Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: High-melting-point crystals of poly(L-lactic acid) (PLLA): the most efficient nucleating agent to enhance the crystallization of PLLA
Hai-Yan
Yin
a,
Xin-Feng
Wei
a,
Rui-Ying
Bao
*a,
Quan-Xiao
Dong
b,
Zheng-Ying
Liu
a,
Wei
Yang
*a,
Bang-Hu
Xie
a and
Ming-Bo
Yang
a
aState Key Laboratory of Polymer Materials Engineering, College of Polymer Science and Engineering, Sichuan University, Chengdu, 610065 Sichuan, China. E-mail: baoruiying2005@126.com; weiyang@scu.edu.cn
bBeijing Engineering Research Center of Architectural Functional Macromolecular Materials, Beijing Building Construction Research Institute, Beijing 100039, People's Republic of China
First published on 10th March 2017
Abstract
Correction for ‘High-melting-point crystals of poly(L-lactic acid) (PLLA): the most efficient nucleating agent to enhance the crystallization of PLLA’ by Hai-Yan Yin et al., CrystEngComm, 2015, 17, 2310–2320.
We reported that high-melting-point crystals of PLLA and PDLA can act as efficient nucleating agents to enhance the crystallization of PLLA in our previous publications (ACS Sustainable Chem. Eng., 2015, 3, 654–661; CrystEngComm, 2015, 17, 2310–2320; CrystEngComm, 2015, 17, 4334–4342), and a possible nucleation process was shown in Fig. 10 of the article CrystEngComm, 2015, 17, 2310–2320.
Inspired by the work conducted by Wittmann, Lotz et al.,1,2 who introduced the polymer decoration technique, we now think that the nucleation via a template of the fold surfaces we proposed and the drawing we made are misleading.
The following corrections to the published version of CrystEngComm, 2015, 17, 2310–2320 are required:
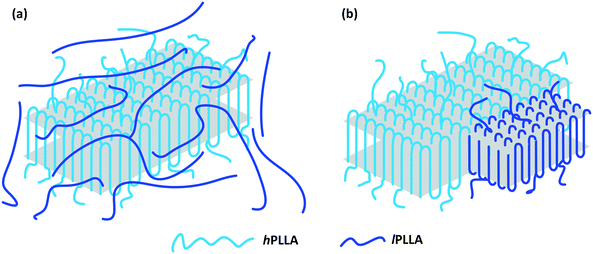
(1) Fig. 10 and its caption on page 2318 should be:
(2) The last paragraph of the “Results and discussion” section on page 2318 and 2319 should be:
Based on the above discussion, it is clear that owing to the similarity of crystal structure between the α-form hPLLA crystallites and lPLLA, nucleation can be induced by the lateral crystalline growth edges of the hPLLA crystallites. As is known, for polymer crystals, the chains are partially crystalline and partially amorphous. It has been established that lPLLA and hPLLA chains are completely miscible due to their identical chemical composition. Therefore, as shown in Fig. 10a, hPLLA crystallites are well dispersed in the molten lPLLA matrix before crystallization. Owing to the same crystal structure, the lPLLA matrix finds a crystalline substrate that is exactly the one it wants to make. The lPLLA chains deposit on the growth front of the hPLLA crystallites, and nucleation is induced by the lateral crystalline, growth edges of the hPLLA crystallites. Then lamellae of lPLLA form rapidly (as shown in Fig. 10b). During this surface induced crystallization process, the complete miscibility i.e., a strong interfacial interaction, between the amorphous chains of the hPLLA crystallites and lPLLA matrix, and the identical crystal structure between the α-form hPLLA crystallites and lPLLA matrix sharply reduce the energy barrier for heterogeneous nucleation.
The authors would like to apologize for this oversight and for any confusion that has arisen as a result.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
References
- J. C. Wittmann and B. Lotz, J. Polym. Sci., Part B: Polym. Phys., 1985, 23, 205–226 CrossRef CAS.
- J. H. Chen, S. Z. D. Cheng, S. S. Wu, B. Lotz and J. C. Wittmann, J. Polym. Sci., Part B: Polym. Phys., 1995, 33, 1851–1855 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |