Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An unprecedented Dianin clathrate structure with Z′(host) = 16†
Christopher S.
Frampton
 *a,
Kamal K.
Ketuly
b,
Hapipah B. M.
Ali
c,
Ainnul H. S.
Azizan
c,
James H.
Gall
d and
David D.
MacNicol
*d
*a,
Kamal K.
Ketuly
b,
Hapipah B. M.
Ali
c,
Ainnul H. S.
Azizan
c,
James H.
Gall
d and
David D.
MacNicol
*d
aWolfson Centre for Materials Processing, Brunel University, Kingston Lane, Uxbridge, Middlesex UB8 3PH, UK. E-mail: chris.frampton@brunel.ac.uk; Fax: +44 (0)1895 203376; Tel: +44 (0)1895 265337
bDepartment of Medical Chemistry, College of Medicine, University of Duhok, Duhok, Kurdistan Region, Iraq
cChemistry Department, Faculty of Science, University of Malaya, 50603 Kuala Lumpur, Malaysia
dDepartment of Chemistry, University of Glasgow, Glasgow G12 8QQ, Scotland, UK. E-mail: david.macnicol@glasgow.ac.uk; Tel: +44 (0)141 330 4479
First published on 5th April 2017
Abstract
The structure of the iso-propanol clathrate of 4-p-hydroxyphenyl 2,2,4-trimethylthiachroman, the direct thia-analogue of Dianin's compound, has been studied by single-crystal X-ray diffraction as a function of temperature from 371 K down to 90 K. The standard Dianin unit cell, observed at high temperature, undergoes two sequential commensurate thermal phase transformations which results at low temperature in a unit cell with 16 times the original volume and with Z′(host) = 16 and Z = 288, the space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) being retained. This ultimate unit cell with a quadrupled a-axis has a = 111.7910(5) Å and c = 10.8568(1) Å and the crystal packing now features not only the prototypal [OH]6 hexamer host unit but also novel hydrogen-bonded octameric host–guest units with respective symmetries Ci and C1. In addition it has been established that the corresponding achiral selena-Dianin's clathrate and the polar chiral quasiracemic iso-propanol clathrate, space group R3, formed from R-Dianin's and S-thia-Dianin's components also exhibit novel related temperature-dependant behaviour.
being retained. This ultimate unit cell with a quadrupled a-axis has a = 111.7910(5) Å and c = 10.8568(1) Å and the crystal packing now features not only the prototypal [OH]6 hexamer host unit but also novel hydrogen-bonded octameric host–guest units with respective symmetries Ci and C1. In addition it has been established that the corresponding achiral selena-Dianin's clathrate and the polar chiral quasiracemic iso-propanol clathrate, space group R3, formed from R-Dianin's and S-thia-Dianin's components also exhibit novel related temperature-dependant behaviour.
Introduction
Elucidation of the crystal structure of the clathrates formed by 4-p-hydroxyphenyl-2,2,4-trimethylchroman 1 (Scheme 1), widely known as Dianin's compound,1 has allowed interpretation of results from very wide ranging studies of these fascinating inclusion compounds.2 In addition, the availability of this detailed structural information has provided the key to the successful design and synthesis of new host molecules related to parent 1, for example 2 and 3. For many years the structural situation appeared completely defined and a basic tenet relating to all the Dianin's clathrates was that they all crystallised in what one may now aptly term the classical clathrate space group, R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) with hexagonal unit cell axes, a ca. 27 Å and c ca. 11 Å, and with universally Z′(host) = 1. A true closed-cage clathrate structure, arising from astute consideration of space group and associated unit cell dimensions alone, was assigned to the adducts of 1 in 1955,3 however it was not until the end of the 1960's that detailed X-ray diffraction studies4 fully characterised the clathrate structure5,6 of 1. Closed hour glass-shaped cavities were found to be formed between C3i [
with hexagonal unit cell axes, a ca. 27 Å and c ca. 11 Å, and with universally Z′(host) = 1. A true closed-cage clathrate structure, arising from astute consideration of space group and associated unit cell dimensions alone, was assigned to the adducts of 1 in 1955,3 however it was not until the end of the 1960's that detailed X-ray diffraction studies4 fully characterised the clathrate structure5,6 of 1. Closed hour glass-shaped cavities were found to be formed between C3i [![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ] symmetric, R66(12), [OH]6 hexameric host units stacked infinitely along the c-axis. At around this time also, exactly parallel and independent results were found7,8 for the closely related isostructural clathrates of thiachroman 2, and the structure9 of the stable isostructural apohost, (empty cage), form of 1 followed immediately from an electron density map phased on the atomic coordinates of the EtOH clathrate of 2. The consistent host packing, in space group R
] symmetric, R66(12), [OH]6 hexameric host units stacked infinitely along the c-axis. At around this time also, exactly parallel and independent results were found7,8 for the closely related isostructural clathrates of thiachroman 2, and the structure9 of the stable isostructural apohost, (empty cage), form of 1 followed immediately from an electron density map phased on the atomic coordinates of the EtOH clathrate of 2. The consistent host packing, in space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) , Z′(host) = 1, found for all the known clathrate structures of these hosts engendered the view that this packing mode was universal for all clathrates of 1 and 2. This perception was buttressed by the knowledge that many host molecules structurally related to 1 and 2 also form isomorphous, although not in all cases isostructural, host lattices which are analogous to those of 1 and 2, invariably with Z′(host) = 1. Examples of such hosts are the selenachroman 3;10 4-p-mercaptophenyl-2,2,4-trimethyl-chroman;11 methyl-substituted counterparts of 2 with 2,2,4,6-and 2,2,4,8-tetramethyl distributions;4 the resorcinol 4-(2,4-dihydroxyphenyl)-2,2,4-trimethylchroman;12 and a 2-nor-methyl analogue13 of 1. The first indication that this packing might not, in fact, represent a unique clathrate potential energy minimum came from the reported unit cell dimensions10 of the EtOH clathrate of 3, which showed relative to the expected dimensions, c essentially remained unchanged but a effectively doubled with a = 57.42(1) Å and c = 10.871(1) Å; however, all attempts to solve this structure were unsuccessful owing to the fact that only X-ray photographic data were available at that time. In 2009 Jacobs and co-workers14 published the structure of the ethylenediamine clathrate of compound 1 which interestingly also demonstrated a unit cell which was doubled in a relative to the archetypal Dianin's cell which contained two distinct unit types wherein one unit contained neutral guest molecules and the second contained partially ionised guests.
, Z′(host) = 1, found for all the known clathrate structures of these hosts engendered the view that this packing mode was universal for all clathrates of 1 and 2. This perception was buttressed by the knowledge that many host molecules structurally related to 1 and 2 also form isomorphous, although not in all cases isostructural, host lattices which are analogous to those of 1 and 2, invariably with Z′(host) = 1. Examples of such hosts are the selenachroman 3;10 4-p-mercaptophenyl-2,2,4-trimethyl-chroman;11 methyl-substituted counterparts of 2 with 2,2,4,6-and 2,2,4,8-tetramethyl distributions;4 the resorcinol 4-(2,4-dihydroxyphenyl)-2,2,4-trimethylchroman;12 and a 2-nor-methyl analogue13 of 1. The first indication that this packing might not, in fact, represent a unique clathrate potential energy minimum came from the reported unit cell dimensions10 of the EtOH clathrate of 3, which showed relative to the expected dimensions, c essentially remained unchanged but a effectively doubled with a = 57.42(1) Å and c = 10.871(1) Å; however, all attempts to solve this structure were unsuccessful owing to the fact that only X-ray photographic data were available at that time. In 2009 Jacobs and co-workers14 published the structure of the ethylenediamine clathrate of compound 1 which interestingly also demonstrated a unit cell which was doubled in a relative to the archetypal Dianin's cell which contained two distinct unit types wherein one unit contained neutral guest molecules and the second contained partially ionised guests.
More recently, Lee et al.15 have studied the iso-propanol (IPA) clathrate of 1 in the temperature range 15 K to 299 K and also found a corresponding cell where doubling of the a axis for this compound occurred just below 180 K with the low temperature form retaining the trigonal space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) , but now with Z′(host) = 4, rather than Z′(host) = 1 characteristic of the archetypal trigonal Dianin's clathrate structure. Cooling to 15 K did not induce any further transformation of the unit cell. We now report novel temperature-dependent behaviour for the 3
, but now with Z′(host) = 4, rather than Z′(host) = 1 characteristic of the archetypal trigonal Dianin's clathrate structure. Cooling to 15 K did not induce any further transformation of the unit cell. We now report novel temperature-dependent behaviour for the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (host
1 (host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest) IPA clathrates of 2 and 3, the direct thia- and selenachroman counterparts of parent 1; and in addition for the remarkable novel polar and chiral (3
guest) IPA clathrates of 2 and 3, the direct thia- and selenachroman counterparts of parent 1; and in addition for the remarkable novel polar and chiral (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) IPA clathrate 6 having quasiracemic host components R-Dianin's compound, 4 and S-thia-Dianin's compound, 5. For 2 and 3 we describe below a further ultimate quadrupling of the a axis compared with standard Dianin's clathrates, producing a hexagonal unit cell with volume increased by sixteen times containing 288 host and 96 IPA guest molecules and featuring 16 host molecules of 2 or 3 in the asymmetric unit, i.e. Z′(host) = 16. Differential scanning calorimetry (DSC) studies have revealed two reversible commensurate phase changes for the IPA clathrate of 2. Above the higher-temperature transition at ca. 368 K, approximately 14 K prior to guest loss and final melting, we have defined the archetypal Dianin's cell, Z′(host) = 1, for 2.
1) IPA clathrate 6 having quasiracemic host components R-Dianin's compound, 4 and S-thia-Dianin's compound, 5. For 2 and 3 we describe below a further ultimate quadrupling of the a axis compared with standard Dianin's clathrates, producing a hexagonal unit cell with volume increased by sixteen times containing 288 host and 96 IPA guest molecules and featuring 16 host molecules of 2 or 3 in the asymmetric unit, i.e. Z′(host) = 16. Differential scanning calorimetry (DSC) studies have revealed two reversible commensurate phase changes for the IPA clathrate of 2. Above the higher-temperature transition at ca. 368 K, approximately 14 K prior to guest loss and final melting, we have defined the archetypal Dianin's cell, Z′(host) = 1, for 2.
Results and discussion
Thia-Dianin's IPA clathrate, 2
For the IPA clathrate of the thiachroman analogue 2, full single crystal X-ray structure datasets were collected at 371 K, 295 K, 200 K and 90 K (Table 1). The crystal structure of the thiachroman clathrate at 295 K is isostructural/isomorphous with the published low temperature forms, (100 K and 15 K), of the IPA clathrate of Dianin's compound15 and with similar unit cell dimensions10 to the EtOH clathrate of 3 with an observed doubling of the a unit cell axis with a = 56.4934(4) Å and c = 10.9041(1) Å and a retention of the trigonal space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) . The asymmetric unit features Z′(host) = 4 and 4/3 IPA guests. In common with the 100 K Dianin's clathrate there exist two distinct hydrogen-bonded host units, illustrated in Fig. 1. The first is a classical [OH]6 hexameric type I host unit, between two of which stacked infinitely along the c-axis, are located two IPA guest molecules close to the 3-fold proper rotation axis, which are statically disordered around a point of
. The asymmetric unit features Z′(host) = 4 and 4/3 IPA guests. In common with the 100 K Dianin's clathrate there exist two distinct hydrogen-bonded host units, illustrated in Fig. 1. The first is a classical [OH]6 hexameric type I host unit, between two of which stacked infinitely along the c-axis, are located two IPA guest molecules close to the 3-fold proper rotation axis, which are statically disordered around a point of ![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) symmetry; the second unit, type II, is an eight-membered hydrogen-bonded composite unit, located on point of
symmetry; the second unit, type II, is an eight-membered hydrogen-bonded composite unit, located on point of ![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) symmetry, comprised of six molecules of 2 and two centrosymmetrically-related guest molecules which are incorporated in the R88(16) hydrogen bonded motif. In this unit there are three crystallographically-independent molecules of 2 and one independent guest molecule, all located in general positions. At 200 K the structure is essentially unaltered; although the
symmetry, comprised of six molecules of 2 and two centrosymmetrically-related guest molecules which are incorporated in the R88(16) hydrogen bonded motif. In this unit there are three crystallographically-independent molecules of 2 and one independent guest molecule, all located in general positions. At 200 K the structure is essentially unaltered; although the ![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) unit's IPA guest component becomes more highly ordered, however, at ca. 180 K autoindexing of a set of orientation frames fails, providing evidence for the incipient commensurate thermal phase transition leading to the formation of a super cell. Further cooling of the crystal down to 90 K leaves the R
unit's IPA guest component becomes more highly ordered, however, at ca. 180 K autoindexing of a set of orientation frames fails, providing evidence for the incipient commensurate thermal phase transition leading to the formation of a super cell. Further cooling of the crystal down to 90 K leaves the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) space group unaltered but results in a further doubling of the a unit cell axis, to a = 111.7910(6) Å, whilst the c-axial dimension remains approximately the same at c = 10.8568(1) Å, a value indicating some modest isotropic contraction owing to the lower temperature. This supercell represents an ultimate quadrupling of the a axis as compared with the standard Dianin clathrates, producing a hexagonal unit cell with volume increased by 16-fold containing 288 thiachroman host molecules and 96 IPA guest molecules and featuring 16 host molecules of 2 in the asymmetric unit, i.e. Z′(host) = 16 and 16/3 IPA guest molecules. The structure of this second super cell is shown in Fig. 2. In this structure, there are now two different types of eight-membered hydrogen-bonded composite (6
space group unaltered but results in a further doubling of the a unit cell axis, to a = 111.7910(6) Å, whilst the c-axial dimension remains approximately the same at c = 10.8568(1) Å, a value indicating some modest isotropic contraction owing to the lower temperature. This supercell represents an ultimate quadrupling of the a axis as compared with the standard Dianin clathrates, producing a hexagonal unit cell with volume increased by 16-fold containing 288 thiachroman host molecules and 96 IPA guest molecules and featuring 16 host molecules of 2 in the asymmetric unit, i.e. Z′(host) = 16 and 16/3 IPA guest molecules. The structure of this second super cell is shown in Fig. 2. In this structure, there are now two different types of eight-membered hydrogen-bonded composite (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) host–guest assemblies present, in addition to the type I [OH]6 hexameric unit which is retained. The first of these is the centrosymmetric type II unit as described above and the second type we denote as type III. There are two crystallographically-independent type III host–guest assemblies and these, unlike the type II units lack any crystallographic symmetry. Notwithstanding, the type III structure, Fig. 2, maintains the R88(16) hydrogen-bonded pattern of type II, although each unit now features six crystallographically independent host molecules, and two IPA molecules which are no longer centrosymmetrically related. Residual density, (∼2.4 e Å−3), is observed close to molecules located in the centrosymmetric type II unit, (point of
2) host–guest assemblies present, in addition to the type I [OH]6 hexameric unit which is retained. The first of these is the centrosymmetric type II unit as described above and the second type we denote as type III. There are two crystallographically-independent type III host–guest assemblies and these, unlike the type II units lack any crystallographic symmetry. Notwithstanding, the type III structure, Fig. 2, maintains the R88(16) hydrogen-bonded pattern of type II, although each unit now features six crystallographically independent host molecules, and two IPA molecules which are no longer centrosymmetrically related. Residual density, (∼2.4 e Å−3), is observed close to molecules located in the centrosymmetric type II unit, (point of ![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) symmetry), possibly indicating a very minor secondary orientation of the host molecule at this temperature, (90 K), or perhaps suggesting the possibility of a further evolution of the structure at lower temperatures, see ESI† part 2. The IPA clathrate of the selenachroman analogue 3 also demonstrates a quadrupling of the unit cell parameters with respect to the archetypal Dianin's compound and is isostructural/isomorphous with the thiachroman analogue described above giving a unit cell at 120 K of a = 113.983(5) Å and c = 10.7937(7) Å and a similar retention of the trigonal space group R
symmetry), possibly indicating a very minor secondary orientation of the host molecule at this temperature, (90 K), or perhaps suggesting the possibility of a further evolution of the structure at lower temperatures, see ESI† part 2. The IPA clathrate of the selenachroman analogue 3 also demonstrates a quadrupling of the unit cell parameters with respect to the archetypal Dianin's compound and is isostructural/isomorphous with the thiachroman analogue described above giving a unit cell at 120 K of a = 113.983(5) Å and c = 10.7937(7) Å and a similar retention of the trigonal space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) .
.
| 2, (371 K) | 2, (295 K) | 2, (200 K) | 2, (90 K) | 3, (120 K) | |
|---|---|---|---|---|---|
| Formula | C18H20O1S1, 0.33(C3H8O1) | C18H20O1S1, 0.33(C3H8O1) | C18H20O1S1, 0.33(C3H8O1) | C18H20O1S1, 0.33(C3H8O1) | C18H20O1Se1, 0.33(C3H8O1) |
| Crystal system | Trigonal | Trigonal | Trigonal | Trigonal | Trigonal |
| Space group |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
| a, Å | 28.246(4) | 56.4934(4) | 56.1623(4) | 111.7910(5) | 113.983(5) |
| b, Å | 28.246(4) | 56.4934(4) | 56.1623(4) | 111.7910(5) | 113.983(5) |
| c, Å | 10.965(3) | 10.9041(1) | 10.8866(1) | 10.8568(1) | 10.7937(7) |
| α, ° | 90 | 90 | 90 | 90 | 90 |
| β, ° | 90 | 90 | 90 | 90 | 90 |
| γ, ° | 120 | 120 | 120 | 120 | 120 |
| V, (Å3) | 7576(3) | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 138.1(5) 138.1(5) |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 738.0(6) 738.0(6) |
117![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 502(1) 502(1) |
121![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 445(14) 445(14) |
| Z′ | 1 | 4 | 4 | 16 | 16 |
| Z | 18 | 72 | 72 | 288 | 288 |
| T, (K) | 371(1) | 295(1) | 200(1) | 90(1) | 120(1) |
| F(000) | 2940 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760 760 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760 760 |
47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 040 040 |
52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 224 224 |
| D calc (g cm−3) | 1.201 | 1.208 | 1.224 | 1.239 | 1.383 |
| λ, resolution (Å) | CuKα, 1.10 | CuKα, 0.80 | CuKα, 0.80 | CuKα, 0.80 | MoKα, 0.80 |
| μ, (mm−1) | 1.686 | 1.696 | 1.719 | 1.740 | 2.226 |
| Crystal size, (mm)3 | 0.30 × 0.30 × 0.25 | 0.50 × 0.49 × 0.47 | 0.55 × 0.53 × 0.53 | 0.55 × 0.53 × 0.53 | 0.45 × 0.45 × 0.40 |
| Number of reflections | 2751 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 313 313 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 182 182 |
269![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 011 011 |
412![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 985 985 |
| Unique reflections, (Rint) | 1311, (0.1779) | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 919, (0.0298) 919, (0.0298) |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 597, (0.0175) 597, (0.0175) |
53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 208, (0.0375) 208, (0.0375) |
49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 107, (0.0860) 107, (0.0860) |
| wR2, all data | 0.4072 | 0.1614 | 0.1435 | 0.1775 | 0.2638 |
| R 1, (I > 2σ(I)) | 0.1493, (711) | 0.0545, (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 538) 538) |
0.0457, (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 305) 305) |
0.0608, (48451) | 0.1092, (29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 966) 966) |
| S on F2 | 1.981 | 1.026 | 1.000 | 1.048 | 1.491 |
| Residual density, (e Å−3) | 0.496, −0.490 | 0.924, −0.739 | 0.554, −0.559 | 2.299, −0.743 | 2.228, −2.908 |
| CCDC number | 1400610 | 1400608 | 1400609 | 1400611 | 1400701 |
Thermal analysis
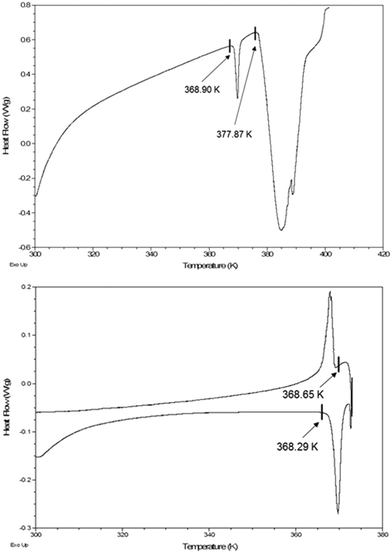
Given that the IPA clathrate of host 2 at 295 K is isostructural with the corresponding clathrate of 1 at 100 K, we have sought to establish if the primary archetypal Dianin cell could be observed for the former clathrate above room temperature and encouraging results were obtained from the DSC traces shown in Fig. 3. The upper trace shows a low energy event of 4.532 J g−1 with onset temperature of 368.90 K compatible with the anticipated transition, with a second larger endothermic event attributable to guest loss and subsequent melting. The origin of the former event was confirmed by the hysteresis shown in the middle trace of Fig. 3, (endotherm onset 368.29 K, energy 4.522 J g−1, exotherm onset 368.65 K, energy 3.364 J g−1). Accordingly, a full crystal structure determination was undertaken on a fresh crystal at 371 K, at which temperature the guest is still retained. The diffraction intensities tailed off markedly at this temperature after 1.0 Å resolution, however the truncated dataset confirmed the existence of the archetypal small cell, space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) , with a = 28.246(4) Å and c = 10.965(3) Å and Z′(host) = 1, with the guest molecule on the three-fold proper rotation axis having the expected statistical disorder as illustrated in Fig. 4. Cooling the crystal back to room temperature gave a unit cell consistent with the 295 K structure and showed that no significant guest loss had occurred during high-temperature data collection. It was also possible to observe the lower temperature reversible phase transition, (lower trace), by cooling the sample to 90 K and allowing the temperature to increase naturally. The exothermic phase transition was observed at 182.23 K with an associated energy of 14.15 J g−1.
, with a = 28.246(4) Å and c = 10.965(3) Å and Z′(host) = 1, with the guest molecule on the three-fold proper rotation axis having the expected statistical disorder as illustrated in Fig. 4. Cooling the crystal back to room temperature gave a unit cell consistent with the 295 K structure and showed that no significant guest loss had occurred during high-temperature data collection. It was also possible to observe the lower temperature reversible phase transition, (lower trace), by cooling the sample to 90 K and allowing the temperature to increase naturally. The exothermic phase transition was observed at 182.23 K with an associated energy of 14.15 J g−1.
Quasiracemate, 6
In a recent paper17 we described the retention of robust clathrate forming ability of a quasiracemate formed from the R-enantiomeric form of Dianin's compound 4, and the S-enantiomeric form of thia-Dianin's compound 5 which were obtained by chiral HPLC chromatography; this quasiracemic system was shown to undergo supramolecular assembly to form a polar clathrate lattice which was stable even in the absence of a consolidating guest component. Seeking a direct parallel with the above novel temperature-dependent phenomena we also investigated the IPA clathrate of this quasiracemic host system, 6, as a function of temperature, see Table 2. The structure at 290 K exactly parallels that of 1 itself at this temperature forming an archetypal Dianin's unit cell with a = 27.658(2) Å and c = 10.956(1) Å, space group R3 with a total of 18 molecules in the unit cell. Lowering the temperature to 90 K results in the formation of the first doubled unit cell with a = 55.0808(9) Å and c = 10.8542(2) Å, space group R3 where there are now four independent chroman hosts, 4, and four independent thiachroman hosts, 5, in the asymmetric unit of the structure. The absolute stereochemistry of the quasiracemate was confirmed at both temperatures through the Flack parameter 0.024(5) and 0.006(7) at 290 K and 90 K respectively, see Fig. 5 and 6.| 6, (290 K) | 6, (90 K) | |
|---|---|---|
| Formula | C18H20O2, C18H20O1S1, 0.66(C3H8O1) | |
| Crystal system | Trigonal | Trigonal |
| Space group | R3 | R3 |
| a, Å | 27.6575(15) | 55.0808(9) |
| b, Å | 27.6575(15) | 55.0808(9) |
| c, Å | 10.9557(14) | 10.8542(2) |
| α, ° | 90 | 90 |
| β, ° | 90 | 90 |
| γ, ° | 120 | 120 |
| V, (Å3) | 7257.7(12) | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 518.6(11) 518.6(11) |
| Z′ | 1 | 4 |
| Z | 9 | 36 |
| T, (K) | 290(1) | 90(1) |
| F(000) | 2868 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 472 472 |
| D calc (g cm−3) | 1.221 | 1.243 |
| λ, resolution (Å) | CuKα, 0.80 | CuKα, 0.80 |
| μ, (mm−1) | 1.182 | 1.203 |
| Crystal size, (mm)3 | 0.25 × 0.25 × 0.25 | 0.25 × 0.25 × 0.25 |
| Number of reflections | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 975 975 |
205![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 222 222 |
| Unique reflections, (Rint) | 6498, (0.0182) | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 917, (0.0343) 917, (0.0343) |
| wR2, all data | 0.1186 | 0.0815 |
| R 1, (I > 2σ(I)) | 0.0397, (6269) | 0.0312, (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 644) 644) |
| S on F2 | 1.006 | 1.004 |
| Residual density, (e Å−3) | 0.260, −0.380 | 0.637, −0.699 |
| Flack parameter | 0.024(5) | 0.006(7) |
| CCDC number | 1400607 | 1400606 |
An important feature of the conformation of all the host molecules described above is the proximal relationship of the p-hydroxyphenyl substituent and its syn related methyl group; it may be noted that this conformation avoids a short contact between the syn related which is present in the structures of the enantiomerically pure non-solvated components.17,19
In all the isomorphous clathrate structures described above there are columns located on the three-fold proper rotation axis, comprised of infinitely stacked hexameric host units. Throughout the transformations, all host molecules of a given conformation maintain a fixed uniform distribution with respect to the c-axial direction. The temperature-dependent cell transformations correspond to a progressive ‘wedging apart’ of the trigonal columns; the column separations for 2 being 16.31 Å, 32.62 Å and 64.54 Å for 371 K, 295 K and 90 K respectively. These columns are shown in green in Fig. 1, 2 and 4 and the emergence of the inter-column molecules suggests the possibility of storage of other molecules, for example pharmaceutical actives, in related clathrates. A recent excellent review20 highlights the current interest in high Z′ structures. For very high Z′ cases, the Cambridge Structural Database (CSD),21,22 Version 5.38 update February 2017, shows that currently out of over 876![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 entries there are only 73 unique crystal structures with Z′ greater or equal to 10 and 21 with Z′ greater or equal to 15. It is of interest, therefore, to record that the value Z′(host) = 16 found for 2 (and 3) represents to our knowledge a current universal maximum for all purely organic clathrates, i.e. not including low density metal organic frameworks and indeed is among the highest known for all purely organic crystals. The value of Z at 288 is also the highest known for all structures in the database, the current maximum being 192.
000 entries there are only 73 unique crystal structures with Z′ greater or equal to 10 and 21 with Z′ greater or equal to 15. It is of interest, therefore, to record that the value Z′(host) = 16 found for 2 (and 3) represents to our knowledge a current universal maximum for all purely organic clathrates, i.e. not including low density metal organic frameworks and indeed is among the highest known for all purely organic crystals. The value of Z at 288 is also the highest known for all structures in the database, the current maximum being 192.
Experimental section
Preparation of compounds and clathrates
Compounds 1,22 (ref. 16) and 3 (ref. 10) were all synthesized according to established procedures and compounds 4 and 5 were obtained via the optical resolution of 1 and 2 respectively according to the procedure in ref. 17. Clathrates of 1, 2 and 3 were prepared by recrystallisation of the unsolvated materials from neat IPA. Cocrystallisation of equimolar quantities of 4 and 5 from neat IPA yields the novel polar quasiracemic clathrate 6.Single crystal X-ray diffraction
Variable temperature single crystal X-ray structure data for the iso-propanol clathrate of 2 and the iso-propanol clathrate of the polar quasiracemic host 6 were collected on an Agilent Supernova Dual Source, Cu at Zero, Atlas CCD diffractometer equipped with an Oxford Cryosystems Cobra cooling device. The data was collected using mirror monochromated CuKα radiation, (λ = 1.54178 Å). Crystal structure data for the iso-propanol clathrate of 3 was collected on a Siemens SMART IK diffractometer equipped with an Oxford Cryosystems Cryostream cooling device. The data were collected using graphite monochromated MoKα radiation, (λ = 0.71069 Å). Structures were typically solved and refined with the SHELX18 suite of programs. Unless otherwise stated, hydrogen atoms attached to carbon were placed geometrically and allowed to refine with a riding isotropic displacement parameter. Hydrogen atoms attached to a heteroatom were located in a difference Fourier synthesis and were allowed to refine freely with an isotropic displacement parameter. Temperature-dependent crystal structure data for compounds 2, 3 and 6 has been deposited at the CCDC 1400608–1400611, 1400701, and 1400606–1400607 respectively.Thermal analysis
Differential scanning calorimetry, (DSC), data were collected on a TA Instruments Q2000 equipped with a 50-position auto-sampler. The calibration for thermal capacity was carried out using sapphire and the calibration for energy and temperature was carried out using certified indium. Typically, 0.5–3 mg of each sample, in a pin-holed aluminium pan, was heated at 10 °C min−1 from 25 °C to 300 °C. A purge of dry nitrogen at 50 ml min−1 was maintained over the sample. The instrument control software was Advantage for Q Series v2.8.0.394 and Thermal Advantage v5.5.3 and the data were analysed using Universal Analysis v4.5A.Conclusions
The temperature-dependence of the structure of members of the Dianin host series, enclathrating iso-propanol, is a general phenomenon extending through the parent, 1 thiachroman 2 and selenachroman 3 individual host members to a polar quasiracemic counterpart 6; and is not limited to a single commensurate thermal phase transition.Acknowledgements
Financial support from the Malaysia HIR MOHE, Grant No.: F000009-21001 is gratefully acknowledged. We also wish to thank Dr Alex. R. Eberlin, Johnson-Matthey (Pharmorphix), Cambridge (UK), for assistance with the DSC experiments.Notes and references
- A. P. Dianin, J. Russe. Phys. Chem. Soc., 1914, 46, 1310–1319 CAS.
- (a) W. Baker, A. J. Floyd, J. F. W. McOmie, G. Pope, A. S. Weaving and J. H. Wild, J. Chem. Soc., 1956, 2010–2017 RSC; (b) W. Baker, J. F. W. McOmie and A. S. Weaving, J. Chem. Soc., 1956, 2018–2020 RSC.
- H. M. Powell and B. D. P. Wetters, Chem. & Ind., 1955, 256–257 CAS.
- See, for example: (a) D. D. MacNicol, in Inclusion Compounds, Vol. 2, ed. J. L. Atwood, J. E. D. Davies and D. D. MacNicol, Academic Press, 1984, ch. 1, pp. 12–32 Search PubMed; (b) P. Finocchiaro and S. Failla, in Comprehensive Supramolecular Chemistry, ed. D. D. MacNicol, F. Toda and R. Bishop, Elsevier, Oxford, 1996, ch. 18, vol. 6, pp. 618–627 Search PubMed.
- J. L. Flippen, J. Karle and I. L. Karle, J. Am. Chem. Soc., 1970, 3749–3755 CrossRef CAS.
- J. L. Flippen and J. Karle, J. Phys. Chem., 1971, 75, 3566–3567 CrossRef CAS.
- D. D. MacNicol, H. H. Mills and F. B. Wilson, J. Chem. Soc. D, 1969, 1332–1333 RSC.
- D. D. MacNicol and F. B. Wilson, J. Chem. Soc. D, 1971, 786–787 RSC.
- F. B. Wilson, Ph.D. Thesis, University of Glasgow, 1971 Search PubMed.
- D. D. MacNicol, P. R. Mallinson, R. A. B. Keates and F. B. Wilson, J. Inclusion Phenom., 1987, 5, 373–377 CrossRef CAS.
- A. D. U. Hardy, J. J. McKendrick, D. D. MacNicol and D. R. Wilson, J. Chem. Soc., Perkin Trans. 2, 1979, 729–734 RSC.
- T. W. Beresford, C. S. Frampton, J. H. Gall and D. D. MacNicol, J. Struct. Chem., 1999, 40, 705–713 CrossRef CAS.
- J. H. Gall, A. D. U. Hardy, J. J. McKendrick and D. D. MacNicol, J. Chem. Soc., Perkin Trans. 2, 1979, 376–380 RSC.
- T. Jacobs, G. O. Lloyd, M. W. Bredenkamp and L. J. Barbour, Cryst. Growth & Des., 2009, 9, 1284–1286 CAS.
- J. J. Lee, R. O. Fuller, A. N. Sobolev, H. F. Clausen, J. Overgaard, G. A. Koutsantonis, B. B. Iversen and M. A. Spackman, Chem. Commun., 2011, 47, 2029–2031 RSC.
- D. D. MacNicol, J. Chem. Soc. D, 1969, 836 RSC.
- C. S. Frampton, K. A. Ketuly, A. H. A. Hadi, J. H. Gall and D. D. MacNicol, Chem. Commun., 2013, 49, 7198–7200 RSC.
- (a) G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64(1), 112–122 CrossRef CAS PubMed; (b) G. M. Sheldrick, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2015, 71, 3–8 CrossRef PubMed.
- C. S. Frampton, D. D. MacNicol and D. R. Wilson, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2011, 67, o188–o191 CAS.
- K. M. Steed and J. W. Steed, Chem. Rev., 2015, 115, 2895–2933 CrossRef CAS PubMed.
- (a) F. H. Allen, Acta Crystallogr., Sect. B: Struct. Sci., 2002, 58, 380–388 CrossRef; (b) F. H. Allen and W. D. S. Motherwell, Acta Crystallogr., Sect. B: Struct. Sci., 2002, 58, 407–422 CrossRef; (c) C. R. Groom, I. J. Bruno, M. P. Lightfoot and S. C. Ward, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2016, 72, 171–179 CrossRef CAS PubMed.
- C. F. Macrae, I. J. Bruno, J. A. Chisholm, P. R. Edgington, P. McCabe, E. Pidcock, L. Rodriguez-Monge, R. Taylor, J. van de Streek and P. A. Wood, J. Appl. Crystallogr., 2008, 41, 466–470 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental details and crystal structure data. CCDC 1400608–1400611 and 1400701, [2 (295 K, 200 K, 371 K and 90 K), 3 (120 K)]. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ce00451f |
| This journal is © The Royal Society of Chemistry 2017 |