Open Access Article
Open Access ArticleA series of Cd(II) coordination polymers based on flexible bis(triazole) and multicarboxylate ligands: topological diversity, entanglement and properties†
Ke
Li
 a,
Vladislav A.
Blatov
a,
Vladislav A.
Blatov
 bc,
Tao
Fan
c,
Tian-Rui
Zheng
a,
Ya-Qian
Zhang
a,
Bao-Long
Li
bc,
Tao
Fan
c,
Tian-Rui
Zheng
a,
Ya-Qian
Zhang
a,
Bao-Long
Li
 *a and
Bing
Wu
a
*a and
Bing
Wu
a
aState and Local Joint Engineering Laboratory for Functional Polymeric Materials, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, PR China. E-mail: libaolong@suda.edu.cn
bSamara Center for Theoretical Materials Science (SCTMS), Samara University, Ac. Pavlov St. 1, Samara 443011, Russia
cSchool of Materials Science and Engineering, Northwestern Polytechnical University, Xi'an, Shaanxi 710072, PR China
First published on 11th September 2017
Abstract
Eight Cd(II) coordination polymers, {[Cd(btrb)(H2O)4][Cd2(btrb)(btec)(H2O)2]2(OH)2·5H2O}n (1·(OH)2·5H2O), {[Cd(btrb)0.5(bptc)0.5]·H2O}n (2·H2O), {[Cd3(btrb)(btc)2]·3H2O}n (3·3H2O), {[Cd(btrb)(ip)(H2O)]·H2O}n (4), {[Cd(btrb)(MeOip)]·H2O}n (5), {[Cd2(btrb)2(1,2-bdc)2]·3H2O}n (6), {[Cd2(btrb)(bpdc)2]2·11H2O}n (7) and {[Cd(btrb)(bpdc)]·H2O}n (8), were synthesized under hydrothermal conditions. 1 shows an unprecedented (4,4,4,4)-connected 3D (electroneutral motif) + 1D (cationic motif) → 3D polythreaded structure. The point symbol of the new 3D motif is (46)2(86)(42·82·102)(43·83)4, or the second mot topology based on the [Cd2(COO)] dimer. 2 shows an unprecedented (4,5,6)-connected 3D network with a point symbol of (42·84)(47·63)2(46·66·83). 3 shows an unprecedented (3,8)-connected self-catenated 3D network based on the Cd(II) trimer cluster [Cd3(COO)2] with a point symbol of (42·6)2(44·611·76·86·9). 4 exhibits a 2D + 2D → 2D interpenetration network based on the 2D sql network. 5 exhibits a 4-connected network with a cds topology. 6 presents the first example of a 2-fold interpenetrating 6-connected cco-6-Pbcm 3D network with a point symbol of (47·68). 7 shows a (3,3,4,4)-connected 2D 3,3,4,4L72 network with a point symbol of (3·4·5)(4·82)(3·4·5·83)(3·4·82·92). 8 presents a (3,5)-connected self-catenated 2D 3,5L2 network with a point symbol of (42·6)(42·67·8). The luminescence and thermal stability of 1–8 were investigated.
Introduction
The synthesis of coordination polymers (CPs) with specific structures is still a great challenge, and has been a hot topic in chemistry for decades due to not only the interesting topologies, but also the multitudinous properties for functional materials in various fields, such as gas adsorption and storage, catalysis, sensors, luminescent materials and so on.1–6 The well-known TOPOS topological databases contain more than 180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 types of topology and more than 1
000 types of topology and more than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700
700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 examples of their occurrences in crystal structures.7,8 Topological analysis of crystal structures can help us better understand the structural features of coordination polymers and allow us to compare them with other coordination polymers. In order to pre-design the synthesis of coordination polymers with specific structures, a good understanding of the structural features of the coordination polymers is necessary.
000 examples of their occurrences in crystal structures.7,8 Topological analysis of crystal structures can help us better understand the structural features of coordination polymers and allow us to compare them with other coordination polymers. In order to pre-design the synthesis of coordination polymers with specific structures, a good understanding of the structural features of the coordination polymers is necessary.
The entanglements of coordination polymers are particularly intriguing because of the presence of periodic entanglements, in which independent motifs are entangled together in different modes. Entangled networks can be classified as interpenetrated, polycatenated, polythreaded or polyknotted.9–12 Interpenetrated and polycatenated networks are the two most common entangled systems, but self-catenated coordination networks are uncommon and less exploited.9,10 Polythreaded structures are characterized by the presence of closed loops, as well as elements that can thread through the loops, and can be considered as extended periodic analogues of molecular rotaxanes and pseudorotaxanes.11,12 The few polythreaded species include 0D → 1D or 2D, 1D → 2D or 1D → 3D, 1D + 2D → 2D, 1D + 2D → 3D, 2D → 3D, and 1D + 3D → 3D.11,12 Polythreaded structures with 3D components are really rare.12
The assembly of CPs is controlled by numerous factors under hydrothermal conditions, such as temperature, the ratio of materials, ligands, pH, solvent and other factors.13 The carboxyl group has flexible coordination modes with metal ions. The various coordination modes of the carboxyl group can be divided into three modes: monodentate, bridging and chelating. 1-Substituted 1,2,4-triazole ligands are widely used in the construction of CPs or MOFs, while 4-substituted 1,2,4-triazole ligands are still less used. The 1,2-nitrogen atoms of 4-substituted 1,2,4-triazole ligands make them strong σ-donors when coordinating with one or two metal ions. The co-ligand (N-donor and carboxylate ligands) system is the most popular way to design, synthesize and regulate CPs and MOFs.14 The combination of multicarboxylate and 1,2,4-triazole co-ligands is a very effective method in the construction and regulation of CPs. Our group focuses on the design and synthesis of novel CPs and MOFs with intriguing topologies and interesting properties, using multicarboxylate ligands and imidazole or triazole co-ligands.15 For example, three MOFs based on a [Co4(μ3-OH)2] cluster were synthesized and connected numbers of [Co4(μ3-OH)2] clusters from 4 to 6 and then 8 were successfully regulated.15d In this work, we focus on the construction and regulation of Cd(II) CPs with intriguing topologies and interesting properties using the 4-substituted bis(1,2,4-triazole) ligand 1,4-bis(1,2,4-triazol-4-ylmethyl)benzene (btrb) and multicarboxylate co-ligands. Eight Cd(II) CPs, {[Cd(btrb)(H2O)4][Cd2(btrb)(btec)(H2O)2]2(OH)2·5H2O}n (1·(OH)2·5H2O), {[Cd(btrb)0.5(bptc)0.5]·H2O}n (2·H2O), {[Cd3(btrb)(btc)2]·3H2O}n (3·3H2O), {[Cd(btrb)(ip)(H2O)]·H2O}n (4), {[Cd(btrb)(MeOip)]·H2O}n (5), {[Cd2(btrb)2(1,2-bdc)2]·3H2O}n (6), {[Cd2(btrb)(bpdc)2]2·11H2O}n (7) and {[Cd(btrb)(bpdc)]·H2O}n (8), were synthesized under hydrothermal conditions (btrb = 1,4-bis(1,2,4-triazol-4-ylmethyl)benzene, H4btec = 1,2,4,5-benzene tetracarboxylic acid, H4bptc = (1,1′-biphenyl)tetracarboxylic acid, H3btc = 1,3,5-benzenetricarboxylic acid, H2ip = isophthalic acid, H2MeOip = 4-methoxyl-isophthalic acid, 1,2-H2bdc = phthalic acid, H2bpdc = (1,1′-biphenyl)-2,2′-dicarboxylic acid). 1 presents an unprecedented (4,4,4,4)-connected 3D (electroneutral motif) + 1D (cationic motif) → 3D polythreaded network with a bli1 topology, where the point symbol of the 3D motif is (46)2(86)(42·82·102)(43·83)4, or a second mot topology based on the [Cd2(COO)] dimer. 2 shows an unprecedented 3D (4,5,6)-connected bli2 topology with a point symbol of (42·84)(47·63)2(46·66·83). 3 shows an unprecedented 3D (3,8)-connected bli3 topology based on the Cd(II) trimer cluster [Cd3(COO)2] with a point symbol of (42·6)2(44·611·76·86·9). 4–8 show topologies devisable by the regulation of the multicarboxylate ligands. The luminescence and thermal stability were investigated.
Experimental
Materials and methods
1,4-Bis(1,2,4-triazol-4-ylmethyl)benzene (btrb) was synthesized according to the reported method.16 All reagents were purchased and used without further purification. Elemental analyses for C, H and N were performed on a Perkin-Elmer 240C analyzer. FT-IR spectra were obtained on a Bruker VERTEX 70 FT-IR spectrophotometer in the 4000–600 cm−1 region. Powder X-ray diffraction (PXRD) was performed on a D/MAX-3C diffractometer with Cu-Kα radiation (λ = 1.5406 Å) at room temperature. Thermogravimetric analysis (TGA) was carried out using a Thermal Analyst 2100 TA Instrument and SDT 2960 Simultaneous TGA-DTA Instrument with nitrogen flow at a heating rate of 10 °C min−1. The luminescence measurements were carried out in the solid state at room temperature and the spectra were collected on a PerkinElmer LS50B spectrofluorimeter.Synthesis of {[Cd(btrb)(H2O)4][Cd2(btrb)(btec)(H2O)2]2(OH)2·5H2O}n (1·(OH)2·5H2O)
A mixture of H4btec (0.04 mmol, 0.010 g), CdSO4·8/3H2O (0.06 mmol, 0.015 g) in 7.5 mL H2O and btrb (0.4 mmol, 0.096 g) in 0.4 mL DMF was adjusted to pH = 8 with dilute NaOH solution. The mixture was added to a 25 mL Teflon-lined stainless autoclave, sealed and then heated at 110 °C for 3 days, then left for one month at room temperature to give colourless block crystals of 1·(OH)2·5H2O (yield: 0.014 g, 34% based on H4btec). Anal. calc. for C56H68Cd5N18O31 (1·(OH)2·5H2O): C, 32.79; H, 3.34; N, 12.29%; found: C, 32.70; H, 3.41; N, 12.19%. IR (cm−1): 3370 (m), 1580 (s), 1538 (vs), 1489 (m), 1423 (m), 1371 (vs), 1207 (w), 1079 (w), 995 (w), 873 (w), 815 (w), 743 (w), 625 (m).Synthesis of {[Cd(btrb)0.5(bptc)0.5]·H2O}n (2·H2O)
A mixture of H4bptc (0.025 mmol, 0.008 g), CdSO4·8/3H2O (0.05 mmol, 0.013 g) in 7.5 mL H2O and btrb (0.05 mmol, 0.012 g) in 0.5 mL DMF was adjusted to pH = 7 with dilute NaOH solution. The mixture was added to a 25 mL Teflon-lined stainless autoclave, sealed and then heated at 90 °C for 3 days, then cooled to room temperature. Colourless stick crystals of 2·H2O were obtained (yield: 0.017 g, 42% based on btrb). Anal. calc. for C14H11CdN3O5(2·H2O): C, 40.65; H, 2.68; N, 10.16%; found: C, 41.39; H, 2.57; N, 10.32%. IR (cm−1): 3389 (w), 1585 (vs), 1539 (s), 1361 (vs), 1159 (s), 1067 (m), 1022 (m), 864 (vs), 831 (s), 791 (s), 727 (s), 667 (s), 627 (vs).Synthesis of {[Cd3(btrb)(btc)2]·3H2O}n (3·3H2O)
The synthesis procedure for 3 is similar to that for 2, except that H3btc (0.04 mmol, 0.008 g) was used instead of H4bptc. Colourless block crystals of 3·3H2O were obtained (yield: 0.014 g, 27% based on btrb). Anal. calc. for C30H24Cd3N6O15 (3·3H2O): C, 34.45; H, 2.31; N, 8.03%; found: C, 34.61; H, 2.31; N, 7.96%. IR (cm−1): 3369 (m), 3102 (w), 1612 (s), 1526 (s), 1434 (s), 1369 (vs), 1213 (w), 1108 (w), 1071 (w), 1028 (w), 765 (s), 720 (vs), 633 (s).Synthesis of {[Cd(btrb)(ip)(H2O)]·H2O}n (4)
The synthesis procedure for 4 is similar to that for 2, except that H2ip (0.05 mmol, 0.008 g) was used instead of H4bptc. Colourless block crystals of 4 were obtained (yield: 0.022 g, 80% based on btrb). Anal. calc. for C20H20CdN6O6 (4): C, 43.41; H, 3.62; N, 15.20%; found: C, 43.24; H, 3.73; N, 15.11%. IR (cm−1): 3324 (m), 3122 (w), 1655 (w), 1609 (m), 1542 (s), 1371 (s), 1181 (m), 1075 (m), 1010 (m), 764 (m), 723 (s), 628 (s).Synthesis of {[Cd(btrb)(MeOip)]·H2O}n (5)
The synthesis procedure for 5 is similar to that for 2, except that H2MeOip (0.05 mmol, 0.010 g) was used instead of H4bptc. Colourless block crystals of 5 were obtained (yield: 0.018 g, 64% based on btrb). Anal. calc. for C21H20CdN6O6 (5): C, 44.62; H, 3.54; N, 14.87%; found: C, 44.41; H, 3.67; N, 14.69%. IR (cm−1): 3421 (m), 3116 (w), 1611 (m), 1562 (s), 1449 (w), 1420 (m), 1377 (s), 1258 (s), 1188 (w), 1136 (w), 1076 (m), 1019 (m), 897 (w), 864 (w), 835 (w), 782 (m), 716 (s), 684 (m), 638 (vs).Synthesis of {[Cd2(btrb)2(1,2-bdc)2]·3H2O}n (6)
The synthesis procedure for 6 is similar to that for 2, except that 1,2-H2bdc (0.05 mmol, 0.008 g) was used instead of H4bptc. Colourless block crystals of 6 were obtained (yield: 0.024 g, 88% based on btrb). Anal. calc. for C40H38Cd2N12O11 (6): C, 44.13; H, 3.49; N, 15.45%; found: C, 43.95; H, 3.62; N, 15.33%. IR (cm−1): 3320 (m), 3110 (w), 1690 (w), 1538 (vs), 1442 (w), 1363 (vs), 1212 (vs), 1161 (m), 1076 (m), 1016 (m), 887 (m), 859 (m), 830 (m), 754 (s), 710 (s), 633 (s).Synthesis of {[Cd2(btrb)(bpdc)2]2·11H2O}n (7)
The synthesis procedure for 7 is similar to that for 2, except that H2bpdc (0.05 mmol, 0.012 g) was used instead of H4bptc. Colourless block crystals of 7 were obtained (yield: 0.024 g, 46% based on btrb). Anal. calc. for C80H78Cd4N12O27 (7): C, 45.99; H, 3.76; N, 8.05%; found: C, 45.87; H, 3.83; N, 7.93%. IR (cm−1): 3387 (m), 1603 (w), 1533 (vs), 1439 (m), 1396 (vs), 1192 (w), 1080 (w), 860 (m), 752 (s), 719 (s), 706(s), 685 (s), 669 (s), 631 (s).Synthesis of {[Cd(btrb)(bpdc)]·H2O}n (8)
The synthesis procedure for 8 is similar to that for 2, except that H2bpdc (0.03 mmol, 0.007 g) was used instead of H4bptc. Colourless plate crystals of 8 were obtained (yield: 0.012 g, 39% based on btrb). Anal. calc. for C26H22CdN6O5 (8): C, 51.12; H, 3.63; N, 13.76%; found: C, 51.01; H, 3.85; N, 13.61%. IR (cm−1): 3466 (m), 3094 (w), 1690 (w), 1612 (m), 1549 (vs), 1466 (w), 1394 (vs), 1190 (m), 1067 (m), 1013 (m), 962 (w), 905 (w), 837 (m), 814 (m), 768 (s), 741 (m), 723 (m), 677 (m), 636 (s).X-ray crystallography
X-ray diffraction data were collected on a Rigaku Saturn 724 CCD, Agilent Gemini Atlas or Bruker D8 Quest diffractometer with graphite monochromated Mo-Kα radiation (λ = 0.71073 Å). The intensities were collected by ω-scan and reduced on the CrystalClear (Rigaku Inc., 2007), CrysAlisPro (Agilent Technologies, 2013) or APEX III (Bruker Corp., 2016) program. A multi-scan absorption correction was applied.17 The positions of the hydrogen atoms were determined by theoretical calculations. The structures were solved and refined by the SHELXTL package.18 The solvent molecules in 1, 2 and 3 were removed with the SQUEEZE procedure in PLATON.19 The ISOR and EADP commands were used and the occupancies of the disordered atoms were determined to make sure that the Ueq values were reasonable. The number of lattice water molecules for 1, 2 and 3 was deduced from the TGA and elemental analysis. The parameters of the crystal data collection and refinement are given in Table S1.† Selected bond lengths, bond angles and hydrogen bonds are given in Tables S2 and S3 in the ESI.†Results and discussion
Synthesis of 1–8
The balanced chemical reaction equations of 1–8 are shown in Fig. S43 in the ESI.†2–8 were all prepared under common hydrothermal conditions, while 1 could not be obtained in the same way. During the preparation of 1, we found that the btec ligand has a very strong bonding ability with Cd(II) atoms, even stronger than the btrb ligand under hydrothermal conditions. We could only obtain [Cd(btec)0.5(H2O)]n (CCDC: 214511)20 at certain ratios and temperatures (from 70 °C to 140 °C). [Cd(btec)0.5(H2O)]n is still the only product except the unknown precipitate, even when the ratio of btrb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) btec = 10
btec = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In order to get a CP in which btrb and btec ligands both coordinate with Cd(II) atoms, we tried to use [Cd(btec)0.5(H2O)]n as the starting material. Crystals of [Cd(btec)0.5(H2O)]n were put into a solution of btrb. After leaving the solution to stand for a long time, the shape of the [Cd(btec)0.5(H2O)]n crystals changed from diamond-like to big blocks, suggesting the formation of 1. Then we improved the steps in the preparation as shown in the synthesis section by a combination of the hydrothermal method and solvent method. This case shows a step-synthesis strategy in a mixed ligand system, especially for those ligands which have big differences in coordination ability. The crystals can be constructed by the metal and strong ligands first and then these crystals can be used as a material to react with a solution of the weak ligand under suitable conditions. There is a good chance of obtaining unexpected products after the reaction. The combination of the hydrothermal/solvothermal method and solvent method is a new thinking in the preparation of unusual CPs.
1. In order to get a CP in which btrb and btec ligands both coordinate with Cd(II) atoms, we tried to use [Cd(btec)0.5(H2O)]n as the starting material. Crystals of [Cd(btec)0.5(H2O)]n were put into a solution of btrb. After leaving the solution to stand for a long time, the shape of the [Cd(btec)0.5(H2O)]n crystals changed from diamond-like to big blocks, suggesting the formation of 1. Then we improved the steps in the preparation as shown in the synthesis section by a combination of the hydrothermal method and solvent method. This case shows a step-synthesis strategy in a mixed ligand system, especially for those ligands which have big differences in coordination ability. The crystals can be constructed by the metal and strong ligands first and then these crystals can be used as a material to react with a solution of the weak ligand under suitable conditions. There is a good chance of obtaining unexpected products after the reaction. The combination of the hydrothermal/solvothermal method and solvent method is a new thinking in the preparation of unusual CPs.
Crystal structure of {[Cd(btrb)(H2O)4][Cd2(btrb)(btec)(H2O)2]2(OH)2·5H2O}n (1·(OH)2·5H2O)
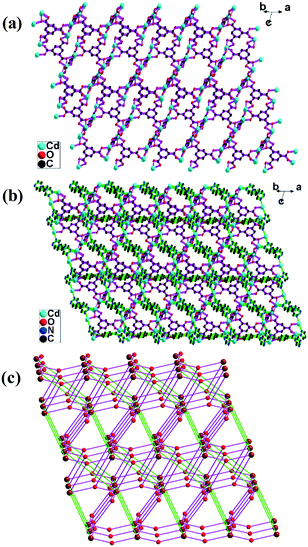
1 is composed of a 3D electroneutral motif and a 1D cationic motif polythreaded together and forms a 1D + 3D → 3D polythreaded network. The 3D motif shows an unprecedented (4,4,4,4)-connected bli1 topology or a second mot topology based on the [Cd2(COO)] dimer. The asymmetric unit of the 3D motif consists of two Cd(II) atoms (Cd1/Cd2), two halves of btec ligands, one btrb ligand and two coordinated water molecules. Cd1 is in a distorted octahedral geometry, coordinated by three carboxylate oxygen atoms (O1/O2/O8A) from two btec ligands, two nitrogen atoms (N1/N5B) from two btrb ligands and one water oxygen atom (O9). Cd2 is in a 7-coordinated pentagonal bipyramidal geometry, coordinated by four carboxylate oxygen atoms (O1/O3/O5/O6) from two btec ligands, two nitrogen atoms (N2/N4B) from two btrb ligands and one water oxygen atom (O10) (Fig. S1 in the ESI†). Two kinds of btec ligand are both coordinated with four Cd(II) atoms in three kinds of coordination mode: chelating bridging (O1O2), monodentate (O3O4/O8O7) and chelating (O5O6) modes (Fig. S2 and S3 in the ESI†). The btrb ligands are all coordinated with four Cd(II) atoms in a cis-configuration. Two btrb ligands (N1–N6/N1B–N6B) connected with the same four Cd(II) atoms form a [Cd4(btrb)2] ring (Fig. S4 in the ESI†). The planes of the benzene rings in the coordination ring are parallel, with a distance of 3.2 Å. The [Cd4(btrb)2] rings are linked by btec ligands and extended to form a nano-tunnel (Fig. 1a, Fig. S5 in the ESI†). The distance between the adjacent parallel nano-tunnels is 13.2 Å. The adjacent nano-tunnels are connected by btec ligands to form a unique 3D network (Fig. 1b). The planes formed by the adjacent parallel nano-tunnels are also parallel, with a distance of 9.8 Å, and the space angle of the nano-tunnels in adjacent planes is 70.5° along the c-axis.Topologically, the Cd1 and Cd2 atoms are both coordinated by two btrb and two btec ligands. Two kinds of btec ligand and the btrb ligands in the 3D motif are all coordinated by four Cd(II) atoms. Thus, the Cd(II) atoms and the btec and btrb ligands can all be described as 4-connected nodes. The structure of the 3D motif is analysed with Toptware.7 The 3D network in 1 can be simplified as a (4,4,4,4)-connected network (Fig. 1c) with a point symbol of (46)2(86)(42·82·102)(43·83)4.21 This topology is unprecedented, and was deposited in the TTD (Topos Topological Database) collection and named bli1.7
To simplify the topologies, if the [Cd2(COO)] dimer is simplified as a 4-connected node, the double btrb ligands in the [Cd4(btrb)2] ring and the btec (O1–O4) ligand are all 2-connected. The btec (O5–O8) ligand can also be considered as a 4-connected node. The 3D structure can be simplified as a (4,4)-connected mot topology with a point symbol of (64·82)2(66) (Fig. 1d). According to the TTO (Topological Types Observed) collection, only one example of the mot topology has been reported before.22
The asymmetric unit of the 1D cationic motif consists of half of a Cd(II) atom (Cd3), half of a btrb ligand and two water molecules (Fig. 1e). The btrb ligands connect the adjacent Cd(II) atoms to form a 1D cationic chain {[Cd(btrb)(H2O)4]2+}n with a Cd⋯Cd distance of 14.5113(31) Å. The disordered hydroxyl ions balance the charge. The 1D cationic chains pass through the nano-tunnels in the 3D motif to form a 1D + 3D → 3D polythreaded network (Fig. 1e). Hydrogen bonding interactions stabilize the polythreading array (Table S3 in the ESI†). The solvent accessible volume calculated by PLATON is 1391.8 Å3, which is 18.6% of the cell volume. The disordered hydroxyl ions and lattice water molecules are located in the void.
Polythreaded structures with finite components are unusual. Polythreaded networks containing 3D components are really rare.9–12 To the best of our knowledge, examples involving 3D component polythreaded frameworks have only rarely been reported. The Wang group reported five polyoxometalate-based polythreaded structures involving 3D components.12a–c [Cu(bbi)2V10O26][Cu(bbi)]2·H2O,12a [CuII(bbi)2(α -Mo8O26)][CuI(bbi)]2, [CuIICuI(bbi)3(α-Mo8O26)][CuI(bbi)],12b [CuI(bix)][(CuIbix)(δ-Mo8O26)0.5] and H(CuIbix)[(CuIbix)2(β-Mo8O26)]·2H2O (bbi = 1,1′-(1,4-butanediyl)bis(imidazol), bix = 1,4-bis(imidazol-1-ylmethyl)benzene)12c exhibit similar 1D + 3D → 3D polythreaded networks based on a 3D polyoxometalate anionic network and 1D cationic chains. Our group synthesized three 1D + 3D → 3D polythreaded networks, which were all constructed by a 3D anionic network and 1D cationic chains.12d–f
In short, the interesting structural features of 1 can be summarized as three aspects: (a) 1 shows a rare 1D + 3D → 3D polythreaded structure; (b) 1 is the first 1D + 3D → 3D polythreaded structure which is constructed by a 3D electroneutral network (not a 3D anionic network) and 1D cationic chains; (c) the 3D electroneutral network exhibits an unprecedented (4,4,4,4)-connected network.
Crystal structure of {[Cd(btrb)0.5(bptc)0.5]·H2O}n (2·H2O)
2 shows an unprecedented (4,5,6)-connected network. The asymmetric unit of 2 consists of one Cd(II) atom, half of a btrb ligand and half of a bptc ligand. Cd1 is in a distorted pentagonal bipyramidal geometry and coordinates five carboxylate oxygen atoms (O1/O1A/O2A/O3/O3B) from three bptc ligands and two nitrogen atoms (N1/N2A) from two btrb ligands (Fig. S6 in the ESI†). The bptc ligands in 2 show chelating bridging and bridging coordination modes with six Cd(II) atoms (Fig. S7 in the ESI†). The Cd(II) atoms are connected by the bptc ligands and extend to form a [Cd(bptc)0.5]n 2D network (Fig. 2a). Each btrb ligand shows an anti-conformation and coordinates four Cd(II) atoms with its 1,2-position triazole nitrogen atoms (Fig. S8 in the ESI†). The [Cd(bptc)0.5]n 2D networks are linked by btrb ligands and construct a 3D network (Fig. 2b).Topologically, each Cd(II) atom is coordinated by three bptc ligands and two btrb ligands and can be described as a 5-connected node (Fig. S9 in the ESI†), while the bptc ligands and btrb ligands are 6-connected and 4-connected nodes, respectively (Fig. S7 and S8 in the ESI†). The 3D structure of 2 can be simplified as a (4,5,6)-connected network (Fig. 2c) with a point symbol of (42·84)(47·63)2(46·66·83).21 This topology is unprecedented, and was deposited in the TTD (Topos Topological Database) collection and named bli2.7
Crystal structure of {[Cd3(btrb)(btc)2]·3H2O}n (3·3H2O)
3 presents an unprecedented (3,8)-connected 3D network based on the Cd(II) trimer cluster [Cd3(COO)2]. The asymmetric unit consists of one (Cd2) and half (Cd1) Cd(II) atoms, half of a btrb ligand (N1–N3) and one btc ligand (O1–O6). Cd1 coordinates with four carboxylate oxygen atoms (O1/O1C/O6A/O6B) from four btc ligands and two nitrogen atoms (N1/N1C) from two btrb ligands in a distorted octahedral geometry. The Cd2 atom is in a distorted octahedral geometry coordinating with five carboxylate oxygen atoms (O1/O2/O3D/O4D/O5B) from three btc ligands and one nitrogen atom (N2) from one btrb ligand (Fig. S10 in the ESI†). One btc ligand is coordinated with five Cd(II) atoms. The three carboxylate groups show three kinds of coordination mode: bidentate bridging for O1O2, bidentate chelating for O3O4 and a bidentate coordination mode for O5O6 (Fig. S11 in the ESI†). The O1 carboxylate oxygen atoms and the O1C atoms from the two btc ligands show a bridging mode and connect two Cd(II) atoms. The Cd(II) trimer cluster [Cd3(COO)2] is formed. The Cd(II) trimer clusters [Cd3(COO)2] are joined by btc ligands and the [Cd3(btc)2]n 3D network is formed (Fig. 3a). Each btrb ligand shows an anti-conformation and connects four Cd(II) atoms. The Cd(II) atoms of the [Cd3(btc)2]n 3D network are linked by btrb ligands and the [Cd3(btrb)(btc)2]n 3D network is constructed (Fig. 3b).Topologically, if the Cd(II) trimer cluster [Cd3(COO)2] is simplified as one node, it connects six btc ligands and two btrb ligands and is 8-connected (Fig. S12 in the ESI†). Each btc ligand coordinates three [Cd3(COO)2] and is 3-connected. Each btrb ligand links two [Cd3(COO)2] and is 2-connected. The 3D network of 3 can be simplified as a (3,8)-connected network (Fig. 3c) with a point symbol of (42·6)2(44·611·76·86·9).21 This topology is unprecedented, and was deposited in the TTD (Topos Topological Database) collection and named bli3.7 The network is a self-catenated 3D network. Self-catenated 3D networks are unusual and few have been reported in the literature.21 The solvent accessible volume calculated by PLATON excluding water molecules is 927.9 Å3, which is 24.6% of the cell volume.
Crystal structure of {[Cd(btrb)(ip)(H2O)]·H2O}n (4)
4 exhibits a 2D + 2D → 2D interpenetration network based on an undulated 2D sql network. The asymmetric unit consists of one Cd(II) atom, two halves of btrb ligands, one ip ligand, one coordinated water molecule and one lattice water molecule. Cd1 is 6-coordinated with three carboxylate oxygen atoms (O1/O2/O4A) from two ip ligands, two nitrogen atoms (N1/N4) from two btrb ligands and one water molecule in a distorted octahedral geometry (CdO4N2) (Fig. S13 in the ESI†).The btrb ligands exhibit an anti-configuration and are 2-connected. Two carboxylate groups of one ip ligand act as monodentate and chelating modes (Fig. S14 in the ESI†). The 2-connected btrb and ip ligands connect the 4-connected Cd(II) nodes to form an undulated 2D sql network (Fig. 4a) with a point symbol of (44·62). Two adjacent undulated 2D networks interpenetrate each other and a 2D + 2D → 2D interpenetration network is formed (Fig. 4b).
Crystal structure of {[Cd(btrb)(MeOip)]·H2O}n (5)
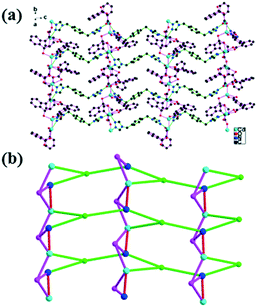
5 exhibits a 4-connected 3D network. 5 crystallizes in the monoclinic system with a P21/n space group. The asymmetric unit consists of one Cd(II) atom, two halves of btrb ligands, one MeOip ligand and one lattice water molecule. Cd1 is 5-coordinated with three carboxylate oxygen atoms (O1/O2/O3A) from two MeOip ligands and two nitrogen atoms from two btrb ligands (N2/N4) in a distorted square pyramidal geometry (CdO3N2) (Fig. S15 in the ESI†). The structural distortion index τ for the Cd1 atom is 0.288, indicating that the coordination environment of the metal atom is closer to a square pyramidal geometry than a trigonal bipyramidal configuration.Two carboxylate groups of one MeOip ligand act as chelating (O1O2) and monodentate (O3O4) modes. The MeOip ligands are 2-connected. The Cd⋯Cd distance separated by the MeOip bridge is 8.9586(26) Å. The btrb ligands exhibit an anti-configuration and are 2-connected. Each Cd(II) atom connects four other Cd(II) atoms through two MeOip and two btrb bridges. The Cd⋯Cd distances separated by the btrb bridges are 14.5312(40) and 13.9854(33) Å. The Cd(II) atoms are connected by MeOip and btrb ligands and extend to construct a self-catenated 3D network (Fig. 5a).
 | ||
| Fig. 5 (a) The 3D network of 5; (b) schematic depiction of the 4-connected 3D network in 5. The pink and bright green sticks represent MeOip and btrb ligands, respectively. | ||
Topologically, each Cd(II) is 4-connected. The MeOip and btrb ligands are 2-connected. The 3D network of 5 can be simplified as a 4-connected 3D network of a cds topology (Fig. 5b) with a point symbol of (65·8).
Crystal structure of {[Cd2(btrb)2(1,2-bdc)2]·3H2O}n (6)
6 presents a 6-connected 3D cco-6-Pbcm network. The asymmetric unit of 6 consists of two Cd(II) atoms, one btrb ligand and two halves of btrb ligands, two 1,2-bdc ligands and three lattice water molecules. Cd1 is in a distorted octahedral geometry coordinated with three carboxylate oxygen atoms (O1/O3/O5C) from two 1,2-bdc ligands and three nitrogen atoms (N1/N8/N11B) from three btrb ligands. Cd2 is in a distorted pentagonal bipyramidal geometry coordinated with four carboxylate oxygen atoms (O1/O2/O7/O8) from two 1,2-bdc ligands and three nitrogen atoms (N4/N7/N12B) from three btrb ligands (Fig. S16 in the ESI†). Two kinds of btrb ligand (N1–N3/N4–N6) act as 2-connected bridges and coordinate two Cd(II) atoms (Fig. S17 in the ESI†). The third kind of btrb ligand (N7–N12) coordinates four Cd(II) atoms with its 1,2-position triazole nitrogen atoms (Fig. S18 in the ESI†). There are two kinds of 1,2-bdc ligand. One kind of 1,2-bdc ligand (O1–O4) shows a chelating bridging (O1O2) and monodentate (O3O4) mode (Fig. S19 in the ESI†). The other kind of 1,2-bdc ligand (O5–O8) shows a monodentate (O5O6) and chelating (O7O8) coordination mode (Fig. S20 in the ESI†).A 1,2-bdc ligand (O1–O4) bridges the Cd1 and Cd2 atoms and forms the [Cd2(1,2-bdc)] dimer. The [Cd2(1,2-bdc)] dimer is linked by a 1,2-bdc ligand (O5–O8) and constructs the [Cd2(1,2-bdc)2]n 1D chain (Fig. S21 in the ESI†). The [Cd2(1,2-bdc)2]n 1D chains are connected by btrb ligands and form the 3D network (Fig. 6a).
Topologically, if the [Cd2(1,2-bdc)] dimer is simplified as one node, it connects two 1,2-bdc ligands and four btrb ligands and is a 6-connected node (Fig. S22 in the ESI†). The 1,2-bdc ligands (O5–O8) and btrb ligands are 2-connected. The 3D structure of 6 can be simplified as a 6-connected 3D cco-6-Pbcm network with a point symbol of (47·68) (Fig. 6b).21 According to the TTO Collection, there are just two examples of this topology among CPs formed by dimeric structural groups,23 but 6 contains two interpenetrating cco-6-Pbcm motifs (Fig. 6c) and is the first such example for this topology.
Crystal structure of {[Cd2(btrb)(bpdc)2]2·11H2O}n (7)
7 shows a (3,3,4,4)-connected 2D network. The asymmetric unit of 7 consists of two Cd(II) atoms, one btrb ligand, two bpdc ligands, three ordered lattice water molecules and two and a half disordered lattice water molecules. Cd1 is in a distorted octahedral geometry coordinated with four carboxylate oxygen atoms (O1/O3/O5A/O6A) from two bpdc ligands and two nitrogen atoms (N1/N4B) from two btrb ligands. Cd2 is seven coordinated with six carboxylate oxygen atoms (O1C/O2C/O3/O4/O7/O8) from three bpdc ligands and one nitrogen atom (N2) from one btrb ligand (Fig. S23 in the ESI†).There are two kinds of bpdc ligand, one of which shows a dual bidentate bridging mode (O1O2/O3O4) and coordinates with three Cd(II) atoms (Fig. S24 in the ESI†). The other shows a dual chelating (O5O6/O7O8) coordination mode and coordinates with two Cd(II) atoms (Fig. S25 in the ESI†). The btrb ligands in 7 are all coordinated with three Cd(II) atoms (Fig. S26 in the ESI†). The Cd(II) atoms are connected by bpdc and btrb ligands and form a 2D network (Fig. 7a).
Topologically, each Cd1 atom links two bpdc and two btrb ligands and is 4-connected. Each Cd2 atom connects one btrb ligand and three bptc ligands and is 4-connected (Fig. S27 in the ESI†). The bpdc (O1–O4) and btrb ligands are 3-connected. The bpdc ligand (O5–O8) is 2-connected. The 2D network of 7 can be simplified as a (3,3,4,4)-connected 2D 3,3,4,4L72 network with a point symbol of (3·4·5)(4·82)(3·4·5·83)(3·4·82·92) (Fig. 7b).21
Crystal structure of {[Cd(btrb)(bpdc)]·H2O}n (8)
8 shows a (3,5)-connected 2D network. The asymmetric unit of 8 consists of half of a Cd(II) atom, half of a btrb ligand, half of a bpdc ligand and half of a disordered lattice water molecule. Cd1 is in a slightly distorted octahedral geometry coordinated with four carboxylate oxygen atoms (O1/O1A/O2B/O2C) from three bpdc ligands and two nitrogen atoms (N1/N1A) from two btrb ligands (Fig. S28 in the ESI†). The bpdc ligands in 8 show a dual bidentate coordination mode and link three Cd(II) atoms (O1O2/O1AO2A) (Fig. S29 in the ESI†). The btrb ligands exhibit an anti-configuration and are 2-connected with their 1-position triazole nitrogen atoms. The Cd(II) atoms are connected by bpdc and btrb ligands to construct a 2D network (Fig. 8a).Topologically, each Cd(II) atom links three bpdc ligands and two btrb ligands and is 5-connected (Fig. S30 in the ESI†). Each bpdc ligand is 3-connected. Each btrb ligand is 2-connected. The 2D network of 8 can be simplified as a (3,5)-connected self-catenated 2D 3,5L2 network with a point symbol of (42·6)(42·67·8) (Fig. 8b).21
Effects of multicarboxylate ligands and the bis(triazole) ligand btrb on the structures of 1–8
In this work, we used Cd(II) as the metal centre, the btrb ligand as the main ligand, and different carboxylate ligands as the auxiliary ligands to tune the structures of the CPs 1–8.Initially, we chose 1,2,4,5-benzenetetracarboxylic acid (H4btec) as an auxiliary ligand, thus the unprecedented (4,4,4,4)-connected 3D (electroneutral motif) + 1D (cationic motif) → 3D polythreaded structure of 1 was obtained. The unprecedented 3D (4,5,6)-connected structure of 2 was synthesized if the tetracarboxylate ligand btec was replaced by (1,1′-biphenyl)tetracarboxylic acid (H4bptc). When the tricarboxylate ligand 1,3,5-benzenetricarboxylic acid (H3btc) was used, an unprecedented 3D (3,8)-connected topology of 3, based on the Cd(II) trimer cluster [Cd3(COO)2], was obtained. If the dicarboxylic ligand isophthalic acid (H2ip) was used, 4 exhibited a 2D + 2D → 2D interpenetration network based on the 2D sql network. 5 exhibited a 4-connected 3D cds network when the dicarboxylic ligand 4-methoxyl-isophthalic acid (H2MeOip) was used. 6 presented the first 2-fold interpenetrating cco-6-Pbcm 3D network when the dicarboxylic ligand phthalic acid (1,2-H2bdc) was used. A (3,3,4,4)-connected 2D network of 7 and a (3,5)-connected self-catenated 2D network of 8 were synthesized when the dicarboxylic ligand (1,1′-biphenyl)-2,2′-dicarboxylic acid (H2bpdc) was used.
The carboxyl in 1–8 shows five kinds of coordination mode: monodentate, chelating, bidentate, monodentate bridging and chelating bridging (Fig. S31 in the ESI†). The ortho-position carboxyl of the 1,2-bdc, btec and bptc ligands makes them easier to chelate with more Cd(II) atoms compared with the meta-position carboxyl of the ip and MeOip ligands. The Cd(II) atoms are more aggregated in 1, 2 and 6 than in 4 and 5. Due to the formation of Cd3(COO)2, the Cd(II) atoms in 3 are the most concentrated. This result suggests an advantage of multidentate ligands in the formation of metal clusters. The coordination modes of the carboxylate ligands and btrb ligands in 4 and 5 are very similar but their structures are quite different. 4 is a typical 2D ‘wave’ sql network, while 5 is a 4-connected 3D cds network with a point symbol of (65·8). The steric hindrance effect of the 4-substituent group in MeOip during assembly may be the main reason for the difference between 4 and 5. One bptc ligand is the combination of two 1,2-bdc ligands. The bigger molecular volume of bptc may limit the coordination of btrb ligands. More btrb ligands join in the coordination in 6 than in 2. The addition of different amounts of bpdc ligands results in the formation of 7 and 8. The formulae of 7 and 8 show the phenomenon that the fewer bpdc ligands are added, the more btrb ligands join in coordination. These cases show the competition effect in co-ligand systems. 1–8 show the topologies devisable by the regulation of multicarboxylate ligands.
The btrb ligand was expected to be tetradentate since it has four donor nitrogen atoms. Indeed, this coordination mode (μ4) is typical; it occurs in complexes 1, 2, 3 and 6. However, the simple bridging mode μ2 is also quite ordinary; it is found in complexes 4, 5, 6 and 8. The intermediate μ3 mode is realized only in 7. Thus, the btrb ligand shows three kinds of coordination mode (Fig. S32 in the ESI†). The μ3 and μ4 modes result in the formation of condensed polynuclear groups of Cd atoms connected by N–N bridges: in 1 and 6 such groups are represented by dimers, in 3 – trimers, and in 2 – chains (Fig. S33 in the ESI†).
Despite rather simple coordination modes, the btrb ligand provides a diverse range of coordination network topologies. Some of them are rare, like mot, which, according to the ToposPro TTO Collection,8a is found only in [(μ4-isophthalato)2(μ3-isophthalato)2(1,10-phenanthroline)3Dy3(NO3)]·2H2O (CEJBEA)22 or 3,3,4,4L72, which is realized only in [(1,3-propanediamine)3Cu3W2(CN)18]·3H2O (IRIRIK),24 while others are unique, like bli2 and bli3. Complex 6 is also unique as it is the first example of a 2-fold cco-6-Pbcm topology. Importantly, in all these cases the btrb ligand forms condensed groups (Fig. S33 in the ESI†); when it plays the role of a bridge, the topologies become quite common, like in 4 (sql) and 8 (3,5L2).
Another important feature of the btrb ligand is that it provides a plethora of catenation (self-catenation in 3 and 8 or interpenetration in 4 and 6). This is obviously caused by its length and twisted geometry.
Thus, the btrb ligand demonstrates a way to obtain new architectures of coordination polymers: the ligand should be polydentate and plays two roles: as a linker in polynuclear complex groups and as a bridge between them. A complicated geometrical form and stretching will lead to catenated topologies.
Solid state photoluminescence properties
The solid-state luminescence spectra of 1–8 were studied at room temperature. The free btrb ligand does not show luminescent properties in the solid state. The possible btrb fluorescence through intra-ligand charge transfer is apparently quenched by the thermal intra-ligand rotations around the C–C and C–N bonds.15a,251–8 have emission band maxima at 444 and 469 nm (ex = 342 nm) for 1, 462 and 525 nm (ex = 345 nm) for 2, 413 and 467 nm (ex = 320 nm) for 3, 421 and 465 nm (ex = 316 nm) for 4, 429 and 464 nm (ex = 323 nm) for 5, 436 and 464 nm (ex = 326 nm) for 6, 437 and 465 nm (ex = 333 nm) for 7, and 445 and 473 nm (ex = 333 nm) for 8, respectively (Fig. 9). Due to the d10 configuration, the Cd(II) ion is difficult to oxidize or reduce. The emissions are neither metal-to-ligand charge transfer (MLCT) nor ligand-to-metal charge transfer (LMCT). The emissions can be attributed to the intra-ligand and ligand-to-ligand charge transitions (LLCT) from the btrb and multicarboxylate ligands.251–8 all show two emission peaks. The free H4btec, H4bptc, H3btc, H2ip, H2MeOip, 1,2-H2bdc and H2bpdc ligands exhibit emissions at 336, 402, 328, 346, 360, 354 and 364 nm, respectively, which are probably attributable to the π* → n or π* → π transitions.26 The emission peaks of 1–8 are listed in Table 1 for comparison. Fixation of the btrb ligand in a coordination network stops the rotation and freezes a single conformation to enable fluorescence; the btrb ligand may show luminescence when it coordinates with Cd(II) atoms.25 One of the emission peaks of 1–8 is near 466 nm, which tentatively results from the btrb ligands. The two benzene rings of the bptc ligand in 2 are in the a plane and have the biggest conjugative effect, while other single benzene ring carboxylate ligands have similar weak conjugative systems. The two benzene rings of the bpdc ligand in 7 and 8 rotate a large angle, 65.8° and 60.0° in 7 and 63.6° in 8. Thus, the bpdc ligands in 7 and 8 have similar conjugative effects compared with single benzene ring carboxylate ligands. The bigger conjugative effect in 2 results in the exceptional emission shift.26c–f [Cd(btc)0.5(phen)]·H2O (H4btc = biphenyl-3,3′,4,4′-tetracarboxylic acid, phen = 1,10-phenanthroline) has an emission peak at 506 nm26g and [Cd2(bptc)(L)2(H2O)2]·5H2O (L = 2-(2-chloro-6-fluorophenyl)-1H-imidazo[4,5-f][1,10]phenanthroline and H4bptc = 3,3′,4,4′-biphenyltetracarboxylic acid) shows a similar emission peak at 518 nm.26h The other emission peaks of 1–8 are attributed to the n → π* or π → π* LLCT from the multicarboxylate ligands. The emissions from carboxylate ligands show different red shift values from 69 to 123 nm. The red-shift is mainly due to the coordination interactions, which effectively increase the rigidity of the ligand and reduce the loss of energy by non-radiative decay of the intra-ligand emission excited state.25
| CP | Emission peak from btrb in CP (nm) | Emission peak from carboxylate in CP (nm) | Emission peak from free carboxylate ligand (nm) | Red shift value of carboxylate in CP (nm) |
|---|---|---|---|---|
| 1 | 469 | 444 | 336 | 108 |
| 2 | 462 | 525 | 402 | 123 |
| 3 | 467 | 413 | 328 | 85 |
| 4 | 465 | 421 | 346 | 75 |
| 5 | 464 | 429 | 360 | 69 |
| 6 | 464 | 436 | 354 | 82 |
| 7 | 465 | 437 | 364 | 73 |
| 8 | 473 | 445 | 364 | 81 |
PXRD and thermal stability
The measured and simulated PXRDs confirm the purity of 1–8 (Fig. S34–S41 in the ESI†). Thermogravimetric analysis (TGA) of 1–8 was performed on crystalline samples heated from rt. to 800 °C in a nitrogen atmosphere (Fig. S42 in the ESI†). The water molecules of 1 were completely lost at 150 °C (calcd: 12.29%, found: 12.30%), and the framework was stable up to 220 °C. The residue of 1 at 800 °C should be CdO (calcd: 31.30%, found: 31.55%). The lattice and coordination water molecules of 2 were completely lost at 118 °C (calcd: 4.36%, found: 2.29%), and the network was stable up to 354 °C. The residue of 2 at 800 °C should be CdO (calcd: 31.04%, found: 32.07%). The lattice and coordination water molecules of 3 were completely lost at 162 °C (calcd: 5.17%, found: 4.57%), and the network was stable up to 394 °C. The residue of 3 at 800 °C should be CdO (calcd: 36.84%, found: 37.42%). The lattice and coordination water molecules of 4–8 were completely lost at 117 °C, 162 °C, 175 °C, 149 °C and 130 °C, respectively (calcd: 6.52%, found: 6.67% for 4, calcd: 3.19%, found: 3.25% for 5, calcd: 4.97%, found: 4.69% for 6, calcd: 9.49%, found: 9.63% for 7 and calcd: 2.95%, found: 2.98% for 8). The anhydrous matters of 4–8 were thermally stable up to 273 °C, 308 °C, 263 °C, 294 °C and 256 °C, respectively. Then weight loss continuously occurred and did not end until 800 °C. The main residues at 800 °C were consistent with CdO (calcd: 23.23%, found: 23.27% for 4, calcd: 22.73%, found: 22.79% for 5, calcd: 23.61%, found: 23.51% for 6, calcd: 24.59%, found: 24.50% for 7 and calcd: 21.02%, found: 21.05% for 8).Conclusions
In short, eight Cd(II) CPs were synthesized using the flexible bis(triazole) ligand 1,4-bis(1,2,4-triazol-4-ylmethyl)benzene and seven multicarboxylate ligands under hydrothermal conditions. 1 presents an unprecedented (4,4,4,4)-connected 3D (electroneutral motif) + 1D (cationic motif) → 3D polythreaded network. 2 shows an unprecedented 3D (4,5,6)-connected structure. 3 shows an unprecedented 3D (3,8)-connected topology based on the Cd(II) trimer cluster [Cd3(COO)2]. 4 exhibits a 2D + 2D → 2D interpenetration network based on the 2D sql network. 5 exhibits a 4-connected cds 3D network. 6 presents the first 2-fold interpenetrating cco-6-Pbcm 3D network. 7 shows a (3,3,4,4)-connected 2D network. 8 presents a (3,5)-connected self-catenated 2D network with a point symbol of (42·6)(42·67·8). The diverse structures demonstrate that the structures of coordination polymers can be successfully tuned by the carboxylate and bis(triazole) co-ligands. 1–3 show unprecedented 3D topologies. The 3D structures of 3 and 8 are self-catenated. The successful syntheses of 1–8 demonstrate that the topologies of coordination polymers can be adjusted and that unprecedented and intriguing topologies can be constructed by bis(1,2,4-triazole) and multicarboxylate co-ligands. This work contributes to the development of crystal engineering and materials science.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work is supported by the National Natural Science Foundation of China (No. 21171126), Natural Science Foundation of Jiangsu Province (No. BK20161212), Priority Academic Program Development of Jiangsu Higher Education Institutions, State and Local Joint Engineering Laboratory for Functional Polymeric Materials and the project of scientific and technological infrastructure of Suzhou (SZS201708). Vladislav A. Blatov is grateful to the Russian Government (Grant 14.B25.31.0005) for support.References
- (a) M. O'Keeffe and O. M. Yaghi, Chem. Rev., 2012, 112, 675 CrossRef PubMed; (b) M. Li, D. Li, M. O'Keeffe and O. M. Yaghi, Chem. Rev., 2014, 114, 1343 CrossRef CAS PubMed; (c) J. R. Li, R. J. Kuppler and H. C. Zhou, Chem. Soc. Rev., 2009, 38, 1477 RSC; (d) J. R. Li, J. Sculley and H. C. Zhou, Chem. Rev., 2012, 112, 869 CrossRef CAS PubMed; (e) J. B. DeCoste and G. W. Peterson, Chem. Rev., 2014, 114, 5695 CrossRef CAS PubMed; (f) M. Du, C. P. Li, C. S. Liu and S. M. Fang, Coord. Chem. Rev., 2013, 257, 1282 CrossRef CAS.
- (a) Q. X. Han, B. Qi, W. M. Ren, C. He, J. Y. Niu and C. Y. Duan, Nat. Commun., 2015, 6, 10007 CrossRef PubMed; (b) H. L. Tang, S. C. Cai, S. L. Xie, Z. B. Wang, Y. X. Tong, M. Pan and X. H. Lu, Adv. Sci., 2016, 3, 1500265 CrossRef PubMed; (c) C. K. Wang, F. F. Xing, Y. L. Bai, Y. M. Zhao, M. X. Li and S. R. Zhu, Cryst. Growth Des., 2016, 16, 2277 CrossRef CAS; (d) Y. J. Yang, M. J. Wang and K. L. Zhang, J. Mater. Chem. C, 2016, 4, 11404 RSC.
- (a) D. Liu, J. P. Lang and B. F. Abrahams, J. Am. Chem. Soc., 2011, 133, 11042 CrossRef CAS PubMed; (b) D. Liu, Z. G. Ren, H. X. Li, J. P. Lang, N. Y. Li and B. F. Abrahams, Angew. Chem., Int. Ed., 2010, 49, 4767 CrossRef CAS PubMed; (c) Z. Z. Lu, R. Zhang, Y. Z. Li, Z. J. Guo and H. G. Zheng, J. Am. Chem. Soc., 2011, 133, 4172 CrossRef CAS PubMed; (d) P. Yang, X. He, M. X. Li, Q. Ye, J. Z. Ge, Z. X. Wang, S. R. Zhu, M. Shao and H. L. Cai, J. Mater. Chem., 2012, 22, 2398 RSC; (e) H. B. Zhou, J. H. Yao, X. P. Shen, H. Zhou and A. H. Yuan, RSC Adv., 2014, 4, 61 RSC; (f) S. Y. Hao, S. X. Hou, K. V. Heckeb and G. H. Cui, Dalton Trans., 2017, 46, 1951 RSC; (g) J. W. Cui, S. X. Hou, K. V. Heckeb and G. H. Cui, Dalton Trans., 2017, 46, 2892 RSC.
- (a) X. X. Liu, C. D. Shi, C. W. Zhai, M. L. Cheng, Q. Liu and G. X. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 4585 CrossRef CAS PubMed; (b) M. X. Li, Y. F. Zhang, X. He, X. M. Shi, Y. P. Wang, M. Shao and Z. X. Wang, Cryst. Growth Des., 2016, 16, 2912 CrossRef CAS; (c) X. Zhao, X. H. Bu, Q. G. Zhai, H. Tran and P. Y. Feng, J. Am. Chem. Soc., 2015, 137, 1396 CrossRef CAS PubMed; (d) L. L. Tan, H. Li, Y. C. Qiu, D. X. Chen, X. Wang, R. Y. Pan, Y. Wang, S. X. A. Zhang, B. Wang and Y. W. Yang, Chem. Sci., 2015, 6, 1640 RSC; (e) X. L. Lv, K. C. Wang, B. Wang, J. Su, X. D. Zou, Y. B. Xie, J. R. Li and H. C. Zhou, J. Am. Chem. Soc., 2017, 139, 211 CrossRef CAS PubMed.
- (a) J. Cepeda and A. Rodríguez-Diéguez, CrystEngComm, 2016, 18, 8556 RSC; (b) Y. L. Li, Y. Zhao, P. Wang, Y. S. Kang, Q. Liu, X. D. Zhang and W. Y. Sun, Inorg. Chem., 2016, 55, 11821 CrossRef CAS PubMed; (c) L. Di, J. J. Zhang, S. Q. Liu, J. Ni, H. J. Zhou and Y. J. Sun, Cryst. Growth Des., 2016, 16, 4539 CrossRef CAS; (d) Z. C. Hu, W. P. Lustig, J. M. Zhang, C. Zheng, H. Wang, S. J. Teat, Q. H. Gong, N. D. Rudd and J. Li, J. Am. Chem. Soc., 2015, 137, 16209 CrossRef CAS PubMed; (e) Q. Zhang, J. Su, D. W. Feng, Z. W. Wei, X. D. Zou and H. C. Zhou, J. Am. Chem. Soc., 2015, 137, 10064 CrossRef CAS PubMed.
- (a) S. Y. Zhang, W. Shi, P. Cheng and M. J. Zaworotko, J. Am. Chem. Soc., 2015, 137, 12203 CrossRef CAS PubMed; (b) L. L. Zhu, C. F. Tan, M. M. Gao and G. W. Ho, Adv. Mater., 2015, 27, 7713 CrossRef CAS PubMed; (c) S. Yuan, T. F. Liu, D. W. Feng, J. Tian, K. C. Wang, J. S. Qin, Q. Zhang, Y. P. Chen, M. Bosch, L. F. Zou, S. J. Teat, S. J. Dalgarno and H. C. Zhou, Chem. Sci., 2015, 6, 3926 RSC; (d) D. K. Maity, B. Bhattacharya, A. Halder and D. Ghoshal, Dalton Trans., 2015, 4, 20999 RSC; (e) B. Bhattacharya, A. Halder, D. K. Maity and D. Ghoshal, CrystEngComm, 2016, 18, 4074 RSC.
- http://topospro.com .
- (a) V. A. Blatov, A. P. Shevchenko and D. M. Proserpio, Cryst. Growth Des., 2014, 14, 3576 CrossRef CAS; (b) S. W. Zhang, J. G. Ma, X. P. Zhang, E. Y. Duan and P. Cheng, Inorg. Chem., 2015, 54, 586 CrossRef CAS PubMed; (c) D. Sun, Z. H. Yan, V. A. Blatov, L. Wang and D. F. Sun, Cryst. Growth Des., 2013, 13, 1277 CrossRef CAS; (d) X. H. Jing, X. C. Yi, E. Q. Gao and V. A. Blatov, Dalton Trans., 2012, 41, 14316 RSC; (e) M. Marmier, G. Cecot, A. V. Vologzhanina, J. L. Bila, I. Zivkovic, H. M. Ronnow, B. Nafradi, E. Solari, P. Pattison, R. Scopellitia and K. Severin, Dalton Trans., 2016, 45, 15507 RSC; (f) G. H. Cui, C. H. He, C. H. Jiao, J. C. Geng and V. A. Blatov, CrystEngComm, 2012, 14, 4210 RSC; (g) H. Q. Ma, D. Sun, L. L. Zhang, R. M. Wang, V. A. Blatov, J. Guo and D. F. Sun, Inorg. Chem., 2013, 52, 10732 CrossRef CAS PubMed.
- (a) S. R. Batten and R. Robson, Angew. Chem., Int. Ed., 1998, 37, 1460 CrossRef; (b) L. Carlucci, G. Ciani and D. M. Proserpio, Coord. Chem. Rev., 2003, 246, 247 CrossRef CAS; (c) E. V. Alexandrov, V. A. Blatov and D. M. Proserpio, CrystEngComm, 2017, 19, 1993 RSC; (d) Y. N. Gong, D. C. Zhong and T. B. Lu, CrystEngComm, 2016, 18, 2596 RSC.
- (a) Y. Gong, J. Li, J. Qin, T. Wu, R. Cao and J. Li, Cryst. Growth Des., 2011, 11, 1662 CrossRef CAS; (b) B. Xu, J. Lu and R. Cao, Cryst. Growth Des., 2009, 9, 3003 CrossRef CAS; (c) G. P. Yang, L. Hou, L. F. Ma and Y. Y. Wang, CrystEngComm, 2013, 15, 2561 RSC; (d) A. L. Chen, N. Liu, Y. F. Yue, Y. W. Jiang, E. Q. Gao, C. H. Yan and M. Y. He, Chem. Commun., 2007, 407 RSC; (e) G. W. Xu, Y. P. Wu, H. B. Wang, Y. N. Wang, D. S. Li and Y. L. Lu, CrystEngComm, 2015, 17, 9055 RSC; (f) J. M. Hu, V. A. Blatov, B. Y. Yu, K. V. Heckec and G. H. Cui, Dalton Trans., 2016, 45, 2426 RSC; (g) V. Martins, J. A. L. C. Resende and C. M. Ronconi, CrystEngComm, 2017, 19, 3103 RSC.
- (a) Q. X. Yao, Z. F. Xu, X. H. Jin and J. Zhang, Inorg. Chem., 2009, 48, 1266 CrossRef CAS PubMed; (b) Y. Y. Liu, Z. H. Wang, J. Yang, B. Liu, Y. Y. Liu and J. F. Ma, CrystEngComm, 2011, 13, 3811 RSC; (c) H. Y. Zang, Y. Q. Lan, G. S. Yang, X. L. Wang, K. Z. Shao, G. J. Xu and Z. M. Su, CrystEngComm, 2010, 12, 434 RSC; (d) B. Zheng and J. Bai, CrystEngComm, 2009, 11, 271 RSC; (e) G. H. Wang, Z. G. Li, H. Q. Jia, N. H. Hu and J. W. Xu, Cryst. Growth Des., 2008, 8, 1932 CrossRef CAS; (f) X. Y. Cao, Q. P. Lin, Y. Y. Qin, J. Zhang, Z. J. Li, J. K. Cheng and Y. Y. Yao, Cryst. Growth Des., 2009, 9, 20 CrossRef CAS; (g) H. Wu, B. Liu, J. Yang, H. Y. Liu and J. F. Ma, CrystEngComm, 2011, 13, 3661 RSC.
- (a) Y. Q. Lan, S. L. Li, Z. M. Su, K. Z. Shao, J. F. Ma, X. L. Wang and E. B. Wang, Chem. Commun., 2008, 58 RSC; (b) Y. Q. Lan, S. L. Li, X. L. Wang, K. Z. Shao, D. Y. Du, H. Y. Zang and Z. M. Su, Inorg. Chem., 2008, 47, 8179 CrossRef CAS PubMed; (c) J. X. Meng, Y. Lu, Y. G. Li, H. Fu and E. B. Wang, Cryst. Growth Des., 2009, 9, 4116 CrossRef CAS; (d) Y. F. Cui, X. Qian, Q. Chen, B. L. Li and H. Y. Li, CrystEngComm, 2012, 14, 1201 RSC; (e) M. Li, S. Zhao, Y. F. Peng, B. L. Li and H. Y. Li, Dalton Trans., 2013, 42, 9771 RSC; (f) M. Li, Y. F. Peng, S. Zhao, B. L. Li and H. Y. Li, RSC Adv., 2014, 4, 14241 RSC.
- (a) B. Zheng, H. Dong, J. F. Bai, Y. Z. Li, S. H. Li and M. Scheer, J. Am. Chem. Soc., 2008, 130, 7778 CrossRef CAS PubMed; (b) D. K. Maity, A. Halder, B. Bhattacharya, A. Das and D. Ghoshal, Cryst. Growth Des., 2016, 16, 1162 CrossRef CAS; (c) M. Chen, Y. Lu, J. Fan, G. C. Lv, Y. Zhao and W. Y. Sun, CrystEngComm, 2012, 44, 2015 RSC; (d) R. Luo, H. Xu, H. X. Gu, X. Wang, Y. Xu, X. Shen, W. W. Bao and D. R. Zhu, CrystEngComm, 2014, 16, 784 RSC; (e) Y. Zhang, J. Yang, Y. Yang, J. Guo and J. F. Ma, Cryst. Growth Des., 2012, 12, 4060 CrossRef CAS; (f) Z. Su, M. Chen, T. Okamura, M. S. Chen, S. S. Chen and W. Y. Sun, Inorg. Chem., 2011, 50, 985 CrossRef CAS PubMed; (g) S. L. Li, K. Tan, Y. Q. Lan, J. S. Qin, M. N. Li, D. Y. Du, H. Y. Zang and Z. M. Su, Cryst. Growth Des., 2010, 10, 1699 CrossRef CAS; (h) J. B. Lin, J. P. Zhang, W. X. Zhang, W. Xue, D. X. Xue and X. M. Chen, Inorg. Chem., 2009, 48, 6652 CrossRef CAS PubMed; (i) C. P. Li and M. Du, Chem. Commun., 2011, 47, 5958 RSC; (j) L. Q. Ma and W. B. Lin, J. Am. Chem. Soc., 2008, 130, 13834 CrossRef CAS PubMed; (k) R. Peng, S. R. Deng, M. Li, D. Li and Z. Y. Li, CrystEngComm, 2008, 10, 590 RSC; (l) D. L. Michael and D. B. Paul, Coord. Chem. Rev., 2006, 250, 3142 CrossRef; (m) P. A. Gale, M. E. Light and R. Quesada, Chem. Commun., 2005, 5864 RSC.
- (a) G. A. Senchyk, A. B. Lysenko, H. Krautscheid, E. B. Rusanov, A. N. Chernega, K. W. Krämer, S. X. Liu, S. Decurtins and K. V. Domasevitch, Inorg. Chem., 2013, 52, 863 CrossRef CAS PubMed; (b) J. H. Zhou, R. M. Cheng, Y. Song, Y. Z. Li, X. T. Chen, Z. L. Xue and X. Z. You, Inorg. Chem., 2005, 44, 8011 CrossRef CAS PubMed; (c) E. C. Yang, B. Ding, Z. Y. Liu, Y. L. Yang and X. J. Zhao, Cryst. Growth Des., 2012, 12, 1185 CrossRef CAS; (d) H. A. Habib, J. Sanchiz and C. Janiak, Inorg. Chim. Acta, 2009, 362, 2452 CrossRef CAS; (e) Y. X. Shi, F. L. Hu, W. H. Zhang and J. P. Lang, CrystEngComm, 2015, 17, 9404 RSC; (f) L. Qin, L. N. Wang, P. J. Ma and G. H. Cui, J. Mol. Struct., 2014, 1059, 202 CrossRef CAS; (g) L. L. Liu, C. X. Yu, F. J. Ma, Y. R. Li, J. J. Han, L. Lin and L. F. Ma, Dalton Trans., 2015, 44, 1636 RSC; (h) X. X. Wang, B. Y. Yu, K. V. Hecke and G. H. Cui, RSC Adv., 2014, 4, 61281 RSC; (i) N. N. Adarsh, P. Sahoo and P. Dastidar, Cryst. Growth Des., 2010, 10, 4976 CrossRef CAS; (j) M. H. Mir, S. Kitagawa and J. J. Vittal, Inorg. Chem., 2008, 47, 7728 CrossRef CAS PubMed; (k) G. H. Cui, C. H. He, C. H. Jiao, J. C. Geng and V. A. Blatov, CrystEngComm, 2012, 14, 4210 RSC; (l) S. Q. Zang, M. M. Dong, Y. J. Fan, H. W. Hou and T. C. W. Mak, Cryst. Growth Des., 2012, 12, 1239 CrossRef CAS; (m) Q. Chu, Z. Su, J. Fan, T. Okamura, G. C. Lv, G. X. Liu, W. Y. Sun and N. Ueyama, Cryst. Growth Des., 2011, 11, 3885 CrossRef CAS.
- (a) J. G. Ding, X. Zhu, Y. F. Cui, N. Liang, P. P. Sun, Q. Chen, B. L. Li and H. Y. Li, CrystEngComm, 2014, 16, 1632 RSC; (b) S. S. Han, L. L. Shi, K. Li, S. Zhao, B. L. Li and B. Wu, RSC Adv., 2015, 5, 107166 RSC; (c) Y. F. Peng, S. Zhao, K. Li, L. Liu, B. L. Li and B. Wu, CrystEngComm, 2015, 17, 2544 RSC; (d) K. Li, X. X. Lv, L. L. Shi, L. Liu, B. L. Li and B. Wu, Dalton Trans., 2016, 45, 15078 RSC; (e) L. Liu, Y. F. Peng, X. X. Lv, K. Li, B. L. Li and B. Wu, CrystEngComm, 2016, 18, 2490 RSC; (f) S. Zhao, X. X. Lv, L. L. Shi, B. L. Li and B. Wu, RSC Adv., 2016, 6, 56035 RSC; (g) X. X. Lv, L. L. Shi, K. Li, B. L. Li and H. Y. Li, Chem. Commun., 2017, 53, 1860 RSC; (h) L. L. Shi, T. R. Zheng, M. Li, L. L. Qian, B. L. Li and H. Y. Li, RSC Adv., 2017, 7, 23432 RSC.
- (a) C. R. Murdock, N. W. McNutt, D. J. Keffer and D. M. Jenkins, J. Am. Chem. Soc., 2014, 136, 671 CrossRef CAS PubMed; (b) R. M. Christopher and M. J. David, J. Am. Chem. Soc., 2014, 136, 10983 CrossRef PubMed.
- (a) R. Jacobson, Private communication to the Rigaku Corporation, Tokyo, Japan, 1998; Search PubMed; (b) CrysAlisPro, Agilent Technologies, Version 1.171.36.32 (release 02-08-2013 CrysAlis171.NET); Search PubMed; (c) L. Krause, R. Herbst-Irmer, G. M. Sheldrick and D. Stalke, J. Appl. Crystallogr., 2015, 48, 3 CAS.
- (a) G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed; (b) G. M. Sheldrick, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2015, 71, 3 CrossRef PubMed.
- A. L. Spek, Acta Crystallogr., Sect. A: Found. Crystallogr., 1990, 46, 194 CrossRef.
- Y. Q. Sun, J. Zhang and G. Y. Yang, J. Coord. Chem., 2004, 57, 1299 CrossRef CAS.
- (a) V. A. Blatov, M. O'Keeffe and D. M. Proserpio, CrystEngComm, 2010, 12, 44 RSC; (b) E. V. Alexandrov, V. A. Blatov, A. V. Kochetkova and D. M. Proserpio, CrystEngComm, 2011, 13, 3947 RSC; (c) M. O'Keeffe and O. M. Yaghi, Chem. Rev., 2012, 112, 675 CrossRef PubMed; (d) V. A. Blatov, A. P. Shevchenko and D. M. Proserpio, Cryst. Growth Des., 2014, 14, 3576 CrossRef CAS.
- P. Wang, R. Q. Fan, Y. L. Yang, X. R. Liu, W. W. Cao and B. Yang, J. Solid State Chem., 2012, 196, 441 CrossRef CAS.
- (a) S. Z. Zhan, D. Li, X. P. Zhou and X. H. Zhou, Inorg. Chem., 2006, 45, 9163 CrossRef CAS PubMed; (b) S. Z. Zhan, M. Li, X. P. Zhou, J. Ni, X. C. Huang and D. Li, Inorg. Chem., 2011, 50, 8879 CrossRef CAS PubMed.
- D. F. Li, L. M. Zheng, X. Y. Wang, J. Huang, S. Gao and W. X. Tang, Chem. Mater., 2003, 15, 2094 CrossRef CAS.
- (a) H. A. Habib, J. Sanchiz and C. Janiak, Inorg. Chim. Acta, 2009, 362, 2452 CrossRef CAS; (b) M. D. Allendorf, C. A. Bauer, R. K. Bhakta and R. J. T. Houk, Chem. Soc. Rev., 2009, 38, 1330 RSC; (c) X. L. Wang, Y. F. Bi, H. Y. Lin and G. C. Liu, Cryst. Growth Des., 2007, 7, 1086 CrossRef CAS; (d) R. Sun, Y. Z. Li, J. F. Bai and Y. Pan, Cryst. Growth Des., 2007, 7, 890 CrossRef CAS; (e) X. W. Wang, J. Z. Chen and J. H. Liu, Cryst. Growth Des., 2007, 7, 1227 CrossRef CAS; (f) X. L. Wang, C. Qin, E. B. Wang, L. Xu, Z. M. Su and C. W. Hu, Angew. Chem., Int. Ed., 2004, 43, 5036 CrossRef CAS PubMed; (g) H. Yersin and A. Vogler, Photochemistry and Photophysics of Coordination Compounds, Springer, Berlin, 1987; Search PubMed; (h) B. Valeur, Molecular Fluorescence: Principles and Application, Wiley-VCH, Weinheim, 2002 Search PubMed.
- (a) Q. Y. Huang, T. Li and X. R. Meng, Inorg. Chem. Commun., 2014, 49, 52 CrossRef; (b) C. C. Wang, H. P. Jing, P. Wang and S. J. Gao, J. Mol. Struct., 2015, 1080, 44 CrossRef CAS; (c) R. H. Cui, G. J. Xu and Z. H. Jiang, J. Coord. Chem., 2011, 64, 222 CrossRef CAS; (d) F. Wang, X. H. Ke, J. B. Zhao, K. J. Deng, X. K. Leng, Z. F. Tian, L. L. Wen and D. F. Li, Dalton Trans., 2011, 40, 11856 RSC; (e) H. Wang, F. Y. Yi, S. Wang, W. G. Tian and Z. M. Sun, Cryst. Growth Des., 2014, 14, 147 CrossRef CAS; (f) G. X. Liu, K. Zhu, H. Chen, R. Y. Huang and X. M. Ren, Z. Anorg. Allg. Chem., 2009, 635, 156 CrossRef CAS; (g) J. J. Wang, M. L. Yang, H. M. Hua, G. L. Xue, D. S. Li and Q. Z. Shi, Z. Anorg. Allg. Chem., 2007, 633, 341 CrossRef CAS; (h) Z. L. Xu, F. Y. Liu, Y. Xu, J. Wang, Y. Li and X. Y. Wang, Chin. J. Struct. Chem., 2014, 33, 1516 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: PXRD patterns, TGA curve and additional figures. CCDC 1554826–1554833. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ce01176h |
| This journal is © The Royal Society of Chemistry 2017 |