Open Access Article
Open Access ArticleSynthetic insect antifreeze peptides modify ice crystal growth habit†
Charles H. Z.
Kong
,
Ivanhoe K. H.
Leung
 and
Vijayalekshmi
Sarojini
and
Vijayalekshmi
Sarojini
 *
*
School of Chemical Sciences, The University of Auckland, Private Bag 92019, Auckland, New Zealand. E-mail: v.sarojini@auckland.ac.nz; Fax: +64 9 373 7422; Tel: +64 9 923 3387
First published on 24th February 2017
Abstract
Antifreeze proteins modify the growth of ice crystals. We present a rational approach towards the design of short peptide analogues of the 8.7 kDa disulfide-bridged β-helical hyperactive insect antifreeze protein. Our synthetic peptides bind and modify the shape of ice crystals, and show enhanced antifreeze activity with the addition of citrate.
Antifreeze proteins (AFPs) are naturally produced by various organisms such as polar fish, terrestrial arthropods, plants and insects.1–3 AFPs inhibit and change the normal growth patterns of ice crystals, restricting their size, morphology and preventing ice recrystallization.3 Ice recrystallization inhibition is important in cryopreservation and AFPs possess this ability at relatively low concentrations, thus making them valuable for use in frozen foods and other cryopreservation applications.4De novo designed AFPs, glycopeptides and other synthetic polymers with ice recrystallization ability have been reported.5–7 Inspired by natural AFPs, researchers have also succeeded in generating tunable onset of ice recrystallization inhibition in proteins and peptides with apparently no sequence homology to natural AFPs.8
Insect AFPs have been reported to exhibit hyperactive antifreeze activity.2,9 The larvae of the beetle Dendroides canadensis produce a family of AFPs (DcAFP) with molecular mass ∼8.7 kDa in various isoforms with varying activity.2,10 DcAFP consists of 6 or 7 repetitive disulfide linked 12- or 13-mer coils each having the conserved sequence (TCTxSxxCxxAx).11 A total of 16 cysteine residues located every 6 residues apart remain internally disulfide bonded forming 8 bridges.12 These disulfide bridges impose significant constraints on secondary structural features, positioning the hydrophilic side-chains for ice-binding.12 On the conserved side of the DcAFP, two precisely arranged parallel rows of threonine–cysteine–threonine (T–C–T) motifs are aligned to form a flat β-sheet along the protein chain (Scheme 1).14 This array of threonine residues form the ice-binding site of the AFP.15–17 Recombinantly expressed DcAFP proteins have been studied to assess their ice-structuring patterns and potential for use in mammalian cell cryopreservation.18 However, their large size and complex nature (e.g. presence of multiple disulfides), make their commercial potential harder to achieve. The development of shorter analogues of DcAFPs that retain the function of the natural protein thus holds promise not only for basic structure–function studies but also from a commercial perspective for several cryopreservation applications.
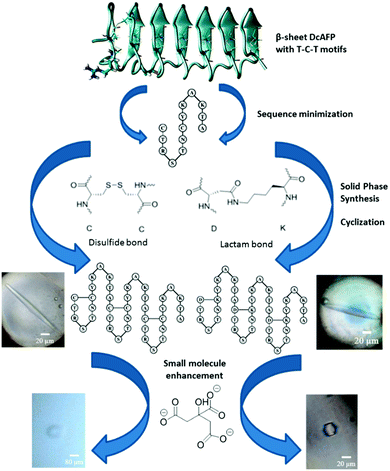 | ||
| Scheme 1 Outline of our approach towards DcAFP antifreeze peptides.11,13 Sequence minimized analogues were designed and synthesized based on the DcAFP with either disulfide or lactam bonds as the intra-coil bridges. DcAFP structure reprinted with permission from ref. 13. Copyright 2009 American Chemical Society. | ||
In the present communication, our focus was to explore, through rational design, whether synthetic peptides significantly shorter than the DcAFP protein amenable through chemical synthesis will retain the ice crystal modification behaviour of the native protein. A 13 amino acid conserved sequence (CTRSTNCYKAKTA) based on the natural Dendroides Canadensis hyperactive AFP forms the basis of our synthetic peptides and consists of two cysteine residues that form the stabilizing disulfides found across the β-sheets in the natural protein.11 The designed sequences include alanine, threonine, serine and arginine, residues that are known to be important for the antifreeze activity of β-helical AFPs,19 as well as critical for the enhancing ability of DcAFP for its interaction with the small molecule enhancers.13 Using solid phase peptide synthesis we generated tailor made analogues (DCR13, DCR26 and DCR39) of the DcAFP protein of varying sequence lengths (13, 26 or 39 residues). Two further analogues replacing all disulfide-bridges of the 26- and 39-mer peptides to lactam-bridges (DCR26 cyclic and DCR39 cyclic) were also synthesized and investigated. The linear precursors of the lactam-bridged peptides were synthesized by substituting alternate cysteines to lysines and aspartic acids with alloc and allyl side-chain protecting groups following a modified literature procedure (Scheme S1) (ESI†).20 HPLC profiles of the cleaved peptides showed one major peak for each peptide, which was identified as the desired lactam-bridged version via high resolution ESI-MS (Fig. S2†). This methodology enabled the introduction of multiple intra-coil lactam bridges in the synthetic analogues to mimic the intra-coil disulfide bridges of the natural DcAFP in an efficient manner. The lactam-bridged peptides have superior product yields, purity and stability. NMR spectra (ESI†) of the lactam-bridged peptides showed sharper peaks (Fig. S6 & S9†) whist DCR26 and DCR39 resonances were broad.
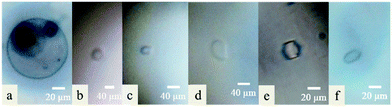
The melting point of pure water (control) is equal to its freezing point. An ice crystal of pure water will either melt or grow at any given temperature. With pure water, a slight drop of temperature below the freezing point induced crystals growing evenly in all directions in a circular form (without faceting) until the whole sample was frozen (Fig. 1a & 4c). As expected, a solution of 0.01 M of DCR13 did not show any faceting (Fig. 1b) and is in agreement with literature findings where sequence length has been reported to be important for antifreeze activity.21,22 Previous studies on the beetle.
 | ||
| Fig. 1 Ice crystal morphology observed in (a) H2O, 0.01 M of (b) DCR13, (c) DCR26, (d) DCR39, (e) DCR26 cyclic, and (f) DCR39 cyclic. | ||
T. molitor AFP (TmAFP) with seven coils reported a reduction in thermal hysteresis (TH) by three quarters when one of the coils was removed. A five coil analogue with two of the coils removed from the original protein resulted in complete loss of antifreeze activity.22 However, our synthetic peptides, DCR26 with only two β-helical coils and DCR39 with three coils, as well as their lactam bridged analogues produced ice crystals that resemble a flat disk or the shape of a rice grain similar to those produced by snow fleas protein23 (Fig. 1c–f). The shapes of these ice crystals are clearly distinct from and never observed with pure water, confirming the ability of these peptides to bind to ice and promote ice shaping. Noticeably these ice crystals are also distinctly different to those produced by the native DcAFP, which forms small lemon-shaped ice crystals with high TH value.17,24,25 The ability of the native protein to bind to both prism and basal planes of ice, because of its large surface area resulting from the several coils, is the key factor that contributes to its hyperactivity. The significant size reduction of our peptides will undoubtedly influence their ability to bind to both prism and basal planes. This is reflected as the absence of measureable TH and lack of extreme ice crystal shaping by the peptides.
Considering the above, we investigated the effects of the addition of small molecules to the activity of the peptides. Certain proteins and small molecules are known to enhance the activity of native AFPs.13,26–29 These enhancers are believed to bind to the AFPs to form larger protein complexes (Fig. 2), thereby increasing their TH activities by blocking larger surface areas of ice. Evans et al.29 and Amornwittawat et al.26 identified that increase in antifreeze activity is proportional to the size of the enhancer. Trisodium citrate was reported as one of the strong enhancers of antifreeze activity in terms of both TH and ice crystal growth modification.30 Citrate also causes the highest increase in the TH of insect AFPs.28
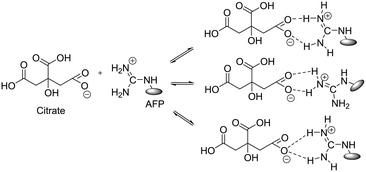 | ||
| Fig. 2 Binding of citrate with the guanidine groups of arginine in DcAFP analogues.13 | ||
The addition of citrate noticeably increased the activity of the DCR analogues (DCR26, DCR39, DCR26 cyclic and DCR39 cyclic), not only promoting extreme crystal shaping resembling the very small ice crystals produced by the native DcAFP protein, but also creating a significant increase in TH (Fig. 3b–e & 4b). This observation is very surprising, as based on literature reports, such small peptide constructs should have no TH activity, and likely no ice shaping.22 No changes were observed with the control (Fig. 3a & 4d) and the shortest peptide analogue DCR13 (Fig. S1a†) upon addition of citrate.
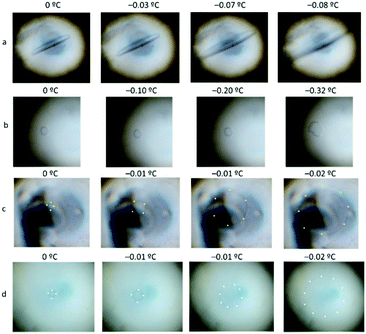 | ||
| Fig. 4 Antifreeze activity shown in (a) 0.01 M DCR39 and (b) 0.01 M DCR39 with 1 M citrate, compared to (c) H2O and (d) citrate. | ||
These results show that with the help of a small molecule enhancer, a rather “non-functional” peptide in terms of negligible TH can be induced to be “functional” with noticeable TH. The enhancement effects can be explained by the fact that citrate induces a “salt-out” effect on the AFPs, disrupting the water structure, and evoking a solubility-induced shift in the distribution pattern of the AFP. Additionally, citrate can bind to the AFP to form a large complex (Fig. 2), thereby increasing the adsorption of the AFP onto the ice surface.13,31 Citrate clearly enhances the ice crystal growth modification activity of the DCR analogues. Additionally, the lactam-bridged peptides showed comparable TH and ice crystal growth modification ability to their corresponding disulfide counterparts (Fig. 3b–e), which underlines the fact that it is not necessary that the intra-coil bridging be accomplished via disulfide bonds to retain the antifreeze property of the DcAFP peptides. The implications of this finding are significant because it opens up the possibility of producing short, robust, non-cysteine analogues of the hyperactive disulfide rich AFPs for use as cryoprotectants in various fields.18,32
We further demonstrate that, even though disulfide bridging is not crucial, an intra-coil bridging of some sort is absolutely essential for antifreeze activity in the DCR family. Linear versions of the 26 and 39-mer peptides were synthesized by substituting the cysteines with alanines as well as a version of the 39-mer peptide with the cysteines in the reduced and alkylated forms. The reduced and alkylated peptide analogue of DCR39 after HPLC purification was found to exist predominantly in the form of 4 out of 6 cysteines alkylated and the remaining 2 in the reduced form as confirmed by MALDI-TOF mass spectrometry. Complete loss of antifreeze activity was observed in both DCR26 A and DCR39 A where circular plates were observed (Fig. S1b & c†) without any TH. The reduced and alkylated form of DCR39 also showed lack of ability for ice crystal modification leading to ice crystals as circular plates (Fig. S1d†) and no TH. This can be due to the removal of disulfide bridges that potentially causes the loss of secondary structure of the peptides, which can detrimentally affect ice-binding. These observations, prove that intra-coil bridging is crucial for antifreeze activity in these peptides. As shown in Scheme 1, the intra-coil bridges likely function to ensure the appropriate alignment of the hydroxyl side-chains of the threonine and serine residues, thereby providing the necessary lattice match and hydrogen bonding between the AFPs and ice.11,12,14
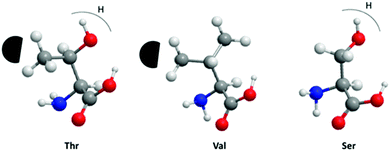
We have also investigated the relative importance of threonine residues for the antifreeze activity of the AFPs. Two DCR39 analogues were synthesized where all the threonines were substituted to either serines (DCR39 S) or valines (DCR39 V) to determine the relative importance of hydroxyl and methyl groups for ice-binding activity in the DCR family. Fig. 5 summarizes the difference between the side-chains of the substituted amino acids and the potential effect of these substitutions on interactions with ice. DCR39 V showed no detectable TH and faceting (Fig. S1e†), indicating no interaction with ice. The lack of activity of DCR39 V, indicates that the loss of the hydroxyl groups would make the peptide less able to secure its anchored-clathrate waters by hydrogen bond. The methyl groups alone are insufficient for ice-binding; thus this shows the importance of the hydroxyl groups of the threonines in modifying ice growth in the DCR peptides.33 DCR39 S gave a very small crystal with measureable TH, upon adding citrate (Fig. 3f). However, the TH of DCR39 S (0.04 °C) was comparatively lower than DCR39 (0.32 °C).
Given the several cysteines in the DcAFP peptide analogues, these peptides likely exist as a mixture of differently folded structures that complicate the analyses because of the lack of sufficient control on disulfide bond formation.12,34 Also, reduction by enzymes such as disulfide isomerases can lead to structural rearrangement and loss of activity, making their potential for in vivo applications less feasible.35 We made use of a simple synthetic route to replace the disulfide bonds in the DcAFP peptide family with lactam bridges resulting in peptides with isopeptide bonds.20 Such a study has not been undertaken in the literature. Lactam bridged DcAFP peptides, unlike their disulfide counterparts are more robust and will be stable to enzymatic and chemical disulfide reductants. The identification of short hyperactive AFPs that retain the ice crystal modification ability of the native DcAFP protein, but are resistant to redox conditions will be beneficial for use in cryopreservation applications and will also help to elucidate the importance of sequence length and intra-coil bridges for ice-binding.
In conclusion, this study reports the first ever observation of ice crystal growth modification in short truncated synthetic DcAFP peptide analogues with only two and three coils, on their own, which gets enhanced with the addition of citrate. Although the TH activity of these AFPs is significantly lower than those of the native protein even at the much higher molar concentration, the ice shaping properties they demonstrate are of more importance for future applications of these peptides as cryoprotectants in various fields. The relatively easy synthesis of these peptides and the enhancing effects on antifreeze activity seen upon addition of citrate are also very promising from a cryoprotectant application point of view. Results from citrate addition proves that the overall antifreeze activity is not determined solely by the presence of AFPs themselves but potentially influenced by other synergistic agents. The addition of enhancers such as citrate can potentiate the antifreeze activity exhibited by the AFPs and broaden their spectrum of applications. Our results also indicate that the intra-coil disulfides present in the original protein can be replaced with the more stable lactam bridges without loss of ice-binding activity. The truncated lactam bridged AFPs reported here have the additional advantage of being stable to redox conditions. These synthetic peptides are readily accessible through solid phase peptide synthesis. Our investigations also prove that while the threonine residues are important for the observed ice crystal modification effects in the peptide, the hydroxyl groups of the threonines play more important roles than the methyl groups.
Acknowledgements
C. K. thanks AgResearch New Zealand for a PhD scholarship. We thank A/P Clive Evans for helpful discussions and support with antifreeze activity studies.Notes and references
- A. L. Devries, Science, 1971, 172, 1152–1155 CAS.
- J. G. Duman, J. Exp. Zool., 1984, 230, 355–361 CrossRef CAS.
- A. L. DeVries, Methods Enzymol., 1986, 127, 293–303 CAS.
- R. E. Feeney and Y. Yeh, Trends Food Sci. Technol., 1998, 9, 102–106 CrossRef CAS.
- M. I. Gibson, C. A. Barker, S. G. Spain, L. Albertin and N. R. Cameron, Biomacromolecules, 2008, 10, 328–333 CrossRef PubMed.
- W. Zhang and R. A. Laursen, J. Biol. Chem., 1998, 273, 34806–34812 CrossRef CAS PubMed.
- D. E. Mitchell, N. R. Cameron and M. I. Gibson, Chem. Commun., 2015, 51, 12977–12980 RSC.
- D. E. Mitchell and M. I. Gibson, Biomacromolecules, 2015, 16, 3411–3416 CrossRef CAS PubMed.
- L. Wang and J. G. Duman, Biochemistry, 2006, 45, 1278–1284 CrossRef CAS PubMed.
- J. Duman, D. Verleye and N. Li, J. Comp. Physiol., B, 2002, 172, 547–552 CrossRef CAS PubMed.
- N. Li, B. S. Kendrick, M. C. Manning, J. F. Carpenter and J. G. Duman, Arch. Biochem. Biophys., 1998, 360, 25–32 CrossRef CAS PubMed.
- N. Li, B. A. Chibber, F. J. Castellino and J. G. Duman, Biochemistry, 1998, 37, 6343–6350 CrossRef CAS PubMed.
- S. Wang, N. Amornwittawat, V. Juwita, Y. Kao, J. G. Duman, T. A. Pascal, W. A. Goddard III and X. Wen, Biochemistry, 2009, 48, 9696–9703 CrossRef CAS PubMed.
- Z. Jia and P. L. Davies, Trends Biochem. Sci., 2002, 27, 101–106 CrossRef CAS PubMed.
- C. B. Marshall, M. E. Daley, L. A. Graham, B. D. Sykes and P. L. Davies, FEBS Lett., 2002, 529, 261–267 CrossRef CAS PubMed.
- N. Pertaya, C. B. Marshall, Y. Celik, P. L. Davies and I. Braslavsky, Biophys. J., 2008, 95, 333–341 CrossRef CAS PubMed.
- S. P. Graether, M. J. Kuiper, S. M. Gagne, V. K. Walker, Z. Jia, B. D. Sykes and P. L. Davies, Nature, 2000, 406, 325–328 CrossRef CAS PubMed.
- D. O. Halwani, K. G. Brockbank, J. G. Duman and L. H. Campbell, Cryobiology, 2014, 68, 411–418 CrossRef CAS PubMed.
- M. E. Daley, S. P. Graether and B. D. Sykes, Biochemistry, 2004, 43, 13012–13017 CrossRef CAS PubMed.
- P. Grieco, P. Gitu and V. Hruby, J. Pept. Res., 2001, 57, 250–256 CrossRef CAS PubMed.
- K. S. Park, W. S. Jung, H. J. Kim and S. Y. Shin, Bull. Korean Chem. Soc., 2010, 31, 3791–3793 CrossRef CAS.
- C. B. Marshall, M. E. Daley, B. D. Sykes and P. L. Davies, Biochemistry, 2004, 43, 11637–11646 CrossRef CAS PubMed.
- L. A. Graham and P. L. Davies, Science, 2005, 310, 461 CrossRef PubMed.
- A. J. Scotter, C. B. Marshall, L. A. Graham, J. A. Gilbert, C. P. Garnham and P. L. Davies, Cryobiology, 2006, 53, 229–239 CrossRef CAS PubMed.
- D. W. Wu, J. G. Duman, C.-H. C. Cheng and F. J. Castellino, J. Comp. Physiol., B, 1991, 161, 271–278 CrossRef CAS.
- S. Wang, N. Amornwittawat, J. Banatlao, M. Chung, Y. Kao and X. Wen, J. Phys. Chem. B, 2009, 113, 13891–13894 CrossRef CAS PubMed.
- R. Peltier, C. W. Evans, A. L. DeVries, M. A. Brimble, A. J. Dingley and D. E. Williams, Cryst. Growth Des., 2010, 10, 5066–5077 CAS.
- J. Duman, J. Comp. Physiol., B, 2002, 172, 163–168 CrossRef CAS.
- R. P. Evans, R. S. Hobbs, S. V. Goddard and G. L. Fletcher, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 2007, 148, 556–561 CrossRef PubMed.
- N. Amornwittawat, S. Wang, J. G. Duman and X. Wen, Biochim. Biophys. Acta, Proteins Proteomics, 2008, 1784, 1942–1948 CrossRef CAS PubMed.
- E. Kristiansen, S. A. Pedersen and K. E. Zachariassen, Cryobiology, 2008, 57, 122–129 CrossRef CAS PubMed.
- M. Griffith and K. V. Ewart, Biotechnol. Adv., 1995, 13, 375–402 CrossRef CAS PubMed.
- M. Bar, Y. Celik, D. Fass and I. Braslavsky, Cryst. Growth Des., 2008, 8, 2954–2963 CAS.
- N. L. Daly, S. Love, P. F. Alewood and D. J. Craik, Biochemistry, 1999, 38, 10606–10614 CrossRef CAS PubMed.
- R. B. Freedman, Nature, 1989, 337, 407–408 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, supplementary figures and videos of ice crystals. See DOI: 10.1039/c7ce00232g |
| This journal is © The Royal Society of Chemistry 2017 |