 Open Access Article
Open Access ArticleThe reasons for the photochemical behaviour of propylamine 4-(2,4,6-triisopropylbenzoyl)benzoate during the Norrish–Yang reaction†
K.
Konieczny
,
J.
Bąkowicz
 *,
T.
Galica
,
R.
Siedlecka
and
I.
Turowska-Tyrk
*,
T.
Galica
,
R.
Siedlecka
and
I.
Turowska-Tyrk
Faculty of Chemistry, Wrocław University of Science and Technology, Wybrzeże, Wyspiańskiego 27, 50-370 Wrocław, Poland. E-mail: julia.bakowicz@pwr.edu.pl; Fax: +48 71 320 33 64
First published on 4th May 2017
Abstract
The path of the Norrish–Yang reaction in crystals of propylamine 4-(2,4,6-triisopropylbenzoyl)benzoate was monitored under ambient pressure by means of single-crystal XRD experiments. The crystal structures containing product and reactant molecules in various proportions, i.e. for various stages of the reaction, were determined. The reaction was successfully monitored until 30% conversion. During the reaction, both o-isopropyl groups created product molecules, however, at different rates. In search of the reasons for such photochemical behaviour of reactant molecules, the intramolecular geometry, size and shape of free space and intermolecular interactions were analyzed. It occurred that the intermolecular interactions had the crucial significance for the reactivity. The progress of the Norrish–Yang reaction in crystals under a high pressure of 1.0 GPa was monitored based on the changes in the cell constants. The high-pressure structure of the pure reactant crystal was determined at 1.0 GPa. Comparison of high and ambient-pressure structures revealed a similarity in the intramolecular geometry and significant changes in the area of molecules, which can influence the photochemical behaviour of the compound at high pressure. The characteristics and the size of the structural changes brought about by the Norrish–Yang reaction are different at ambient and high pressures.
Introduction
For the Norrish–Yang reaction of salts of 4-(2,4,6-triisopropylbenzoyl)benzoic acid, each o-isopropyl can take part in and create a four-membered ring, since both groups are chemically equivalent. The equation for this reaction is given in Scheme 1. However, in crystals, these o-isopropyl groups usually have a different surrounding environment and slightly different orientation in a molecule, and because of this, they have different photochemical reactivity.1,2 The prediction of the proceeding and the direction of the Norrish–Yang reaction in crystals of 2,4,6-triisopropylbenzoates can be carried out based on the intramolecular geometrical parameters describing the reaction centre and/or the size and shape of free space near reactive molecular fragments.1–3In the scientific literature, there are two known documented structures of 4-(2,4,6-triisopropylbenzoyl)benzoates forming product molecules in more than one way during the Norrish–Yang reaction in crystals, namely, methylamine 4-(2,4,6-triisopropylbenzoyl)benzoate (CCDC 1520028)3 and phenylmethylamine 4-(2,4,6-triisopropylbenzoyl)benzoate (refcode SOFPEJ).4 The first mentioned compound gave two products as a result of the Norrish–Yang reaction of two o-isopropyl groups (certainly, only one group in one molecule). For the above-mentioned second compound, both o-isopropyl groups also took part in the Norrish–Yang reaction, but additionally in some molecules, one group created a five-membered ring. The amounts of products in crystals were not equal and in both cases, the above-mentioned reasons for photochemical behaviour were involved in the explanation of this observation, namely: for the methylamine salt, due to the significant change in the volume of free space near both o-isopropyl groups during the reaction and for the phenylmethylamine salt, owing to the geometrical differences between the reaction centres at 2- and 6-isopropyls.
In this paper, we present the next compound of which two o-isopropyl groups are photochemically reactive to a different degree: propylamine 4-(2,4,6-triisopropylbenzoyl)benzoate, compound 1 (see Scheme 1), and describe another reason for the observed differences in the photochemical reactivity.
Experimental
4-(2,4,6-Triisopropylbenzoyl)benzoic acid was prepared via acylation of 1,3,5-triisopropylbenzene with 4-carbomethoxybenzoyl chloride followed by the basic hydrolysis of the obtained product, according to a known procedure.5 Propylamine was added to a solution of 4-(2,4,6-triisopropylbenzoyl)benzoic acid (44.4 mg, 0.126 mmol) in absolute ethanol in a stoichiometric ratio. After evaporation of the solvent at room temperature, crystals of compound 1 were obtained.The Norrish–Yang reaction in the studied crystals was induced by irradiation using a 100 W Hg lamp. In order to conduct the reaction in a homogenous manner, a BG39 filter, which transmits wavelengths from a low-energy absorption tail of the compound, was used.6,7 The transmittance for the applied filter is: 0% for 320 > λ > 620 nm, 55% for ∼350 nm and 95% for ∼460 nm. In order to prevent the crystals from heating, a water filter was used. All the experiments were conducted in darkness.
Crystal 1 was examined under ambient conditions. Its irradiation times were 0, 5, 15, 35 and 50 min in total. After each irradiation, the XRD experiment was carried out using a diffractometer equipped with a CCD detector operated by the CrysAlisPro software.8 After each data collection, the unit cell parameters were determined. The data allowed us to determine the structures of crystal 1 irradiated for 0, 5, 15 and 35 min.
In turn, crystal 2 was examined at high pressure. The high pressure was generated using a Boehler-Almax diamond anvil cell (DAC).9 The DAC was mounted on the diffractometer and aligned using the gasket-shadow centering procedure.10 As a hydrostatic medium, a glycerine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (vol. 3
water (vol. 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) mixture was used. A single crystal of quartz was used as a pressure sensor and the value of pressure was determined on the basis of changes in the unit cell parameters.11 The pressure generated in the DAC was 1.0(1) GPa. The times of irradiation for crystal 2 were 0, 5, 15, 25 and 45 min in total. After each time of irradiation, data collections were carried out and the values of the unit cell parameters were determined. However, the quality of the obtained data allowed us to determine the crystal structure of the pure reactant only, i.e. before irradiation.
2) mixture was used. A single crystal of quartz was used as a pressure sensor and the value of pressure was determined on the basis of changes in the unit cell parameters.11 The pressure generated in the DAC was 1.0(1) GPa. The times of irradiation for crystal 2 were 0, 5, 15, 25 and 45 min in total. After each time of irradiation, data collections were carried out and the values of the unit cell parameters were determined. However, the quality of the obtained data allowed us to determine the crystal structure of the pure reactant only, i.e. before irradiation.
Crystal structures were solved and refined using the SHELXS and SHELXL software.12 In the structures containing reactant molecules only (i.e. after 0 and 5 min of irradiation), hydrogen atoms were found in a difference Fourier map, except hydrogen atoms of the cation and the methyl groups of the anion, which were positioned geometrically with Uiso = 1.5Ueq for the parent carbon atom, and Uiso = 1.2Ueq for atoms C25A, C26A, C25B and C26B.
For the crystal structures obtained after 15 and 35 min of irradiation, characterized by disorder being a consequence of the presence of reactant and product molecules, a set of restraints was used, namely, DFIX, DANG, SIMU and FLAT. For both structures, the anion of the major component was refined anisotropically and the anion of the minor components, isotropically. The cation was refined anisotropically. All hydrogen atoms were treated as riding ones. The hydrogen atom in a hydroxyl group was omitted. In the case of all structures determined at ambient pressure, the cation was disordered. The carbon atoms in the cation were positioned in two occupancy sites, whereas the nitrogen atoms were given the same coordinates and ADPs. The disorder had a dynamic character and was not observed under conditions of high pressure. Because of this, the site occupancy factor (SOF) of the cation at ambient pressure was refined independently of reactant/product anions' SOFs.
For the high-pressure crystal structure, DFIX and SIMU restraints were used. The carbon atoms in the methyl groups in the anion and the oxygen atoms were refined anisotropically. The remaining atoms were refined isotropically. The hydrogen atoms connected to the nitrogen atom were treated as riding and rotating and the remaining hydrogen atoms were refined as riding.
The crystallographic and experimental data for all determined crystal structures are given in Table 1.
| Ambient pressure (0.1 MPa) | 1.0 GPa | ||||
|---|---|---|---|---|---|
| 0 min | 5 min | 15 min | 35 min | 0 min | |
| Reactant content/% | 100 | 100 | 92.6(4) | 69.9(3) | 100 |
| P product content/% | 0 | 0 | 7.4(4) | 23.3(3) | 0 |
| Z product content/% | 0 | 0 | 0 | 6.8(3) | 0 |
| Chemical formula | C26H37O3N | C26H37O3N | C26H37O3N | C26H37O3N | C26H37O3N |
| Formula weight | 411.56 | 411.56 | 411.56 | 411.56 | 411.56 |
| Crystal dimensions/mm | 0.56 × 0.15 × 0.13 | 0.56 × 0.15 × 0.13 | 0.56 × 0.15 × 0.13 | 0.56 × 0.15 × 0.13 | 0.13 × 0.12 × 0.10 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21/c | P21/c | P21/c | P21/c | P21/c |
| a/Å | 24.3213(8) | 24.2843(7) | 24.3127(8) | 24.429(2) | 23.695(8) |
| b/Å | 6.2222(3) | 6.21701(19) | 6.2174(2) | 6.1940(6) | 6.000(9) |
| c/Å | 16.8774(8) | 16.8622(6) | 16.8560(6) | 16.7949(19) | 16.575(3) |
| β/° | 93.894(4) | 93.924(3) | 93.778(3) | 93.294(9) | 95.50(2) |
| V/Å3 | 2548.19(19) | 2539.81(14) | 2542.45(15) | 2537.1(4) | 2346(4) |
| Z | 4 | 4 | 4 | 4 | 4 |
| D x /mg m−3 | 1.073 | 1.076 | 1.075 | 1.077 | 1.165 |
| μ/mm−1 | 0.069 | 0.069 | 0.069 | 0.069 | 0.075 |
| T/K | 299(2) | 299(2) | 299(2) | 299(2) | 299(2) |
| Reflections collected | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 026 026 |
9781 | 9102 | 8673 | 9527 |
| Reflections independent | 5012 | 4998 | 4479 | 4446 | 1383 |
| Reflections observed | 3040 | 3229 | 2942 | 2317 | 441 |
| R int | 0.031 | 0.024 | 0.024 | 0.048 | 0.289 |
| R, wR (F2 > 2σ(F2)), S | 0.064, 0.148, 1.02 | 0.064, 0.147, 1.04 | 0.059, 0.137, 1.03 | 0.112, 0.275, 1.07 | 0.125, 0.317, 1.02 |
| Δρmax, Δρmin/e Å−3 | 0.27, −0.17 | 0.28, −0.21 | 0.22, −0.16 | 0.16, −0.17 | 0.18, −0.20 |
Results
In the case of crystals of compound 1, both o-isopropyl groups created product molecules along with the progress of the Norrish–Yang reaction (see Scheme 1). However, they did not take part in the reaction leading to a product containing a five-membered ring. The reason for this fact is connected with the unsuitable geometry of the reaction centre, i.e. the distance between potentially reactive carbon atoms is too large for the reaction to occur in crystals (above 3.7 Å). For the purposes of further discussion, the product molecules created by the 6-isopropyl and 2-isopropyl groups were named P and Z, respectively. The reaction rate for compound 1 was different for each o-isopropyl group. The product molecules were at first created by the 6-isopropyl group, and after 15 min of UV irradiation, its content was 7.4%. At this stage of the reaction, the content of the product created by the 2-isopropyl group was too small to be taken into account during the crystal structure determination. After 35 min of UV irradiation, the content of the P product was 23.3% while that of the Z product was only 6.8%. The product content was determined on the basis of the site occupancy factor (SOF). The determined crystal structures are presented in Fig. 1.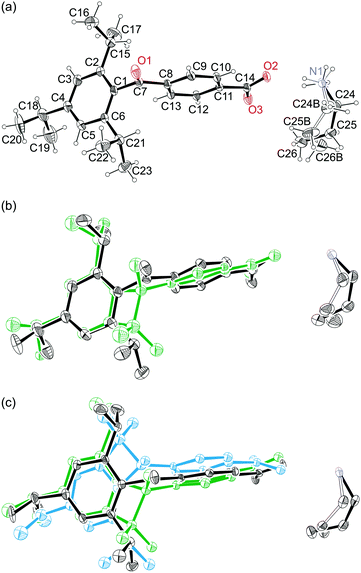 | ||
| Fig. 1 The ORTEP13 view of the crystal containing (a) 100% of the reactant, (b) 92.6% of the reactant and 7.4% of the P product and (c) 69.9% of the reactant, 23.3% of the P product and 6.8% of the Z product. The atomic displacement parameters were drawn at a 20% probability level. Hydrogen atoms were omitted in plots (b) and (c) for clarity. | ||
Further crystal irradiation caused the further progress of the reaction, which was evidenced by the changes in the unit cell parameters (see Fig. 2a). Nevertheless, this also decreased the quality of the sample, which consequently did not allow us to determine the crystal structure and to determine the products' content.
 | ||
| Fig. 2 The relative changes in the unit cell parameters with the time of UV irradiation at (a) ambient pressure and (b) 1.0 GPa. | ||
A question has arisen on why the two chemically equivalent o-isopropyl groups have different photochemical behaviour. The answer is not as straightforward as it was in the case of methylamine 4-(2,4,6-triisopropylbenzoyl)benzoate studied by us previously.3
One of the factors influencing the photochemical reactivity of compounds in crystals is connected with their molecular geometry. The susceptibility to the Norrish–Yang reaction can be estimated based on the following geometrical parameters: the distances between atoms directly taking part in the reaction, i.e. between γ-hydrogen and carbonyl oxygen (d) and between γ-carbon and carbonyl carbon (D), angles C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H (Δ), C–H⋯O (Θ) and torsion between a γ-hydrogen atom and a plane of a carbonyl group (ω).14–16 When we take into account the ranges known from the literature of the above geometrical parameters (see Table 2), we can state that for compound 1, both o-isopropyl groups can take part in the Norrish–Yang reaction, because the values of their geometrical parameters are within the literature ranges. Further analysis of the geometry around the 2- and 6-isopropyl groups indicates that parameters d, D and ω are very similar for both groups, but parameters Δ and Θ have slightly more favourable values in the case of the 2-isopropyl group, i.e. the group creating less product molecules. However, the differences between the values of parameters Δ and Θ might be too small to determine the reactivity of each group.
O⋯H (Δ), C–H⋯O (Θ) and torsion between a γ-hydrogen atom and a plane of a carbonyl group (ω).14–16 When we take into account the ranges known from the literature of the above geometrical parameters (see Table 2), we can state that for compound 1, both o-isopropyl groups can take part in the Norrish–Yang reaction, because the values of their geometrical parameters are within the literature ranges. Further analysis of the geometry around the 2- and 6-isopropyl groups indicates that parameters d, D and ω are very similar for both groups, but parameters Δ and Θ have slightly more favourable values in the case of the 2-isopropyl group, i.e. the group creating less product molecules. However, the differences between the values of parameters Δ and Θ might be too small to determine the reactivity of each group.
| d [Å] | D [Å] | Θ [°] | Δ [°] | ω [°] | |
|---|---|---|---|---|---|
| a The range of the parameters is taken from a reference.3 | |||||
| 0.1 MPa | |||||
| Compound 1, 2-isopropylCompound 1, 6-isopropyl | 2.8922.904 | 2.924(4)2.910(3) | 116.1113.9 | 54.954.3 | 81.582.8 |
| 1.0 GPa | |||||
| Compound 1, 2-isopropylCompound 1, 6-isopropyl | 2.882.85 | 2.90(2)2.86(3) | 117117 | 5552 | 8387 |
| Ideal values | <2.7 | 180 | 90–120 | 0 | |
| Literature rangea | 2.39–3.07 | 2.82–3.12 | 104.3–131.6 | 52.0–88.0 | 50.8–85.5 |
Since the geometrical parameters of the reaction centre do not reveal the reasons for the different reaction rates for the 2- and 6-isopropyl groups, we were looking for the reasons for such behaviour in the surrounding environment of the reaction centre. In general, the size, shape and elasticity of a reaction cavity influence the reaction path and kinetics.17–20 We analyzed the volume and the shape of free space near both o-isopropyl groups. We stated that the amount of free space is relatively small within close proximity to both of them. Nevertheless, the void near the less reactive 2-isopropyl group is slightly bigger (by 8 Å3; calculated by means of the Platon software21 using the radius of a rolling ball of 0.6 Å and a grid of 0.2 Å), which is contrary to the expectations and does not rationalize the observed differences between the photochemical behaviours of both o-isopropyl groups. The difference in the shapes and sizes of the voids is not big enough to determine the reactivity. Moreover, the formation of a four-membered ring in the reaction requires a shift of the whole o-isopropyl group, including the methyl groups. According to this, there is a necessity of free space also on the way from these methyl groups towards the carbonyl group. We found that there is sufficient volume of free space enabling the methyl groups of both o-isopropyl groups to shift.
All the above-mentioned arguments did not allow us to explain the photochemical behaviour of molecules in crystals. For both o-isopropyl groups, the differences in the geometrical parameters and in the free space are nuances rather than a real discrepancy. Nevertheless, both o-isopropyl groups react differently and a reason for this has to exist. In the case of previously studied compounds,1,3,22 there were always crucial factors influencing the molecular reactivity and they were directly connected with free space and/or intramolecular geometrical parameters.
In the case of compound 1, the reason for the differences in the reaction rates of both o-isopropyl groups is almost definitely the intermolecular interactions in crystals. The 6-isopropyl group takes part in a larger number of interactions than the 2-isopropyl group. In order to compare the intermolecular interactions, we carried out analysis of fingerprints for both o-isopropyl groups (see Fig. 3). In the case of 6-isopropyl, there are easily noticeable short C–H⋯O contacts between C23, H23c and the carbonyl oxygen O1i (i = x, −1 + y, z) and a short H⋯H contact between H22a and H12ii (ii = x, 3/2 − y, 1/2 + z) (see Fig. 4 and Table 3). The distances of H23c⋯O1i, C23⋯O1i and H22a⋯H12ii are 2.635, 3.591 and 2.350 Å, respectively. All these contacts can influence the photochemical reactivity. The mentioned C–H⋯O interactions, which are attractive, favour the movement of the methyl group towards its position occupied in the product molecule. On the other hand, the mentioned H⋯H contact seems to be repulsive, since the distance between the hydrogen atoms is short. This repulsion can support the movement of the methyl groups during the reaction. The relationship between the course of photochemical reactions in crystals and the intermolecular interactions was also observed in compounds undergoing linkage isomerization.25–29
 | ||
| Fig. 3 The fingerprint plots23,24 for (a) the 2-isopropyl and (b) 6-isopropyl groups for the crystal structure before UV irradiation at ambient pressure. The red arrows indicate the intermolecular interactions discussed in the paper. | ||
 | ||
| Fig. 4 The intermolecular interactions influencing the photochemical reactivity of compound 1. For clarity, the cation and some of the hydrogen atoms were omitted. | ||
| H23c⋯O1i [Å] | C23⋯O1i [Å] | C23–H23c⋯O1i [°] | |
|---|---|---|---|
| Symmetry codes: i = x, −1 + y, z, ii = x, 3/2 − y, 1/2 + z. | |||
| 0.1 MPa | 2.635 | 3.591(4) | 174.37 |
| 1.0 GPa | 2.49 | 3.44(3) | 170 |
| H22a⋯H12ii | |||
|---|---|---|---|
| 0.1 MPa | 2.350 | ||
| 1.0 GPa | 2.27 |
Along with the time of UV irradiation, the unit cell parameters change, which is an indication of the photochemical reaction proceeding in the crystals. The relative changes in a, b, and c are shown in Fig. 2a. Parameter a increases during the photochemical transformation of the crystal while parameters b and c decrease. All these changes are smooth.
The results obtained in the high pressure experiments show that the Norrish–Yang reaction of compound 1 proceeds also at 1.0 GPa, as evidenced by the changes in the unit cell parameters (see Fig. 2b). The characteristics of the changes in parameters a and c are the same as those under ambient pressure; however, parameter b is statistically constant at a 3σ level, which is opposite to the ambient behaviour of compound 1. The differences in the characteristics of such changes were also observed by us in the case of other compounds.30,31 Moreover, parameter a changes more quickly and parameter c, more slowly at ambient pressure than at high pressure. All this indicate that the structural changes brought about by the Norrish–Yang reaction at ambient and high pressures are different.
The intramolecular geometrical parameters describing the susceptibility of compound 1 to the Norrish–Yang reaction do not significantly change with the increase of pressure (see Table 2) and the same result is valid for the geometry of a whole molecule. The high pressure decreases the volume of free space in the crystal from 464 Å3 at ambient pressure to 301 Å3 at 1.0 GPa, which is shown in Fig. 5a. In connection to this, intermolecular distances also become shorter, including those in which the reactive o-isopropyl groups participate (see Fig. 5b and Table 3). All these changes indicate that the reaction path at 1.0 GPa might be different from that at ambient pressure. The increase of intermolecular interactions can influence the ratio of product molecules P and Z, since the intermolecular interactions were responsible for the difference between the contents of both products at ambient pressure. Moreover, the decrease of the volume of free space in the crystal at high pressure might cause the decrease of the photochemical reactivity of compound 1. Such a situation was observed in the case of benzylammonium 4-(2,4,6-triisopropylbenzoyl)benzoate.2 In general, high pressure causes changes in crystal structures. In particular, it modifies the intermolecular interactions and the size, shape and flexibility of reaction cavities. In this way, high pressure can influence the reactivity of compounds in crystals. The effect of high pressure on photochemical reactions was presented in our previous papers for several compounds2,30,31,34,35 and by Boldyreva and co-workers.36
 | ||
| Fig. 5 (a) The free space in the crystal at ambient pressure and 1.0 GPa calculated by means of the Mercury software32 using the radius of a rolling ball of 0.6 Å and the grid of 0.2 Å. (b) The Hirshfeld surface of the anionic species for the crystals studied at ambient pressure and 1.0 GPa for two opposite sides of the molecules, calculated as dnorm using the CrystalExplorer software.24,33 | ||
Conclusion
The path of the Norrish–Yang reaction in crystals of propylamine 4-(2,4,6-triisopropylbenzoyl)benzoate was studied. The reaction proceeds at ambient pressure and at 1.0 GPa. Under ambient pressure, the path of the reaction was monitored until 30% conversion by means of the changes in the molecular geometry, unit cell parameters and content of the products. Along with the irradiation time, molecules of two products are formed: the first one is created by the 6-isopropyl group and the second one by the 2-isopropyl group. The majority of the molecules are formed by the 6-isopropyl group. In order to explain why the 6-isopropyl group is favoured in the reaction, we analyzed the size and shape of the reaction cavity by means of the intramolecular geometrical parameters, the size and shape of the free space near the reaction centre and the remote environment of the reactive fragments by means of the intermolecular geometrical parameters. In the case of the studied compound, the intermolecular interactions had the crucial importance for defining the reactivity of both o-isopropyl groups.The high-pressure structure of the pure reactant crystal at 1.0 GPa was determined. The high pressure did not significantly change the geometry of the reaction centre and molecules, but it considerably decreased the volume of the free space and the distances of intermolecular interactions. The proceeding of the Norrish–Yang reaction at high pressure was evidenced by the progressing changes in the unit cell parameters along with UV irradiation. Moreover, the characteristics and the size of these changes are different at ambient and high pressures.
Acknowledgements
This work was financed by the statutory activity subsidy from the Polish Ministry of Science and Higher Education for the Faculty of Chemistry of the Wroclaw University of Science and Technology.References
- J. Bąkowicz, J. Olejarz and I. Turowska-Tyrk, J. Photochem. Photobiol., A, 2014, 273, 34–42 CrossRef.
- K. Konieczny, J. Bąkowicz and I. Turowska-Tyrk, CrystEngComm, 2015, 17, 7693–7701 RSC.
- K. Konieczny, J. Bąkowicz, R. Siedlecka, T. Galica and I. Turowska-Tyrk, Cryst. Growth Des., 2017, 17, 1347–1352 CAS.
- H. Koshima, Y. Ide, M. Fukano, K. Fujii and H. Uekusa, Tetrahedron Lett., 2008, 49, 4346–4348 CrossRef CAS.
- Y. Ito, G. Kano and N. Nakamura, J. Org. Chem., 1998, 63, 5643–5647 CrossRef CAS.
- V. Enkelmann, G. Wegner, K. Novak and K. B. Wagener, J. Am. Chem. Soc., 1993, 115, 10390–10391 CrossRef CAS.
- K. Novak, V. Enkelmann, G. Wegner and K. B. Wagener, Angew. Chem., Int. Ed. Engl., 1993, 32, 1614–1616 CrossRef.
- CrysAlisPro Software System, version 1.171.38.41, Rigaku Corporation, Oxford, U.K., 2016 Search PubMed.
- R. Boehler, Rev. Sci. Instrum., 2006, 77, 1–4 CrossRef.
- A. Budzianowski and A. Katrusiak, High-Pressure Crystallography, Kluwer Academic Publishers, Dordrecht, Boston, London, 2004 Search PubMed.
- R. J. Angel, D. R. Allan, R. Miletich and L. W. Finger, J. Appl. Crystallogr., 1997, 30, 461–466 CrossRef CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 CrossRef PubMed.
- L. J. Farrugia, J. Appl. Crystallogr., 2012, 45, 849–854 CrossRef CAS.
- H. Ihmels and J. R. Scheffer, Tetrahedron, 1999, 55, 885–907 CrossRef CAS.
- A. Natarajan, J. T. Mague and V. Ramamurthy, J. Am. Chem. Soc., 2005, 127, 3568–3576 CrossRef CAS PubMed.
- W. Xia, J. R. Scheffer, M. Botoshansky and M. Kaftory, Org. Lett., 2005, 7, 1315–1318 CrossRef CAS PubMed.
- Y. Ohashi, A. Uchida, Y. Sasada and Y. Ohgo, Acta Crystallogr., Sect. B: Struct. Sci., 1983, 39, 54–61 CrossRef.
- T. Luty and C. J. Eckhardt, J. Am. Chem. Soc., 1995, 117, 2441–2452 CrossRef CAS.
- E. Boldyreva, Solid State Ionics, 1997, 101–103, 843–849 CrossRef CAS.
- T. Luty, Mol. Cryst. Liq. Cryst. Sci. Technol., Sect. A, 2001, 356, 539–548 CrossRef CAS.
- A. L. Spek, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2009, 65, 148–155 CrossRef CAS PubMed.
- K. Konieczny, J. Bakowicz and I. Turowska-Tyrk, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 410–414 CAS.
- M. A. Spackman and J. J. McKinnon, CrystEngComm, 2002, 4, 378–392 RSC.
- S. K. Wolff, D. J. Grimwood, J. J. McKinnon, M. J. Turner, D. Jayatilaka and M. A. Spackman, CrystalExplorer (Version 3.1), University of Western Australia, 2012 Search PubMed.
- E. V. Boldyreva, Mol. Cryst. Liq. Cryst. Sci. Technol., Sect. A, 1994, 242, 17–52 CrossRef.
- N. Masciocchi, A. Kolyshev, V. Dulepov, E. Boldyreva and A. Sironi, Inorg. Chem., 1994, 33, 2579–2585 CrossRef CAS.
- N. E. Kashcheeva, D. Y. Naumov and E. V. Boldyreva, Z. Kristallogr., 1999, 214, 534–541 CAS.
- E. Boldyreva, Russ. J. Coord. Chem., 2001, 27, 297–323 CrossRef CAS.
- P. Coppens, I. Novozhilova and A. Kovalevsky, Chem. Rev., 2002, 102, 861–883 CrossRef CAS PubMed.
- J. Bakowicz and I. Turowska-Tyrk, CrystEngComm, 2014, 16, 6039–6048 RSC.
- T. Galica, J. Bakowicz, K. Konieczny and I. Turowska-Tyrk, CrystEngComm, 2016, 18, 8871–8879 RSC.
- C. F. Macrae, P. R. Edgington, P. McCabe, E. Pidcock, G. P. Shields, R. Taylor, M. Towler and J. van de Streek, J. Appl. Crystallogr., 2006, 39, 453–457 CrossRef CAS.
- M. A. Spackman and D. Jayatilaka, CrystEngComm, 2009, 11, 19–32 RSC.
- K. Konieczny, J. Bakowicz and I. Turowska-Tyrk, J. Photochem. Photobiol., A, 2016, 325, 111–115 CrossRef CAS.
- J. Bakowicz and I. Turowska-Tyrk, CrystEngComm, 2016, 18, 8898–8905 RSC.
- B. A. Zakharov, A. S. Marchuk and E. V. Boldyreva, CrystEngComm, 2015, 17, 8812–8816 RSC.
Footnote |
| † CCDC 1539843–1539847. For crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ce00558j |
| This journal is © The Royal Society of Chemistry 2017 |

