Open Access Article
Open Access ArticleTwo magnetic Δ-chain-based Mn(II) and Co(II) coordination polymers with mixed carboxylate–phosphinate and μ3-OH− bridges†
Zi-Yi
Du
a,
Ling
Zhang
a,
Bao-Ying
Wang
b,
Sui-Jun
Liu
 *c,
Bo
Huang
b,
Cai-Ming
Liu
*d and
Wei-Xiong
Zhang
*b
*c,
Bo
Huang
b,
Cai-Ming
Liu
*d and
Wei-Xiong
Zhang
*b
aCollege of Chemistry and Chemical Engineering, Gannan Normal University, Ganzhou 341000, China
bSchool of Chemistry, Sun Yat-Sen University, Guangzhou 510275, China. E-mail: zhangwx6@mail.sysu.edu.cn
cSchool of Metallurgy and Chemical Engineering, Jiangxi University of Science and Technology, Ganzhou 341000, China. E-mail: liusuijun147@163.com
dBeijing National Laboratory for Molecular Sciences, Center for Molecular Science, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: cmliu@iccas.ac.cn
First published on 17th January 2017
Abstract
The hydrothermal reaction of Mn(II) or Co(II) ions with 2-carboxyethyl(phenyl)phosphinic acid (H2L) afforded Mn(II) and Co(II) carboxylate–phosphinates containing μ3-OH− as a co-ligand, namely [M3(L)2(OH)2] (M = Mn (1) or Co (2)). Such two compounds feature a new layered structure in which Δ-type chains built from alternating corner- and edge-sharing M3(μ3-OH) triangles are further connected via the Y-shaped “–(CH2)2–C(–O–)–” spacers. Magnetic studies reveal that there are dominant antiferromagnetic interactions between the metal ions. In 1, the complicated magnetic couplings in the Δ-type chains result in spin competition, displaying spin-glass behaviors. In 2, spin fluctuation behavior was observed and the critical field at 2 K is 25 kOe.
Introduction
During the past few decades, molecule-based magnetic materials with rich magnetic couplings have attracted considerable attention in coordination chemistry, owing to their intriguing magnetic phenomena as well as their potential applications in quantum computation and information storage.1–3 Among the classes of magnetic materials, frustrated magnets and spin fluctuation complexes are of particular theoretical interest.On the one hand, in frustrated magnets, localized magnetic moments, or spins, interacting through competing exchange interactions cannot be simultaneously satisfied, which may lead to some interesting phenomena such as spin liquid at low temperature.4 As one of the archetypes of spin frustration, Δ-type chain magnets that contain chains of corner- and/or edge-sharing triangles of M3(μ3-bridge) may provide a theoretical model to study magneto-structural correlations and act as secondary building blocks to construct novel frustrated materials. However, up to now, examples of coordination polymers (CPs) with Δ-type chain structures are still limited in number.5–7 To create magnetic CPs with Δ-type chain structures, the introduction of a μ3-OH− co-ligand has been proved to be an effective strategy.
On the other hand, metamagnetic or spin fluctuation behaviour may occur if the couplings are weak enough to be overcome by an external field and enter into another magnetic state along with the reorientation of spin.8 Frequently, such couplings are not strong and are exhibited in intermolecule, interchain, and interlayer interactions mediated by long linkers, blocking groups, hydrogen bonds, and π–π interactions.8a,9
Recently, we have undertaken research works on the coordination chemistry of a bifunctional ligand, namely 2-carboxyethyl(phenyl)phosphinic acid (H2L, see Scheme 1), which features two formally analogous carboxylate and phosphinate moieties (i.e., both contain two potential O donors) separated by a flexible ethylene spacer. The use of such a conformationally flexible ligand with multiple donor sites has demonstrated the structural diversity for the construction of CPs.10,11 In our current studies on magnetic CPs of metal carboxylate–phosphinates, here we present two unique examples of magnetic materials with Δ-type chain structures, i.e. [Mn3(L)2(OH)2] (1) and [Co3(L)2(OH)2] (2). Herein, we report their syntheses, crystal structures, and magnetic properties.
Experimental
Materials and instrumentation
2-Carboxyethyl(phenyl)phosphinic acid was prepared using a published procedure.12 All other chemicals were obtained from commercial sources and used without further purification. FT-IR spectra were recorded on a Nicolet 5700 spectrometer using KBr pellets in the range of 4000–400 cm−1. Powder X-ray diffraction (PXRD) patterns (Cu-Kα) were collected on a Bruker Advance D8 θ–2θ diffractometer. Thermogravimetric analysis (TGA) was carried out on a TA Q50 system at a heating rate of 10 °C min−1 under a nitrogen atmosphere. Variable-temperature magnetic susceptibility, zero-field ac magnetic susceptibility, and field dependence of magnetization were measured on a Quantum Design MPMS-XL5 (SQUID) magnetometer. Diamagnetic corrections were estimated from Pascal's constants for all constituent atoms.Synthesis of 1
A mixture of Mn(CH3COO)2·4H2O (0.30 mmol), H2L (0.32 mmol) and urea (0.38 mmol) in 10 mL distilled water was sealed in a Parr Teflon-lined autoclave (23 mL) and heated at 150 °C for 3 days. The final pH value was about 5.5 and colourless plate-shaped crystals of 1 were collected in ca. 55% yield based on Mn. The purity of the as-grown crystals was confirmed by PXRD (Fig. S1a, ESI†). IR data (KBr, cm−1; see Fig. S3, ESI†): 3649(m), 3426(m), 3074(m), 3049(m), 2907(m), 2360(m), 1586(vs), 1415(s), 1299(m), 1190(m), 1147(s), 1122(s), 1034(s), 1134(s), 956(m), 925(m), 764(m), 729(m), 699(m), 634(m), 601(m), 529(s), 495(m).Synthesis of 2
A mixture of CoCl2·6H2O (0.30 mmol), H2L (0.32 mmol) and urea (0.30 mmol) in 10 mL distilled water was sealed into a Parr Teflon-lined autoclave (23 mL) and heated at 150 °C for 3 days. The final pH value was about 5.0 and pink plate-shaped crystals of 1 were collected in ca. 85% yield based on Co. The purity of the as-grown crystals was confirmed by PXRD (Fig. S1b, ESI†). IR data (KBr, cm−1; see Fig. S3, ESI†): 3448(s), 3071(m), 2968(m), 2816(m), 2659(m), 2376(m), 1632(vs), 1595(s), 1516(m), 1387(m), 1349(s), 1114(s), 1044(m), 730(m), 646(m), 538(m).Single-crystal structure determination
Data collections for 1 and 2 were performed on a Smart ApexII CCD diffractometer equipped with a graphite-monochromated Mo-Kα radiation source (λ = 0.71073 Å). Their intensity data were collected at 296 K. The data sets were corrected for Lorentz and polarization factors as well as for absorption by the SADABS program.13 Crystal structures were solved by direct methods and refined by full-matrix least-squares fitting on F2 with the SHELXTL program package.14 The final difference Fourier maps for 1 showed the highest residual peak of 1.41 e Å−3 (1.01 Å from the Mn1 atom) and the deepest hole of −0.99 e Å−3 (0.67 Å from the H2A atom). The residuals for 2 (0.68 and −0.65 e Å−3) are quite small. C-bound H atoms were generated geometrically while O-bound H atoms were located in the difference Fourier map. All non-hydrogen atoms were refined with anisotropic thermal parameters, whereas all hydrogen atoms were refined isotropically. Crystallographic data and structural refinements for 1 and 2 are summarized in Table 1. Selected bond lengths are listed in Table 2. More details on the crystallographic studies as well as atom displacement parameters are given in the X-ray crystallographic files in CIF format.| Compound | 1 | 2 |
|---|---|---|
| a R 1 = ∑‖Fo| − |Fc‖/∑|Fo|, wR2 = {∑w[(Fo)2 − (Fc)2]2/∑w[(Fo)2]2}1/2. | ||
| Empirical formula | C18H20O10P2Mn3 | C18H20O10P2Co3 |
| Formula weight | 623.10 | 635.07 |
| Space group | P21/n | P21/n |
| a (Å) | 5.6315(4) | 5.4625(4) |
| b (Å) | 6.7154(5) | 6.5901(6) |
| c (Å) | 29.169(3) | 29.293(3) |
| β/deg | 91.174(6) | 90.889(6) |
| V/Å3 | 1102.88(16) | 1054.38(15) |
| Z | 2 | 2 |
| D calcd/g cm−3 | 1.876 | 2.000 |
| μ/mm−1 | 1.890 | 2.541 |
| GOF on F2 | 1.083 | 1.001 |
| R 1, wR2 [I > 2σ(I)]a | 0.0887, 0.1933 | 0.0414, 0.0824 |
| R 1, wR2 (all data) | 0.1072, 0.2018 | 0.0603, 0.0895 |
| 1 | |||
|---|---|---|---|
| Symmetry codes: #1 x − 1, y, z; #2 −x + 1, −y + 1, −z; #3 −x + 1, −y, −z. | |||
| Mn(1)–O(2)#1 | 2.138(6) | Mn(1)–O(5)#2 | 2.156(6) |
| Mn(1)–O(4) | 2.168(7) | Mn(1)–O(5) | 2.217(7) |
| Mn(1)–O(1) | 2.258(6) | Mn(1)–O(1)#3 | 2.268(6) |
| Mn(2)–O(5) | 2.097(6) | Mn(2)–O(3)#3 | 2.213(6) |
| Mn(2)–O(1)#1 | 2.266(7) | ||
| 2 | |||
| Co(1)–O(2)#1 | 2.054(2) | Co(1)–O(5)#2 | 2.058(2) |
| Co(1)–O(4) | 2.096(3) | Co(1)–O(5) | 2.136(3) |
| Co(1)–O(3)#3 | 2.171(3) | Co(1)–O(1) | 2.224(3) |
| Co(2)–O(5) | 2.009(3) | Co(2)–O(3)#3 | 2.138(2) |
| Co(2)–O(1)#1 | 2.172(2) | ||
Results and discussion
Synthesis and thermogravimetric analysis
The syntheses of 1 and 2 were carried out using a hydrothermal process in the presence of urea, which slowly hydrolyses at the temperature of the reaction, releasing ammonia and slowly raising the pH of the solution. Their thermal stability was examined by TGA under a nitrogen atmosphere, showing that 1 and 2 are stable up to 325 and 303 °C, respectively, whereupon they start decomposing (Fig. S2, ESI†).Description of the crystal structure
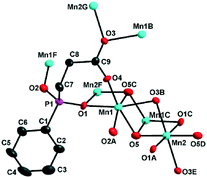
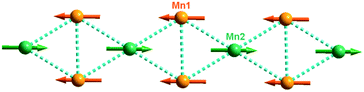
Compounds 1 and 2 crystallize in the monoclinic space group P21/n and they are isomorphous in structure, hence only the structure of 1 will be discussed in detail as a representative. The asymmetric unit of 1 contains two types of Mn2+ ions, one L2− ligand, and one OH− group. The +2 oxidation state of the manganese ions was confirmed by bond valence sum (BVS) analysis (1.995 for the Mn1 ion and 2.062 for the Mn2 ion).15 While the Mn1 ion lying on a general position is octahedrally coordinated by two OH− ligands, two phosphinate O atoms and two carboxylate O atoms of three L2− ligands, the Mn2 ion located at an inversion centre is half-occupied and octahedrally coordinated by two symmetry-related OH− groups as well as two phosphinate O atoms and two carboxylate O atoms from two pairs of symmetry-related L2− ligands (Fig. 1). The Mn–O distances (2.097(6)–2.268(6) Å) are in the normal range (Table 1). The unique L2− ligand adopts a [5.2121324511] coordination mode according to the Harris notation.16 By utilizing all of its four O donors, it chelates one Mn2+ ion and bridges four other Mn2+ ions, with one phosphinate O atom and one carboxylate O atom acting as μ2-O bridges, respectively (Fig. 1). For 2, a similar coordination mode can be observed, except that the Co–O distances (2.009(3)–2.224(3) Å) are slightly shorter than the Mn–O distances of 1. The +2 oxidation state of the cobalt ions was also confirmed by BVS analysis (1.897 for the Co1 ion and 1.995 for the Co2 ion).The interconnection of the Mn2+ ions by the L2− and μ3-OH− ligands results in the formation of a complicated layered structure. As shown in Fig. 2, one Mn2 and two equivalent Mn1 ions are connected by the μ3-OH− group, forming a Mn3(μ3-OH) triangle unit. In such a triangle unit, the Mn⋯Mn distances (Å) are 3.4407(2) for Mn1⋯Mn1A, 3.2520(2) for Mn1⋯Mn2, and 3.3468(2) for Mn1A⋯Mn2, respectively. The Mn1–O5–Mn1A, Mn1–O5–Mn2 and Mn1A–O5–Mn2 angles (°) are 103.762(2), 97.790(2) and 103.783(2), respectively. The Mn1⋯Mn2 and Mn1A⋯Mn2 edges are also bridged by the carboxylate oxygen (O3) and phosphinate oxygen (O1A) of the L2− ligand, respectively. The corresponding Mn1–O3–Mn2 and Mn1A–O1A–Mn2 angles (°) are 93.053(2) and 95.419(2), respectively. The neighbouring Mn3(μ3-OH) triangle units are both corner-shared at the Mn2 site and edge-shared at the Mn1⋯Mn1A borderline, thus leading to an infinite Δ-type chain running along the a-axis (Fig. 2, top). The neighbouring chains are further bridged by the Y-shaped “–(CH2)2–C(–O–)–” spacers to form a layer in the ab plane. The shortest inter-chain Mn⋯Mn distance within the layer is 4.6801(3) Å.
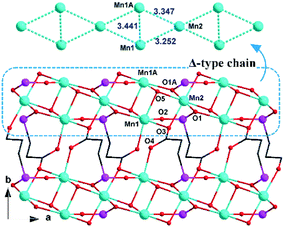 | ||
| Fig. 2 View of the layered structure of 1 down the c-axis. Phenyl groups of the L2− ligands and all hydrogen atoms have been omitted for clarity. For other display details, see Fig. 1. | ||
Furthermore, the layers in 1 are assembled through C–H⋯π interactions between the phenyl groups of the L2− ligands at two adjacent layers, forming a three-dimensional supramolecular structure (Fig. 3). The inter-layer distance is 14.929(1) Å.
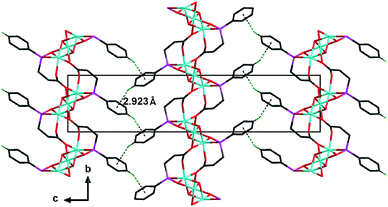 | ||
| Fig. 3 View of the three-dimensional supramolecular structure of 1 down the a-axis. All hydrogen atoms except for the H4A atom have been omitted for clarity. The C–H⋯π interactions between the inter-layer L2− ligands are drawn as dashed lines. The dihedral angle of two adjacent inter-layer phenyl rings is ca. 75.4°. For other display details, see Fig. 1. | ||
Magnetic property studies
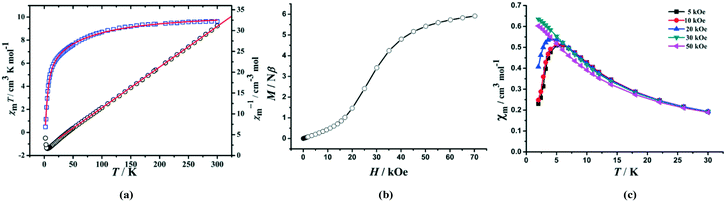
The temperature-dependent magnetic susceptibility was measured at 1000 Oe in the range of 1.8 (2.0)–300 K on crushed crystalline samples of 1 and 2.For 1, the χmT value for a Mn3 triangle unit at 300 K is 12.1 cm3 mol−1 K, which is smaller than the spin-only value for three Mn(II) ions (S = 5/2, 13.12 cm3 mol−1 K for g = 2.0). With decreasing temperature, the χmT value first decreases gradually to a minimum value of ca. 5.85 cm3 mol−1 K at 18 K, then increases sharply to a maximum value of 34.2 cm3 mol−1 K at 4 K, and finally drops down to 26.0 cm3 mol−1 K at 2 K (Fig. 4a). The data at 30–300 K can be fitted to the Curie–Weiss law, giving C = 13.61 cm3 mol−1 K and θ = −34.0 K. The negative θ value indicates that there are dominant antiferromagnetic interactions between the Mn(II) ions. On the other hand, the increase of the χmT value below 18 K implies the uncompensating magnetic moment owing to the spin-frustrated/spin-competing interaction in μ3-OH−-bridged Mn3 units; whereas, the decrease of the χmT value below 4 K is likely due to a saturation effect (vide infra) and/or a weak interchain antiferromagnetic interaction.
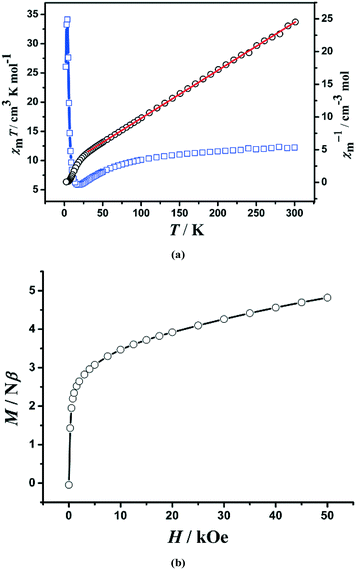 | ||
Fig. 4 (a) Temperature dependence of χmT ( ) and χm−1 (○) for the microcrystalline sample of 1. The red solid line is Curie–Weiss fitting; (b) magnetization measured at 2 K for 1. ) and χm−1 (○) for the microcrystalline sample of 1. The red solid line is Curie–Weiss fitting; (b) magnetization measured at 2 K for 1. | ||
The field dependence of the magnetization at 2.0 K is shown in Fig. 4b. The magnetization curve shows a rapid increase at very small external fields, and then exhibits a linear increase at the high-field range. The value (4.82 Nβ) at 5 T per Mn3 unit is close to the moment of one Mn(II) ion (5 Nβ), suggesting a non-compensating resultant moment of one Mn(II) ion per Mn3 unit, a typical characteristic of topological ferrimagnets.17 No significant remnant magnetization and coercive field were observed in the hysteresis loop at 2.0 K, suggesting a soft magnetic characteristic for 1 (Fig. S4, ESI†). Moreover, the FC and ZFC curves diverge at below 3.6 K (Fig. S5, ESI†), suggesting the occurrence of irreversibility of magnetization. AC susceptibility measurements revealed that both in-phase (χ′) and out-of-phase (χ′′) signals (Fig. S6, ESI†) are somewhat frequency-dependent, which should be ascribed to spin-glass behaviours that often happen on triangle spin-competing systems.18
The complicated magnetic behaviour at low temperature should be mainly ascribed to the complicated magnetic exchange topology in 1. As shown in Fig. 2, a Δ-type chain is built from alternating corner- and edge-sharing Mn3(μ3-OH) triangles with the carboxylate and phosphinate as co-bridges. As revealed by temperature susceptibilities at high temperature, the intra-triangle magnetic interaction should be of antiferromagnetic type, which is consistent with the Mn–O–Mn angles in the range of 92.9(3)–103.9(4)°. In such a Δ-type chain, spin competition is expected as the intrachain magnetic interactions cannot be satisfied at the same time (Fig. 5). This spin-competing behaviour is also supported by a moderate value of the frustration parameter (f = |θ|/TN = 9.2) in 1.19 As the Mn(II) ion usually has a weak magnetic anisotropy, the magnetic structure in such a Δ-type chain is strongly determined by the coupling between the sites. For this case, a single phase transition from the paramagnetic state to a magnetically ordered state was predicted.20 Taking into consideration the large χ′T value (131 cm3 mol−1 K) observed at 3.8 K and the magnetization of 4.82 Nβ at 5 T, one of the possible magnetic structures of a single chain at low temperature can be proposed for 1. As shown in Fig. 6, for each single chain, the moments on the edge-sharing (Mn1) and vertex-sharing (Mn2) sites form two individual sub-lattices, which are anti-parallel to each other, and thus, a non-compensating resultant moment of one Mn(II) ion per Mn3 unit could be formed, leading to a topologically ferrimagnetically ordered state for 1.
For 2, the χmT value at room temperature is 9.63 cm3 mol−1 K, which is much larger than the expected spin-only value (5.625 cm3 mol−1 K) for three magnetically isolated Co(II) ions (S = 3/2, g = 2) and this phenomenon can be attributed to the strong spin–orbit coupling of Co(II) ions. As the temperature decreases, the value of χmT slowly decreases at high temperature and quickly decreases down to a minimum value of 0.48 cm3 mol−1 K at 2.0 K. Curie–Weiss fitting of the magnetic data from 2 K to 300 K for 2 results in C = 10.23 cm3 mol−1 K and θ = −17.3 K. Notably, the negative θ value does not indicate dominant antiferromagnetic coupling between the metal centers because of the strong spin–orbit coupling of Co(II) ions, which itself can lead to a negative θ value and a decrease of χmT at high temperature.21 The strength of the antiferromagnetic exchange interaction caused by the spin–orbit coupling of Co(II) at high temperature was estimated based on eqn (1).22
χT = A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp exp![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (−E1/kT) + B (−E1/kT) + B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp exp![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (−E2/kT) (−E2/kT) | (1) |
In eqn (1), A + B equals the Curie constant, and E1 and E2 are the spin–orbit coupling constant and activation energy of antiferromagnetic interactions, respectively. The best fit of the experimental data gives A + B = 10.53 cm3 mol−1 K, −E1/k = −90.24 K, and −E2/k = −4.98 K for 2 (Fig. 6a). The negative value of −E2/k indicates the dominant antiferromagnetic interactions between adjacent Co(II) ions.22
The field-dependent magnetization of 2 at 2 K has a value of 5.92 Nβ (far from the saturation value) at 7 T and exhibits a pronounced sigmoid shape at low field. The latter implies spin fluctuation behaviour for 2 that a magnetic transition occurs from the AF interaction at low field to a FM state at high field (Fig. 6b), and the critical field defined as dM/dH at 2 K is 25 kOe. To investigate the nature of the spin fluctuation of 2, the low temperature magnetic susceptibilities at different fields were measured. The χmvs. T plot (Fig. 6c) shows a cusp around 5.0 K when the applied fields are lower than 25 kOe. Below 5.0 K, χm drops sharply toward zero, suggesting that the ground state of the system is S = 0. The cusp disappears at higher fields and the antiferromagnetic couplings mediated by μ3-OH could be overcome at external fields larger than 25 kOe, from which 2 turns into a ferromagnetic state. In addition, the antiferromagnetic behavior is further confirmed by the temperature-dependent in-phase (χ′) magnetic susceptibilities (Fig. S7, ESI†), but no out-of-phase (χ′′) signals could be observed.
As is known, the usual coordination modes of carboxylates to transfer magnetism could be divided into four types: two of which are syn–syn-μ2-η1:η1 and anti–anti-μ2-η1:η1 modes, which likely produce antiferromagnetism between CoII ions; one is a syn–anti-μ2-η1:η1 mode, which probably produces weak ferromagnetism; the last one is a μ2-η2 mode, which provides more opportunities for generating ferromagnetism.21b A common rule that they transfer ferromagnetic interactions when the Co–O–Co angle is smaller than 110° was widely recognized. For 2, there exists an anti–syn–anti-μ3-η1:η2 mode of carboxylate groups, phosphinate and μ3-OH−. The Co–O–Co angles of 93.54° and 94.51° (O atoms are from phosphinate and carboxylate) and anti–syn–anti-μ3-η1:η2 carboxylates are favorable for the transference of ferromagnetism, however, the existence of a μ3-OH− magnetic exchange pathway may play a key role in the antiferromagnetic interaction between CoII ions.
Conclusions
In summary, we report the first example of metal carboxylate–phosphinates with the μ3-OH− bridge featuring magnetic Δ-chains. Magnetic studies on such compounds show that there are dominant antiferromagnetic interactions between the Mn(II) ions and the Co(II) ions. Moreover, the complicated magnetic exchange topology results in spin competition in 1, showing spin-glass behaviours. Complex 2 exhibits spin fluctuation behaviours with the critical field of 25 kOe at 2 K. Further research work is in progress to explore other μ3-O-bridged coordination polymers exhibiting various magnetic Δ-chains.Acknowledgements
This work was supported by the NSFC (21301198, 21361002, 21471154 and 21501077), the NSF of Jiangxi Province of China (20151BAB213003 and 20161ACB21013), the China Postdoctoral Science Foundation (2016M592107), and the Young Scientists Training Program of Jiangxi Province (20122BCB23020).Notes and references
- O. Kahn, Molecular Magnetism, VCH, Weinheim, Germany, 1993 Search PubMed; D. Gatteschi, R. Sessoli and J. Villian, Molecular Nanomagnets, Oxford University Press, Oxford, 2006 Search PubMed.
- (a) M. Sakamoto, K. Manseki and H. Ökawa, Coord. Chem. Rev., 2001, 219–22, 379–414 CrossRef; (b) M. N. Leuenberger and D. Loss, Nature, 2001, 410, 789 CrossRef CAS PubMed; (c) D. Gatteschi and R. Sessoli, Angew. Chem., Int. Ed., 2003, 42, 268–297 CrossRef CAS PubMed.
- (a) R. E. P. Winpenny, Angew. Chem., Int. Ed., 2008, 47, 7992–7994 CrossRef CAS PubMed; (b) L. Bogani and W. Wernsdorfer, Nat. Mater., 2008, 7, 179–186 CrossRef CAS PubMed.
- (a) L. Balents, Nature, 2010, 464, 199–208 CrossRef CAS PubMed; (b) F. H. Aidoudi, D. W. Aldous, R. J. Goff, A. M. Z. Slawin, J. P. Attfield, R. E. Morris and P. Lightfoot, Nat. Chem., 2011, 3, 801–806 CrossRef CAS PubMed; (c) Y.-M. Li, C.-Y. Xiao, X.-D. Zhang, Y.-Q. Xu, H.-J. Lun and J.-Y. Niu, CrystEngComm, 2013, 15, 7756–7762 RSC.
- (a) J. J. Borrás-Almenar, E. Coronado, C. J. Gómez-Garcia, R. Georges and C. Muñz-Roca, Chem. Phys. Lett., 1991, 186, 410–414 CrossRef; (b) J. J. Borrás-Almenar, E. Coronado, J. C. Gallart, R. Georges and C. J. Gómez-Garcia, J. Magn. Magn. Mater., 1992, 104–107, 835–836 CrossRef.
- (a) C. Ruiz-Pérez, M. Hernández-Molina, P. Lorenzo-Luis, F. Lloret, J. Cano and M. Julve, Inorg. Chem., 2000, 39, 3845–3852 CrossRef; (b) S. O. H. Gutschke, D. J. Price, A. K. Powell and P. T. Wood, Angew. Chem., Int. Ed., 2001, 40, 1920–1923 CrossRef CAS; (c) S. M. Humphrey and P. T. Wood, J. Am. Chem. Soc., 2004, 126, 13236–13237 CrossRef CAS PubMed; (d) H. Kikuchi, Y. Fujii, M. Chiba, S. Mitsudo and T. Idehara, Polyhedron, 2005, 24, 2835–2838 CrossRef CAS; (e) X.-N. Cheng, W.-X. Zhang, Y.-Z. Zheng and X.-M. Chen, Chem. Commun., 2006, 3603–3605 RSC.
- (a) X.-N. Cheng, W. Xue and X.-M. Chen, Eur. J. Inorg. Chem., 2010, 3850–3855 CrossRef CAS; (b) E.-C. Yang, Z.-Y. Liu, X.-Y. Wu and X.-J. Zhao, Chem. Commun., 2011, 47, 8629–8631 RSC; (c) Y.-Q. Wang, X.-M. Zhang, X.-B. Li, B.-W. Wang and E.-Q. Gao, Inorg. Chem., 2011, 50, 6314–6322 CrossRef CAS PubMed; (d) W.-C. Song, J. Tao, T.-L. Hu, Y.-F. Zeng and X.-H. Bu, Dalton Trans., 2011, 40, 11955–11959 RSC; (e) D. S. Liu, Y. Sui, T. W. Wang, C. C. Huang, J. Z. Chen and X. Z. You, Dalton Trans., 2012, 41, 5301–5306 RSC; (f) R.-X. Yao, Y.-L. Qin, F. Ji, Y.-F. Zhao and X.-M. Zhang, Dalton Trans., 2013, 42, 6611–6618 RSC; (g) Z.-S. Cai, S.-S. Bao, M. Ren and L.-M. Zheng, Chem. – Eur. J., 2014, 20, 17137–17142 CrossRef CAS PubMed.
- (a) Q. Yu, Y.-F. Zeng, J.-P. Zhao, Q. Yang, B.-W. Hu, Z. Chang and X.-H. Bu, Inorg. Chem., 2010, 49, 4301–4306 CrossRef CAS PubMed; (b) A. Das, G. M. Rosair, M. Salah El Fallah, J. Ribas and S. Mitra, Inorg. Chem., 2006, 45, 3301–3306 CrossRef CAS PubMed.
- (a) P. S. Mukherjee, S. Dalai, E. Zangrando, F. Lloretc and N. R. Chaudhuri, Chem. Commun., 2001, 1444–1445 RSC; (b) T. Liu, Y. Zhang, Z. Wang and S. Gao, Inorg. Chem., 2006, 45, 2782–2784 CrossRef CAS PubMed.
- (a) Y.-H. Sun, X. Xu, Z.-Y. Du, L.-J. Dong, C.-C. Zhao and Y.-R. Xie, Dalton Trans., 2011, 40, 9295–9298 RSC; (b) L.-J. Dong, C.-C. Zhao, X. Xu, Z.-Y. Du, Y.-R. Xie and J. Zhang, Cryst. Growth Des., 2012, 12, 2052–2058 CrossRef CAS; (c) C.-C. Zhao, Z.-G. Zhou, X. Xu, L.-J. Dong, G.-H. Xu and Z.-Y. Du, Polyhedron, 2013, 51, 18–26 CrossRef CAS; (d) C.-C. Zhao, J.-W. Zhang, Z.-G. Zhou and Z.-Y. Du, J. Mol. Struct., 2013, 1033, 253–257 CrossRef CAS; (e) Y.-P. Zhao, J.-W. Zhang, C.-C. Zhao and Z.-Y. Du, Inorg. Chim. Acta, 2014, 414, 121–126 CrossRef CAS.
- (a) W. Yang, H. Wang, W.-G. Tian, J. Li and Z.-M. Sun, Eur. J. Inorg. Chem., 2014, 5378–5384 CrossRef CAS; (b) L. Guan, H.-F. Sheng and Y. Wang, Phosphorus, Sulfur Silicon Relat. Elem., 2015, 190, 372–379 CrossRef; (c) J. Li, C.-C. Xue, S. Liu and Z.-X. Wang, Solid State Sci., 2016, 61, 111–115 CrossRef CAS; (d) Z.-Y. Du, H.-R. Wen, C.-M. Liu, Y.-H. Sun, Y.-B. Lu and Y.-R. Xie, Cryst. Growth Des., 2010, 10, 3721–3726 CrossRef CAS.
- G. H. Birumand and R. F. Jansen, U.S. Pat., 4081463, 1978. See also: http://www.google.com/patents/US4081463 Search PubMed.
- Bruker, APEX2, SADABS and SAINT, Bruker AXS Inc., Madison, Wisconsin, USA, 2008 Search PubMed.
- G. M. Sheldrick, SHELX-96 Program for Crystal Structure Determination, 1996 Search PubMed.
- N. E. Brese and M. O'Keeffe, Acta Crystallogr., Sect. B: Struct. Sci., 1991, 47, 192–197 CrossRef.
- R. A. Coxall, S. G. Harris, D. K. Henderson, S. Parsons, P. A. Tasker and R. E. P. Winpenny, J. Chem. Soc., Dalton Trans., 2000, 2349–2356 RSC.
- (a) M. A. M. Abu-Youssef, M. Drillon, A. Escuer, M. A. S. Goher, F. A. Mautner and R. Vicente, Inorg. Chem., 2000, 39, 5022–5027 CrossRef CAS PubMed; (b) M.-H. Zeng, M.-C. Wu, H. Liang, Y.-L. Zhou, X.-M. Chen and S.-W. Ng, Inorg. Chem., 2007, 46, 7241–7243 CrossRef CAS PubMed; (c) J.-Y. Zou, W. Shi, N. Xu, L.-L. Li, J.-K. Tang, H.-L. Gao, J.-Z. Cui and P. Cheng, Chem. Commun., 2013, 49, 8226–8228 RSC.
- C.-M. Liu, D.-Q. Zhang and D.-B. Zhu, Chem. Commun., 2008, 368–370 RSC.
- (a) J. L. Manson, E. Ressouche and J. S. Miller, Inorg. Chem., 2000, 39, 1135–1141 CrossRef CAS PubMed; (b) S. H. Lee, C. Broholm, C. Ratcliff, G. Gasparovic, Q. Huang, T. H. Kim and S. W. Cheong, Nature, 2002, 418, 856–858 CrossRef CAS PubMed; (c) A. Harrison, J. Phys.: Condens. Matter, 2004, 16, S553–S572 CrossRef CAS.
- (a) R. A. Mole, J. A. Stride, P. L. F. Henry, M. Hoelzel, A. Senyshyn, A. Alberola, C. J. G. Garcia, P. R. Raithby and P. T. Wood, Inorg. Chem., 2011, 50, 2246–2251 CrossRef CAS PubMed; (b) R. A. Mole, M. A. Nadeem, J. A. Stride, V. K. Peterson and P. T. Wood, Inorg. Chem., 2013, 52, 13462–13468 CrossRef CAS PubMed.
- (a) C. Y. Niu, X. F. Zheng, X. S. Wan and C. H. Kou, Cryst. Growth Des., 2011, 11, 2874–2888 CrossRef CAS; (b) S.-J. Liu, L. Xue, T.-L. Hu and X.-H. Bu, Dalton Trans., 2012, 41, 6813–6819 RSC; (c) D. R. Xiao, G. J. Zhang, J. L. Liu, L. L. Fan, R. Yuan and M. L. Tong, Dalton Trans., 2011, 5680–5683 RSC.
- Q. C. Chu, Z. Su, J. Fan, T. A. Okamura, G. C. Lv, G. X. Liu, W. Y. Sun and N. Ueyama, Cryst. Growth Des., 2011, 11, 3885–3894 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: PXRD patterns, TGA curves and magnetic characterization of 1 and 2. CCDC 1054109 and 1418238. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ce02537d |
| This journal is © The Royal Society of Chemistry 2017 |