Highlights from the Faraday Discussion: Bio-resources: Feeding a Sustainable Chemical Industry, 19–21 June 2017, London, UK
Agnieszka
Brandt-Talbot
 *ab and
Lisa
Weigand
*ab and
Lisa
Weigand
 b
b
aDepartment of Chemistry, Imperial College London, UK. E-mail: agi@imperial.ac.uk
bDepartment of Chemical Engineering, Imperial College London, UK
First published on 17th November 2017
Introduction and opening lecture
The Faraday Meeting on ‘Bio-resources: Feeding a Sustainable Chemical Industry’ took place at the elegant home of the Royal Society of Chemistry in Burlington House, Central London, on 19–21 June 2017 (Fig. 1). It addresses a rapidly expanding, interdisciplinary field of research: developing technologies that enable the transformation of the chemical industry from a fossil fuel dominated industry into a renewable industry. | ||
| Fig. 1 Participants of the Faraday Discussion on ‘Bio-resources: Feeding a Sustainable Chemical Industry’ in the library at the Royal Society of Chemistry in London, UK. | ||
The final volume containing the papers, records of general discussions, the poster list and a list of participants are available online.† This meeting was divided into four areas of interest: the development of bio-based materials, the development of bio-based chemicals, lignocellulose conversion technologies, and feedstocks and analysis. At Faraday meetings, the majority of the presentation time is dedicated to debate and questions from the audience. Discussions are continued at the end of the sessions by a panel of speakers and in a poster session, as well as informally during numerous breaks and at a conference dinner, and the meeting is framed by inspiring opening and closing lectures.
Prof. Bruce Dale from Michigan State University was invited to present a thought-provoking opening lecture (DOI: 10.1039/C7FD00173H). He set out to challenge a number of assumptions encountered within the community in order to provide stimulus for critically discussing the meeting's individual contributions. Prof. Dale began by highlighting the strong link between energy use and prosperity, concluding that a rate of energy consumption which enables prosperous healthy living is 5 kW per person per day (Fig. 2). He highlighted that excessive energy consumption occurred in a few economies (Prof. Dale coined the term ‘energy La La Land’), and equally that a proportion of countries have not developed such a rate, yet, but that they would hopefully get there one day. He also made it clear that despite the obvious dominance of fossil fuel use in our current economic system, it clearly had an ‘expiration date’ sooner or later, and that society would become poorer if we did not shift to sustainable feedstocks. The new feedstock base would very likely entail a substantial contribution from biomass (suggested to be around 25%). Prof. Dale pointed out that the task was an urgent one, as only a few decades were left in order to accomplish a profound global transformation, before a breaking point for our planet and our society might be reached.
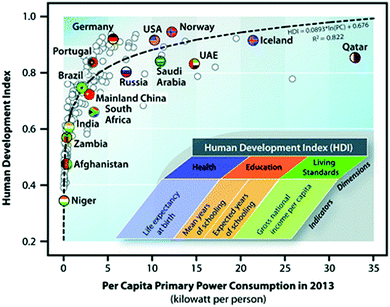 | ||
| Fig. 2 The link between energy consumption and human prosperity (DOI: 10.1039/C7FD00173H). | ||
The first key aspect that the field should focus on more, according to Prof. Dale, is considering the whole production chain when developing bio-based chemical technologies. In particular, he urged the community to ‘not forget the farmers’, who are at the very beginning of the value chain. Prof. Dale challenged the assumption that lignocellulosic biomass was very cheap or even free. He made it clear that the provision of large quantities of feedstock needed a meaningful incentive, calling for a higher feedstock price than historically assumed. He further highlighted the new relationship that the chemical industry has to develop with the agricultural sector by highlighting that fuel, chemical and food production will likely be integrated in the future system. Farms will provide an array of products and services: food for humans, feed for livestock, bio-products, bio-energy and environmental services. Also, many biorefineries will be built close to the farm, forest or waste source, as the low density of lignocellulosic feedstock restricts transportation range. Prof. Dale also challenged the common belief that ‘first-generation’ biofuels and chemicals (from food-based sugars and oils) are subject to a straightforward ‘food versus fuel’ competition. On the contrary, food-crop-based biofuel markets have been shown to encourage farmers to grow more, providing a positive buffer for food supply when farm yields decrease due to environmental fluctuations.
Bio-based materials (session 1)
The majority of the output from the chemical industry ends up in materials such as packaging, furniture, coatings and paints. Since this is unlikely to change, developing viable bio-based materials is an essential part of transitioning the chemical industry from a fossil feedstock base to a renewable feedstock base.The first contribution of the meeting was presented by Prof. Matharu from the University of York. The work demonstrated the isolation of the carbohydrate polymer pectin from mango peel using hot water and flow reactor technology (DOI: 10.1039/C7FD00035A). The work demonstrated that reactor design has an effect on yields, highlighting the impact that engineering can have on making the sustainable chemical industry effective. Discussion of the work focused on the need to evaluate solvent use throughout the entire process (a variety of volatile organic solvents were used before and after extraction), finding uses for the post-extraction residue and the effect the process has on the characteristics of the isolated pectin.
Simo Sarkanen from the University of Minnesota used his contribution to challenge the assumption in the field that lignin is universally a poor polymer precursor. His message was that, as long as suitable purification and casting strategies are in place, good polymer materials with characteristics comparable to common petroleum-derived polymers can be created from any lignin. The paper (DOI: 10.1039/C7FD00083A) characterises polymer materials created from ball-milled lignins. The discussion of this work showed that achieving desired properties can come at a high price, as energy and chemical intensive extraction, purification and modification of lignin could be potential road-blocks to commercial success, if not addressed.
Work from the lab of Mark Mascal at University of California Davis represented the production of polyesters from carbohydrate-derived diols and diacids (DOI: 10.1039/C7FD00057J). Polyesters are a group of industrially important polymers that are particularly suited to being derived from biomass, due to the presence of oxygenated functional groups in diols and diacids. The study expanded the knowledge base around the use of chloromethylfurfural (CMF) as a platform chemical. Polyesters from branched diols, such as 2,5-hexandiol and 2,7-octandiol, were synthesised and characterised. The discussion focused on the product yields and the toxicity of the CMF precursor. It became evident that renewability can clash with other Green Chemistry principles, such as reducing health hazards or environmental toxicity. The participants remained undecided on whether or not compromises in terms of toxicity should be made when searching for economically attractive plant-derived chemical intermediates.
Next, Xingdong Mu from the Qingdao Institute of Bioenergy and Bioprocess Technology presented work on the production of catalytically active carbon materials based on bamboo shoots, which were used as a catalyst support for platinum (DOI: 10.1039/C7FD00041C). The solid catalyst was applied in the hydrogenation of the bio-derived platform molecule furfural to furfuryl alcohol or cyclopentanone. The discussion focused on the mechanistic effect of heteroatom doping originating from the relatively high protein content in bamboo shoot biomass, as well as the effect biomass composition may have on the characteristics of the resulting material. A leading theme of the discussion was the important but also difficult aspect of designing appropriate control materials, which could help pinpoint characteristics of the lignocellulosic feedstock that lead to the advantageous properties of the resulting catalyst material.
Jakob Albert from FAU Erlangen-Nürnberg presented new aspects of using polyoxometallate catalysts for co-producing cellulose and formic acid from lignocellulosic biomass (DOI: 10.1039/C7FD00047B). Formic acid can be used as a C1 chemical building block or as a source of bio-derived hydrogen gas. The latter is needed to synthesise reduced chemical products from often highly oxygenated biomass building blocks. In this work, a semi-selective POM catalyst was identified that leaves the cellulose as a solid, while converting the hemicellulose and lignin portions to formic acid. It was shown that the produced cellulose retained the characteristics of high quality cellulose, and hence it could be used for material applications rather than as a source of glucose. The discussion centred on the reasons for the exhibited selectivity and the properties of the isolated cellulose.
At the end of the materials session, Gadi Rothenburg from the Dutch start-up company Plantics told the audience a tale about turning an accidental discovery in an academic lab into products made from a unique renewable polyester resin, including plant pots, water-sensitive glues and insulation (DOI: 10.1039/C7FD00054E). He demonstrated that novel materials need to be matched carefully with their applications, giving the example that Plantics’ glycerol citric acid polyester (Fig. 3) is hydro- and biodegradable, making it suitable for applications where degradability is valuable, while applications such as in outdoor furniture, where long-term stability is desired, may be less appropriate.
The session was followed by the official poster session, for which a prize was given out on the second day of the meeting during the conference dinner (Fig. 4).
 | ||
| Fig. 4 Prof. James Clark from the University of York with the winners of the poster competition, Servann Herou and Anna Zhenova. | ||
Bio-based chemicals (session 2)
The second key area that was covered by the meeting was the development of bio-based chemicals. Such small molecules can be used as fuels, solvents and monomers for producing polymers, and building blocks for complex chemicals, such as drugs and agrochemicals.The first contribution of the bio-based chemicals session was by Xiaoming Huang from TU Eindhoven, and it focused on the so-called lignin first approach, which aims to generate small aromatic molecules from lignin by contacting lignocellulose with hydrogen, a solvent and a catalytic system (DOI: 10.1039/C7FD00039A). Here, reductive cleavage was carried out in a system composed of methanol, hydrogen, palladium and a co-catalyst. It was found that strong acids such as hydrochloric acid (HCl) and sulfuric acid (H2SO4) were suitable inexpensive replacements for the Lewis acidic metal triflate. Mechanistic aspects were discussed, such as the impact of acid strength, the amount of monomers versus dimers and oligomers created, the fate of the hemicellulose and ash components and the practicalities of the final process, including catalyst recovery and the number and order of reaction steps.
Andrew Hunt from the University of York showcased a screening method aimed at accelerating the discovery of renewable solvents, consisting of computer- and lab-based selection tests applied in sequence (DOI: 10.1039/C7FD00049A). A number of candidates were selected based on solvent polarity parameters. The story came with a twist as the key candidate molecule selected for lab testing turned out to be unexpectedly toxic in later tests. This highlighted the challenge in finding a bio-derived molecules that can satisfy a long list of demands regarding its chemical, economic and environmental properties. The presenter and the audience debated whether and how the screening could be made more effective, for example by changing the order of the steps, or whether the steps were or could be carried out in parallel.
Marta Coma from the University of Bath presented the work of an academic biorefinery consortium aiming to integrate biological and chemical conversion technologies in one plant and enable the conversion of a heterogenic bio-resource. Here, anaerobic digestion and hydrothermal treatment were used to convert lignocellulosic by-products, food residues, algal biomass and plastic into bio-based products (DOI: 10.1039/C7FD00070G, Fig. 5). The primary chemical products were short alcohols, in addition to energy, oil and ‘crude’ products. Questions were mainly of a technical nature, for example the effect that algae addition had on plastic liquefaction, the rationale behind the selection of products and their anticipated purification.
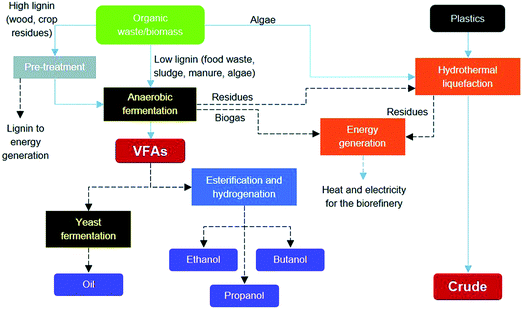 | ||
| Fig. 5 The integrated biorefinery concept proposed by Coma et al. (DOI: 10.1039/C7FD00070G). | ||
The next speaker was Changwei Hu from Sichuan University. His work focused on converting parts of algal bloom biomass into bio-oil using pyrolysis (DOI: 10.1039/C7FD00065K). The study focused on how the separation of the main components (lipids and saccharides) prior to pyrolysis affected the bio-oil product. The contribution prompted a lively discussion about the definition of the word ‘waste’. A consensus appeared to emerge that usage of the word required care, as many waste products have value today (albeit low), and words such as ‘side stream’ or ‘by-product’ may be more appropriate in many instances. The audience also queried choices made by the experimenters, for example the use of pyrolysis versus catalytic upgrading, and enquired about the properties of the resulting product fractions.
The contribution also prompted a lively debate on the general economic viability of algal biofuels. Prof. Dale was unsure how the requirements of a sunny climate, large amounts of flat land, concentrated CO2 and plentiful fresh water could be found in sufficient locations around the world to match the scale of biofuel demand. Material-intensive growing conditions were also cited as a concern. There was a debate on whether research in areas with so many challenges in terms of scale and economic feasibility should continue, and whether genetic modification/biotechnology could be the answer to these fundamental challenges.
Jose-Vitor Bomtempo from the Federal University of Rio de Janeiro presented a paper exploring how a company or entity developing a novel bio-based platform molecule could act to capture maximum value from their efforts (DOI: 10.1039/C7FD00052A). The authors defined the key chemical characteristics that an effective platform chemical should exhibit. Parallels were drawn with technological platforms in the car manufacturing and IT industries, rather than with platforms in the petrochemical industry. It was suggested that a bio-based platform chemical needs to result in several derivatives. This can be achieved through the presence of more than one functionality, although the need for multiple functionalities was debated later. Other important features are the potential to be produced at competitive cost due to few and efficient processing steps, and the potential for a large market volume, which can be a mixture of smaller scale/higher value and larger scale/lower value applications. The study concluded that the platform molecule producer should become involved in the product-side before the molecule becomes a commodity to maximise their own benefit.
Conversion technologies (session 3)
In order to produce chemicals and materials, new physical, chemical and biological conversion technologies must be developed. A selection of such conversion technologies was highlighted and debated in the third session of the meeting.George Huber from the University of Wisconsin-Madison presented a biorefinery process for producing the polyester building blocks 1,5-pentanediol and 1,6-hexanediol from sugars. They are currently both produced from petroleum (DOI: 10.1039/C7FD00036G). The work was exemplary in breadth as it included experimental data all the way from pretreatment to the final products, as well as a techno-economic analysis. The economic model assumed that the two diols could be co-produced from biomass (one from hemicellulose via furfural and one from cellulose via HMF), with lignin providing process heat and fossil gas providing a low-cost but non-renewable source of hydrogen for several hydrogenation reactions. The discussion focused on reaction conditions, yields and by-products.
The second contribution of the session was by Christoph Seidel from ETH Zürich, and it put the spotlight on one of the most commonly applied lignocellulose pretreatment technologies in the nascent cellulosic bio-product industry, steam explosion (DOI: 10.1039/C7FD00066A). The work explored how the decompression/explosion aspect of the treatment affected the subsequent sugar release by hydrolytic enzymes (cellulases). The discussion was mostly of a technical nature, enquiring how cellulose and hemicellulose are affected by the explosion, or the effect feedstock porosity prior to the explosion may have.
The next discussion was led by Joseph Same from Stockholm University and was based on his review article, which explored the current state of analytical methods for characterising lignocellulosic materials before and after pretreatment (DOI: 10.1039/C7FD00046D). The speaker pointed out that the state-of-the-art in feedstock analysis is currently derived from the paper pulping industry which focuses on cellulose, while the bio-based chemical industry will require additional analytical tools, for example methods that characterise hemicellulose and lignin in more detail. Speaker and audience debated the need for a novel, generally accepted ‘standard biomass’, and how such a standardisation may be achieved. Another part of the discussion revolved around establishing a minimum data set that is required for academic publications in order to pass peer review.
Valentina Matveeva from Tver State Technical University presented work on producing enzyme preparations immobilised on inorganic magnetic supports to convert glucose to gluconic acid efficiently (DOI: 10.1039/C7FD00042A). Enzymes are often major cost contributors to bio-based chemical processes, but supporting enzymes in such a way could reduce the cost by improving the stability and recyclability of the biocatalyst. A comment pointed out that there may be problems if the low substrate and product concentrations are not increased. The concentration was limited due to inhibition by glucose, so protein design may be needed to overcome this for a commercial process.
The next contribution to the conversion technologies theme was presented by Eero Kontturi from Aalto University. His work explored the preparation of dispersions of cellulose nano-crystals, which were produced using a novel, economically attractive acid vapour process (DOI: 10.1039/C7FD00053G). It was shown that the nanocrystals are well-formed but that the desired dispersion can only be achieved in very few solvents, otherwise the crystals need to be functionalised by oxidation. The discussion focused on the fact that formic acid was a uniquely good dispersion solvent for the unmodified nanocrystals. There was also a debate on the lack of commercial success of nanocellulose materials in the past, which was attributed to the high cost of production for the currently dominant process and the lack of sufficiently developed applications.
Next was a paper by Jason Hallett from Imperial College London, who presented work on a comparatively new pretreatment technology, ionoSolv pretreatment, which fractionates lignocellulosic biomass using low cost ionic liquids (DOI: 10.1039/C7FD00059F). The pretreatment yields a hydrolysed solid lignin and a cellulose product that is amenable to enzymatic hydrolysis. The work investigated the impact of a number of processing parameters on a commercially relevant willow feedstock. Questions were asked about ionic liquid residues left in the product fraction and lignocellulose fragments left in the ionic liquid after processing. The feedstock session also included a debate on the lack of a feedstock agnostic/independent pretreatment. On this occasion, it was noted that the term ‘feedstock independent’ is better than ‘feedstock agnostic’. It was suggested by the speaker that the ionoSolv pretreatment may fall into this category, as it allows high sugar release from grass, hardwood and softwood biomass.
The last contribution of the conversion session was by Vitaliy Budarin from the University of York, and it looked at another lignocellulose pretreatment technology, termed microwave-assisted acidolysis (DOI: 10.1039/C7FD00102A). This approach combines dilute aqueous acids and microwave irradiation, which solubilises the carbohydrate fraction while leaving the lignin as an undissolved solid. The broth also contains organic acids, oligosaccharides and furans. Two oil-producing yeasts with known tolerances to inhibitors such as acids and furans were used to ferment the solution. The questions were aimed at the properties of the lignin and the feasibility of microwave treatment on a large scale.
Important general comments were made during this session (Fig. 6). For example, it was highlighted that there was a lack of comprehensive, high quality studies comparing several pretreatment technologies applied to one or more feedstocks. The need for applying lower, industrially relevant enzyme loadings was also mentioned, and it was discussed that studies looking at chemical and biological conversions of biomass components should ideally include pretreatment, as by-products generated in the pretreatment step can affect the performance of subsequent conversion steps.
 | ||
| Fig. 6 A general discussion during the conversion technologies session, led by Karen Wilson from Aston University. | ||
Feedstocks and analysis (session 4)
The last session of the meeting addressed a breadth of different sustainable chemical feedstocks, such as dedicated lignocellulosic crops, agricultural by-products, waste paper and even CO2. It also showcased the development of novel purification and analytical strategies.Keith Waldron from the Quadram Institute Bioscience in Norwich started the last session of the meeting with a comparison study conditioning eight lignocellulosic feedstocks with one pretreatment technology to release sugars for fermentation. The pretreatment technology used was microwave-assisted liquid hot water pretreatment (DOI: 10.1039/C7FD00044H). A highlight of the study was the large data set, as eight feedstocks, selected to represent different botanical origins, were each subjected to conditions with 26 levels of severity. This allowed them to look for trends and determine conditions that would lead to a maximum in enzymatic sugar release. It was confirmed that feedstock groups react differently to aqueous pretreatment, with softwood responding generally poorly, irrespective of severity conditions. The discussion probed the general roadblocks to the commercial success of biorefineries, such as separation, the unfavourable price competition with petroleum-derived chemicals and fuels, and the historic dominance of petroleum in the chemical market.
The next contribution was by Roberto Rinaldi from Imperial College London, with a second contribution to the ‘lignin first’ approach towards fractionating and valorising lignocellulosic feedstocks (DOI: 10.1039/C7FD00069C). In this work, the solvent participates as an H-transfer reagent in lieu of molecular hydrogen. This study compared the effect of using two alcohol solvents and a number of solid-supported Ni catalysts. It was shown that using methanol instead of isopropanol resulted in different yields and lignin oil compositions. The discussion focused on various aspects of the work and technology, for example identifying and isolating the final lignin-derived molecules, the role of hemicellulose during CUB treatment and the stability of the catalyst.
This was followed by a contribution by Gary Lye from University College London, which showcases conceptual work into extending the product range of an existing UK-based sugar beet biorefinery (DOI: 10.1039/C7FD00094D). The project looked at converting the three main monosaccharides present in sugar beet (in the form of cellulose, hemicellulose and pectin). A preliminary process design was shown. Participants asked whether the purified biopolymers themselves could be products versus breaking them down and converting the monomers into bio-derived chemicals. Other questions addressed purification and separation, and the timing of LCA analysis (should it be carried out before or after the experimental proof of concept?).
Next, a contribution by Deepak Pant from the Flemish Institute of Technological research put the spotlight on the production of chemicals from carbon dioxide by microbial electrosynthesis (DOI: 10.1039/C7FD00050B). Microbial electrosynthesis is an alternative to CO2 reduction by natural photosynthesis and non-biological (light, heat or electricity-driven) technologies. This study focused on the production of acetic acid and comparing the performance of the process using a reactor operated in batch mode versus in continuous mode. The participants were interested in knowing more about the general aspects of the microbial electrosynthesis strategy for valorising CO2, such as the suitable sources of CO2 (atmospheric versus point source), CO2 purity requirements, energy balance, economics and product isolation at this scale.
Vânia G. Zuin from the Federal University of Sao Carlos presented work on isolating plant-derived molecules through absorption and desorption using renewable porous carbon materials (e.g. Starbon, DOI: 10.1039/C7FD00056A). The sorbents were produced from the carbohydrate polymers starch, alginic acid and pectin. They were used to investigate the isolation of ten bioactive phenolic compounds from an aqueous solution. The phenolic compounds were selected on the basis of their presence in a variety of fruits, seeds and vegetables, and their chemical functionality. The key factors that determined the recovery efficiency were the micro-to-mesoporous surface area ratio, and the oxygen, hydrogen and ash contents of the carbonaceous materials, as these determine the interaction with the target molecules. Participants asked questions about the performance of the bio-derived sorbents compared to commercial sorbents, and the consistency of the material characteristics across batches, among others.
The next speaker was Daniel Hayes from Celignis Limited, based in Limerick. He presented work on using near-infrared spectroscopy as a fast low-cost analytical method for quantifying the major components in lignocellulosic biomass. Such an analytical procedure could potentially be used in biorefineries to monitor their feedstock intake. In this study, the focus was on waste paper and cardboard feedstocks, which were divided into sub-groups, including kitchen roll, till receipts and birthday cards (DOI: 10.1039/C7FD00081B). The paper waste was analysed with a standard, labour-intensive wet analysis method and the results were compared against the composition obtained with the infrared method. Statistical analysis was used to test the reliability of the new method. It was shown that paper waste can vary substantially in composition, depending on its origin, and it was concluded that the model is suitable for the quantification of the sugars glucose, xylose, and lignin, but it had problems with components such as mannose, extractives and ash. The contribution had a positive reception among the participants, showing the great desire in the field for faster and easier compositional analysis methods. The questions probed aspects such as the effect of sample heterogeneity and the interference from non-wood organic matter such as glues.
The last contribution of this discussion was dedicated to the rapid identification of promising biorefinery value chains using data mining by Alexei Lapkin from the University of Cambridge. The presented tool could guide research and speed up the implementation of the sustainable chemical industry. Automated synthesis routes were compiled using data deposited in a chemical reaction data base (REAXYS). The presented case study was a multi-step synthesis of paracetamol from limonene (DOI: 10.1039/C7FD00073A). Synthetic pathways were created and filtered using a newly developed algorithm. A process model of a selected synthetic pathway was then designed ‘by hand’. The contribution triggered a lively discussion, touching on topics such as the effect that false reporting and over-exaggeration may have on pathway generation. Prof. Lapkin expressed hope that the scientific community will soon require the codification of experimental protocols and the use of standardised equipment and methods to help make literature results more reproducible. Requests to expand the model were also issued by some participants, such as starting with complex (mixed) starting molecules, or the integration of enzymatic and metabolic reactions and processes. A concern was expressed regarding restricting the creativity of organic chemists, but the speaker was convinced that using the tool correctly should help organic chemists, for example by identifying new reaction pathways that they may not have thought of, or by pointing them towards areas where a new reaction is needed. Although this method requires more development, it appeared to resonate well with the participants with experimental background, as a convenient tool like this could help them appreciate the whole value chain and engineering aspects more easily and earlier on in the project.
Closing lecture
The closing lecture was given by Andrzej Stankiewicz from Delft University of Technology (DOI: 10.1039/C7FD00178A). He used the Penrose triangle as a metaphor for the challenge that the envisaged change in the chemical industry poses (Fig. 7). | ||
| Fig. 7 The Penrose triangle for a successful transition from a petroleum-based to a bio-based industry. | ||
Andrzej Stankiewicz pointed out that biorefineries will be subject to conditions that are largely unfamiliar to the bulk petrochemical industry, for example handling low density solid feedstocks, dilute aqueous solutions, microbial transformations and enzymatic reactions. These differences mean that new features have to emerge and be optimised, which takes time and effort.
He also highlighted that many contributions to this Faraday Discussion would have been suited to more than one session, due to the multidisciplinary work required to develop sustainable chemical processes which often span feedstocks, pre-processing, product generation, product separation and analytical methods. To overcome this Prof. Stankiewicz presented an alternative ordering that grouped the papers according to questions/challenges that were being addressed, for example: how to increase the market share of a bio-based product?
He finally made a link to Michael Faraday, the person the discussions were named after, who substantially advanced the field of electromagnetism and electrochemistry, by highlighting that the sustainable chemical industry will also require sustainable electricity (Fig. 8).
 | ||
| Fig. 8 Artist’s impression of a bio-product plant based on LEGO: small, modular and driven by green electricity (DOI: 10.1039/C7FD00178A). | ||
Many discussions naturally focused on the immediate research presented in the papers, but the debate regularly returned to the bigger picture set out by Bruce Dale in the opening lecture, some of which Andrzej Stankiewicz summarised in his closing remarks. The papers that were selected to be part of this Faraday meeting cannot completely represent a very broad research theme that spans academic institutions and businesses across the world. Nevertheless this Discussion appeared to success in highlighting where research and development efforts are being focussed at the moment, and where the challenges lie.
Concluding impressions
A key theme running through the meeting was economic feasibility, emphasised in the contributions and debates alike, a sign that the academic community developing sustainable chemical processes has become acutely aware that the economic aspect is crucial for success. Despite this, many appeared to feel that we should not lose sight of other (green) metrics, most importantly energy balances, but also waste factors, safety and environmental impact.Many questions posed during the discussions of research contributions related to the separation and purification of the intermediates and products. There was a feeling that many bio-based value chains suffer from high energy consumption, and that more attention should be paid to energy- and material-efficient separation.
The need for collaboration with engineering was also felt many times during the discussions: for optimising reaction and separation performance, integrating energy use and providing economy of scale. Experimental work should increasingly be complemented with screening and modelling, especially life cycle assessment for energy balances and economic modelling for feasibility.
The conference also showed that the multi-faceted challenges have resulted in the formation of academic biorefinery consortia, supported and guided by industry players. An exciting observation was that papers were presented by companies, and that a number of academic research papers were accompanied by the formation of start-up companies, showing that there is a drive in the community to bring research out of the lab into industrial reality.
It was also uplifting to see that the research field is looking widely for inspiration to tackle its many challenges. Inspiration is being taken from the petrochemical industry, the bio-based industry's main predecessor but also its main competitor. However, it was felt that sometimes comparisons between the bio-based industry and the petrochemical industry are not helpful. For example, the scales and locations of biorefineries will very likely be different. There were also thoughts on the transition period that would see coexistence of the sustainable chemical industry with the petroleum-based chemical industry. Even the possibility of ‘green-brown’ hybrid processes (for example the use of non-renewable heat, hydrogen and electricity, and even some reagents and building blocks) was contemplated.
Other adjacent industries that came into focus during this meeting were the forestry, paper pulping and agricultural sectors. Bruce Dale urged everyone to ‘remember the farmer’ and pointed out that not a single one was present at the meeting. Someone added to also ‘remember the miner’, referring to the reliance of many bio-refining processes on catalysis with expensive and rare metals (e.g. Pt, Co and Pd).
Conference organiser Christian Stevens delivered an important concluding remark, in which he reminded the participants to also not forget the public, but to set aside time to discuss with them the challenges and advantages connected to biorefining: “We have to inform consumers about, and discuss with them, ‘food versus fuel’ issues or ‘land use change’ issues related to the production of materials for non-food use, in order to fight the use of false arguments for blocking viable sustainable solutions.”
This Faraday discussion demonstrated in a powerful way not only the variety of processes that are being developed but also the variety of feedstocks that are being processed: the projects dealt with waste paper, algal bloom biomass, lignocellulosic energy crops (e.g. poplar, willow and pine), agricultural residues (e.g. straw and beet pulp), food waste residues (e.g. mango peel) and even plastic as a co-feed. This illustrates the challenge that the bio-based chemical industry faces regarding feedstock variability and reproducibility. A common criticism for the processing of lignocellulosic biomass was that the analytical methods applied in biorefining were not sufficiently standardised, yet. The discussions also showed that CO2, a by-product of petroleum and biorefining alike, can also have a place as a feedstock for the chemical industry.
The discussions also highlighted the fact that several emerging technologies are trying to solve the same problem and are being developed in parallel; it is unclear whether one or all of them will be part of the matured biorefining industry, and to what extent. As an example, the first step in lignocellulose processing, the pretreatment operation, is being developed in many forms, for example as the reductive lignin-first approach, the hydrolytic aqueous or solvent-based fractionation approaches or as pyrolysis followed by upgrading. It appears that this is a type of tension that the community has to cope with at this early development stage. Alexei Lapkin captured the unpredictability of the development phase: “Regarding … the shape of the future industry, as the industry is a complex hierarchical system, it cannot easily be designed from first principles.”…“We need to explore this. Like in any complex system, its dynamic equilibrium state will be arrived at by evolution, not by design.”
Hence it will be interesting to see how the field will have ‘evolved’ when a follow-up Faraday meeting takes place in 5–7 years. Until then, everyone will likely be busy back at work, developing and exploring sustainable chemical industry technologies as best as they can, hopefully heeding Prof. Dale's advice to ask “Are we doing something that makes a difference?” As conference organiser David Constable said in his concluding remarks: “The challenges are great, but the opportunities are also great.”
Footnote |
| † http://pubs.rsc.org/en/journals/journalissues/fd?_ga=2.191962787.86908288.1509543208-764267119.1498721628#!issueid=fd017202&type=current&issnprint=1359-6640 |
| This journal is © The Royal Society of Chemistry 2017 |

