol−1) |
|---|---|---|---|---|---|
| k 1E (mol h−1) | 0.027 | 0.12 | 0.24 | 0.44 | 141 |
| k 2E (mol h−1) | — | — | 0.014 | 0.023 | — |
| k 3E (mol h−1) | 0.0045 | 0.020 | 0.043 | 0.097 | 152 |
| Reaction temperature (K) | 523 | 548 | 560 | 573 | Activation energy (kJ mol−1) |
|---|---|---|---|---|---|
| k 1TL (mol h−1) | 0.073 | 0.42 | 0.91 | 4.1 | 193 |
| k 2TL (mol h−1) | — | — | — | 0.025 | — |
| k 3TL (mol h−1) | 0.018 | 0.085 | 0.20 | 0.94 | 190 |
| k 1TDL (mol h−1) | 0.036 | 0.16 | 0.29 | 0.80 | 150 |
| k 2TDL (mol h−1) | — | — | — | 0.011 | — |
| k 3TDL (mol h−1) | 0.0064 | 0.033 | 0.060 | 0.19 | 165 |
4. Conclusions
The intramolecular dehydration of biomass-derived sugar alcohols D-sorbitol, D-mannitol, galactitol, xylitol, ribitol, L-arabitol, erythritol, L-threitol, and DL-threitol was carried out in high-temperature water at 523–573 K without the addition of any acid catalysts. We estimated the kinetic parameters of the dehydration reactions. D-Sorbitol and D-mannitol were dehydrated into anhydrohexitols and then dehydrated into isosorbide and isomannide, respectively, as dianhydrohexitol products. Conversely, galactitol was dehydrated into anhydrogalactitols; however, the anhydrogalactitols could not be dehydrated into dianhydrogalactitol products because the hydroxyl groups at the C-3 and C-6 positions were oriented in the opposite directions across the tetrahydrofuran ring. Pentitols such as xylitol, ribitol, and L-arabitol were dehydrated into several types of anhydropentitols. The dehydration rates of the compounds containing hydroxyl groups in the trans form, which remained as hydroxyl groups in the product tetrahydrofuran, were larger than those containing hydroxyl groups in the cis form because of the structural hindrance during the dehydration process. In the case of tetritol dehydration, the order of the rate constants for the dehydration was L-threitol (k1TL) > DL-threitol (k1TDL) > erythritol (k1E). The dehydration of erythritol was slower than the dehydration of threitol, which could be explained by the structural hindrance of the hydroxyl groups. The rate constant for the dehydration of L-threitol is larger than that of DL-threitol, indicating that the dehydration reaction of L-threitol or D-threitol proceeds with an interaction with the other chiral molecule. We propose that molecular clusters formed between the sugar alcohols in water via hydrogen bonding affect the effect the dehydration.References
- G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106, 4044–4098 CrossRef CAS PubMed.
- J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539–554 RSC.
- C. O. Tuck, E. Pérez, I. T. Horváth, R. A. Sheldon and M. Poliakoff, Science, 2012, 337, 695–699 CrossRef CAS PubMed.
- R. D. Cortright, R. R. Davda and J. A. Dumesic, Nature, 2002, 418, 964–967 CrossRef CAS PubMed.
- PNNL, Top value added chemicals from biomass. Volume I: Results of screening for potential candidates from sugars and synthesis gas, Report Report, Pacific Northwest National Laboratory (PNNL) and National Renewable Energy Laboratory (NREL), 2004.
- N. Yan, C. Zhao, C. Luo, P. J. Dyson, H. Liu and Y. Kou, J. Am. Chem. Soc., 2006, 128, 8714–8715 CrossRef CAS PubMed.
- A. Fukuoka and P. L. Dhepe, Angew. Chem., Int. Ed., 2006, 45, 5161–5163 CrossRef CAS PubMed.
- C. Luo, S. Wang and H. Liu, Angew. Chem., Int. Ed., 2007, 46, 7636–7639 CrossRef CAS PubMed.
- L.-N. Ding, A.-Q. Wang, M.-Y. Zheng and T. Zhang, ChemSusChem, 2010, 3, 818–821 CrossRef CAS PubMed.
- S. Van de Vyver, J. Geboers, M. Dusselier, H. Schepers, T. Vosch, L. Zhang, G. Van Tendeloo, P. A. Jacobs and B. F. Sels, ChemSusChem, 2010, 3, 698–701 CrossRef CAS PubMed.
- H. Kobayashi, Y. Ito, T. Komanoya, Y. Hosaka, P. L. Dhepe, K. Kasai, K. Hara and A. Fukuoka, Green Chem., 2011, 13, 326–333 RSC.
- A. M. Liu, K. Hidajat, S. Kawi and D. Y. Zhao, Chem. Commun., 2000, 1145–1146 RSC.
- S. Van de Vyver, J. Geboers, W. Schutyser, M. Dusselier, P. Eloy, E. Dornez, J. W. Seo, C. M. Courtin, E. M. Gaigneaux, P. A. Jacobs and B. F. Sels, ChemSusChem, 2012, 5, 1549–1558 CrossRef CAS PubMed.
- S. K. Guha, H. Kobayashi, K. Hara, H. Kikuchi, T. Aritsuka and A. Fukuoka, Catal. Commun., 2011, 12, 980–983 CrossRef CAS.
- A. Yamaguchi, O. Sato, N. Mimura, Y. Hirosaki, H. Kobayashi, A. Fukuoka and M. Shirai, Catal. Commun., 2014, 54, 22–26 CrossRef CAS.
- A. Yamaguchi, O. Sato, N. Mimura and M. Shirai, Catal. Today, 2016, 265, 199–202 CrossRef CAS.
- A. Yamaguchi, N. Hiyoshi, O. Sato and M. Shirai, Green Chem., 2011, 13, 873–881 RSC.
- A. Yamaguchi, O. Sato, N. Mimura and M. Shirai, RSC Adv., 2014, 4, 45575–45578 RSC.
- Y. Morita, S. Furusato, A. Takagaki, S. Hayashi, R. Kikuchi and S. T. Oyama, ChemSusChem, 2014, 7, 748–752 CrossRef CAS PubMed.
- H. Kobayashi, H. Yokoyama, B. Feng and A. Fukuoka, Green Chem., 2015, 17, 2732–2735 RSC.
- F. Fenouillot, A. Rousseau, G. Colomines, R. Saint-Loup and J.-P. Pascault, Prog. Polym. Sci., 2010, 35, 578–622 CrossRef CAS.
- H. Kobayashi and A. Fukuoka, Green Chem., 2013, 15, 1740–1763 RSC.
- M. Yabushita, H. Kobayashi, A. Shrotri, K. Hara, S. Ito and A. Fukuoka, Bull. Chem. Soc. Jpn., 2015, 88, 996–1002 CrossRef CAS.
- K. Bock, C. Pedersen and H. Thogersen, Acta Chem. Scand., Ser. B, 1981, 35, 441–449 CrossRef.
- G. Fleche and M. Huchette, Starch, 1986, 38, 26–30 CrossRef CAS.
- R. Otomo, T. Yokoi and T. Tatsumi, Appl. Catal., A, 2015, 505, 28–35 CrossRef CAS.
- P. E. Savage, Chem. Rev., 1999, 99, 603–622 CrossRef CAS PubMed.
- A. Yamaguchi, N. Hiyoshi, O. Sato, K. K. Bando and M. Shirai, Green Chem., 2009, 11, 48–52 RSC.
- A. Yamaguchi, N. Hiyoshi, O. Sato and M. Shirai, ACS Catal., 2011, 1, 67–69 CrossRef CAS.
- N. Akiya and P. E. Savage, Chem. Rev., 2002, 102, 2725–2750 CrossRef CAS PubMed.
- K. G. Childers, S. D. Dreher, J. Lee and J. M. Williams, Org. Process Res. Dev., 2006, 10, 934–936 CrossRef CAS.
- S. Selifonov, F. Jing and N. Zhou, WO2010138842, 2010.
- M. E. Jung and O. Kretschik, J. Org. Chem., 1998, 63, 2975–2981 CrossRef CAS.
- C. Montassier, J. C. Ménézo, J. Moukolo, J. Naja, L. C. Hoang and J. Barbier, J. Mol. Catal., 1991, 70, 65–84 CrossRef CAS.
- M. Kurszewska, E. Skorupowa, J. Madaj, A. Konitz, W. Wojnowski and A. Wiśniewski, Carbohydr. Res., 2002, 337, 1261–1268 CrossRef CAS PubMed.
- M. Kurszewska, E. Skorupowa, J. Madaj and A. Wiśniewski, J. Carbohydr. Chem., 2004, 23, 169–177 CrossRef CAS.
- R. Barker, J. Org. Chem., 1970, 35, 461–464 CrossRef CAS.
- A. Wisniewski, E. Skorupowa and J. Sokolowski, J. Carbohydr. Chem., 1991, 10, 77–90 CrossRef CAS.
- M. Kurszewska, E. Skorupa, R. Kasprzykowska, P. Sowiński and A. Wiśniewski, Carbohydr. Res., 2000, 326, 241–249 CrossRef CAS PubMed.
- R. A. Provencal, R. N. Casaes, K. Roth, J. B. Paul, C. N. Chapo and R. J. Saykally, J. Phys. Chem. A, 2000, 104, 1423–1429 CrossRef CAS.
- R. K. Lam, J. W. Smith and R. J. Saykally, J. Chem. Phys., 2016, 144, 191103 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cp06831f |
| This journal is © the Owner Societies 2017 |