Exploring the science of thinking independently together: Faraday Discussion Volume 204 – Complex Molecular Surfaces and Interfaces, Sheffield, UK, July 2017
M.
Samperi
 *a,
B. E.
Hirsch
*a,
B. E.
Hirsch
 *b and
Y. A.
Diaz Fernandez
*b and
Y. A.
Diaz Fernandez
 *c
*c
aGSK Carbon Neutral Laboratories for Sustainable Chemistry, The University of Nottingham, Triumph Road, NG7 2TU, UK. E-mail: mario.samperi@nottingham.ac.uk
bDivision of Molecular Imaging and Photonics, Department of Chemistry, KU Leuven, Celestijnenlaan 200F, 3001 Leuven, Belgium. E-mail: brandon.hirsch@kuleuven.be
cOpen Innovation Hub for Antimicrobial Surfaces, Surface Science Research Centre, University of Liverpool, L69 3BX, UK. E-mail: yuridiaz@liverpool.ac.uk
First published on 15th November 2017
Abstract
The 2017 Faraday Discussion on Complex Molecular Surfaces and Interfaces brought together theoreticians and experimentalists from both physical and chemical backgrounds to discuss the relevant applied and fundamental research topics within the broader field of chemical surface analysis and characterization. Main discussion topics from the meeting included the importance of “disordered” two-dimensional (2D) molecular structures and the utility of kinetically trapped states. An emerging need for new experimental tools to address dynamics and kinetic pathways involved in self-assembled systems, as well as the future prospects and current limitations of in silico studies were also discussed. The following article provides a brief overview of the work presented and the challenges discussed during the meeting.
In July 2017, the University of Sheffield hosted the 194th edition of the Faraday Discussion meeting. The meeting brought together more than 60 researchers from diverse backgrounds, i.e., physics, chemistry, as well as theory and experiment, to create a common conversation around contemporary topics within the broader surface science field. The Edge, creatively named for its location at the “edge” of the university, played host to the discussion during some pleasant yet still unpredictable English weather.
The Faraday Division of the Royal Society of Chemistry has a long and rich history (more than 100 years) of organizing high impact discussion focused meetings. Faraday Discussions are international conferences that focus on emerging fields with modern research topics including photochemistry,1 single-molecule microscopy,2 and catalysis.3 The engaging format allows presenters only five minutes to give an overview of their research articles, which have been written and submitted approximately four months prior to the meeting. The articles are initially reviewed by the organizing committee and subsequently made available for all delegates to read prior to the discussion. As such, the meeting is mainly focused on a debate amongst the community of researchers, rather than on traditional lectures with brief question and answer sessions.
This Discussion was devoted to complex molecular surfaces and interfaces. Placing the Discussion in an historical context, the 2007 Nobel Prize awarded to Gerhard Ertl for “his studies of chemical processes on solid surfaces” remained central to the theme of this meeting. A decade after Ertl's fundamental insights, surface science research has transitioned towards premeditated design of targeted functional outputs.
The variety of research topics reflecting both applied and fundamental aims were categorized within four sessions of different themes: [1] supramolecular effects in self-assembled monolayers; [2] supramolecular systems at liquid–solid interfaces; [3] preparing macromolecular systems on surfaces; and [4] probing properties of molecule-based interfacial systems. The attendees of the meeting were confronted with the challenge of finding a common language amongst the diversity of modern surface science studies. In the following, we give a brief overview of the research highlighted during the meeting from each of these sections.
Lunch preceded welcoming remarks by the chair of the scientific committee, D. Amabilino (University of Nottingham, UK) and co-organizer, S. Tait (Indiana University, USA). After the format of the conference was explained by R. Zadik and L. Murphy of the Royal Society of Chemistry, the conference was framed by an opening lecture from R. Raval (University of Liverpool, UK).
Opening lecture
The opening lecture of this Faraday Discussion was given by Prof. Rasmita Raval (DOI: 10.1039/C7FD90072D), whose pioneering work on chiral separation at metal surfaces helped establish new fundamental principles in the field.4–6 Prof. Raval took the audience through the history and recent developments in understanding the surface behavior of achiral molecules, which are able to display chiral self-assemblies at the solid–gas interface.7 Prof. Raval then discussed how complexity builds-up within these molecular self-assemblies when intrinsically chiral molecules are investigated8 and two manifestations of chirality arises: one from the molecular handedness of a chiral molecule, and the other from the footprint chirality (Fig. 1). Professor Raval et al. coined the term “footedness” for this concept,9 rationalizing the complexity of these systems in an elegant framework of simple surface selection rules.10 | ||
| Fig. 1 (a) Depiction of the dual manifestation of chirality at surfaces created by both handedness (R and S) and footedness (λ and δ); (b) description of overall surface chirality as CHF where the organisation of handedness is depicted by H (superscript) and of footedness by F (subscript) (adapted from ref. 10 with permission from the Royal Society of Chemistry). | ||
Raval's lecture then addressed the emerging branch of on-surface covalent assembly of macromolecules, focusing on the complexity of poly-porphyrin organometallic oligomers and block-co-oligomers obtained by coupling reactions on metal surfaces.11–13 For these supramolecular systems, the current spatial resolution of STM techniques allows the identification of molecular motives that can be correlated to the binding energies of the poly-macrocyclic structures.12 Similarly, the diversity of macromolecules that can be formed by coupling reactions between different molecular building blocks was illustrated by crosslinking reactions between 2H-porphyrin, Zn(II)-diphenylporphyrin, pentacene, tetramesitylporphyrin, perylene, rubrene and coronene on Cu(110).13
Finally, Raval showed an exciting example of the potential application of these surface synthesized oligomers as bottom-up self-assembly scaffolds for bidimensional molecular actuators.14 By using a divalent bis(imidazolyl) molecule, it was possible to confine the movement of single molecules across the close-packed Cu(110) rows, acting as a linear track for motion. This movement was then restricted by porphyrin oligomers synthesized directly on the surface in an orthogonal orientation to the tracked movement, thus creating a “fence” to confine the motion in a single dimensional. This early work helps establish a conceptual understanding for more sophisticated molecular machines.
Session 1: Supramolecular effects in self-assembled monolayers
The first session of this Faraday Discussion meeting was devoted to the evergreen topic of supramolecular chemistry and its manifestations within molecular systems confined to a surface. Prof. Talat S. Rahman showed the strength of first principles calculations for interpreting and predicting experimental properties of metallic complexes. The system studied by Rahman and Le (DOI: 10.1039/C7FD00097A) was the metal–organic network formed by platinum-dipyridyl tetrazine on the Au(100) surface, described before experimentally by Skomski et al.15 The authors demonstrated that within this system, the strong interaction between the organic ligands and the Pt center stabilizes the network when the Pt atoms donate electrons to the ligands, resulting in a +2 oxidation state on Pt. They also observed a significant interaction between the dz2 orbitals of Pt and the gold surface, leading to hybridization and modification of the electronic structure and properties of the Pt center. These findings allowed for the identification of key factors affecting the chemical properties of the metal centers, opening the way towards better design rules for the next generation of single-site metal catalysts.The leitmotif of chiral self-assembly at surfaces was introduced during this session by Dr Vincent Humblot from Pierre and Marie Curie University, Paris. Combining polarization-modulation reflection–absorption infrared spectroscopy (PM-RAIRS), XPS, and STM data, Humblot et al. (DOI: 10.1039/C7FD00116A) demonstrated that the dipeptide Gly-Pro showed different behavior after adsorption on Au(110) or Ag(110). The differences were manifested on two levels: a change of the molecular form and a change in surface organization of the molecules. The adsorption of the dipeptide on Au(110) displayed a coverage and time dependent behavior, changing from neutral to zwitterionic forms, whereas on Ag(110) only the anionic form of the dipeptide was observed. These changes in the molecular state were associated with a reorganization of the molecules at the surface, and the comparison with previously published data on Cu(110)16 evidenced the key role of the metal substrate on the resultant self-assembled structure. This work highlighted the difficulties of extrapolating data for single amino acids and small peptides from one surface to another, since a myriad of completely different scenarios could emerge upon changing of chemistry of the same crystallographic plane.
Increasing complexity is one of the major challenges in current surface science, and a good example of a cutting edge system was presented by Prof. Pol Besenius from Johannes Gutenberg-Universität Mainz, Germany. The system studied by Besenius et al. (DOI: 10.1039/C7FD00100B) combines two molecular building blocks of opposite charges to construct three-dimensional supramolecular polymer brushes directly on a gold surface.17 The multicenter intermolecular interactions between the co-monomers imparted remarkable chemical stability on the self-assembled multilayered system that could be unfolded only by alternating extreme pH conditions, as shown by Surface Plasmon Resonance (SPR) measurements (Fig. 2) and Quartz Crystal Microbalance (QCM-D) experiments. The authors demonstrated thermo-responsive behaviors, showing a non-linear temperature dependence attributed to the desolvation of the molecules which induced stronger binding of the co-monomers at higher temperatures. Dissipation data extracted from QCM-D measurements suggested that the polymer brushes lie within the viscoelastic regime, opening potential applications for optoelectronics.
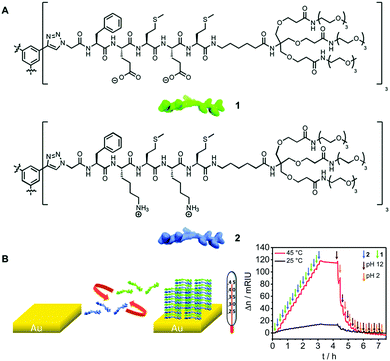 | ||
| Fig. 2 Sequential growth of surface-confined supramolecular copolymers: (A) molecular structure of the two building blocks used; (B) step-by-step sequential strategy for preparation of intercalated copolymer brushes and Surface Plasmon Resonance (SPR) sensorgram of the sequential addition of the two co-monomers 1 and 2 (green and blue arrows, respectively) and subsequent removal of the layers alternating pH = 12 (brown arrows) and pH = 2 (orange arrows) (adapted from DOI: 10.1039/C7FD00100B with permission from the Royal Society of Chemistry). | ||
Other contributions to this session covered a wide range of systems, with different experimental and theoretical approaches. Prof. Nian Lin from The Hong Kong University of Science and Technology introduced an interesting example of a demi-regular lattice formed by a bimodal metal–organic framework (DOI: 10.1039/C7FD00088J). Prof. Giovanni Costantini from the University of Warwick discussed the use of several complementary techniques to probe the interplay between geometric and electronic structures within a charge transfer systems (DOI: 10.1039/C7FD00093F), and Prof. Ioan Baldea from Heidelberg University illustrated the use of theoretical chemistry methods to investigate charge transport in self-assembled monolayers (DOI: 10.1039/C7FD00101K). The session concluded with lightning presentations from the delegates presenting posters, reflecting the wide spectrum of disciplines gathered.
Session 2: Supramolecular systems at the liquid–solid interface
The first day of welcoming introductions and discussion on supramolecular self-assembly principles provided a smooth transition into the next morning's session, which highlighted work on “supramolecular systems at liquid–solid interfaces.” Professor Amar Flood (Indiana University, USA), presented the first work of the morning detailing the two-dimensional self-assembly of selective anion receptors at octanoic acid–graphite interfaces. This contribution (DOI: 10.1039/C7FD00104E) outlined the stability and mobility of anions bound to physisorbed supramolecular receptors underneath the STM tip tunneling junction. A simple model based on electrostatic and vdW interactions was developed to describe the potential energy landscape experienced by an anion. As predicted by the theoretical model, STM self-assembly studies of these receptors reveal dynamic behavior of the anion inside the tunneling junction. Overall, this work attempts to rationalize an increasingly general observation in the field of liquid–solid 2D-assemblies, dynamic host–guest binding, which is often assigned to electric field effects created by the STM interrogation process itself.Manfred Buck (University of St. Andrews, UK) delivered the second paper of the morning (DOI: 10.1039/C7FD00115K) on work involving sequentially nested assemblies on Au(111) substrates. Using the nanoporous assembly of melamine and 3,4,9,10-perylenetretracarboxylic diimide (PTCDI), molecular guests of different shape, dimension, and chemical character were sequentially adsorbed inside the network. Dynamic switching observations of three-armed guests were made between two equivalent adsorption confirmations within the pores. Switching rates were modified by appending hydroxyl groups to the guest compound. The nanoporous network and the three-armed guests were then used to template the chemisorption of adamantine thiol molecules and physisorption of fullerene guests to form nested self-assemblies. This work extends the complexity of rationally designed nanoporous networks (with guests) used to template the adsorption of thiols on gold.
The final talk before morning tea consisted of a contribution from Natalia Martsinovich (University of Sheffield, UK). This work (DOI: 10.1039/C7FD00112F) detailed another combined theoretical and experimental approach that provides an in-depth analysis of the 2D self-assembly of terephthalic acid (TPA) at interfaces between heptanoic acid–graphite, as well as, 1-phenyloctane–graphite. Comparisons of the TPA assembly were made with the assemblies of hydroxylated analogues of TPA. Molecular mechanics and dynamics calculations provide energetic differences between the three TPA variations and their resultant assemblies. Slight dissimilarities can be observed in the self-assembly as shown by STM. These differences are rationalized through the energetic preferences arising from the hydroxyl appendages. A Born–Haber cycle was constructed to rationalize the self-assembled monolayers observed at these interfaces.
Steven De Feyter (KU Leuven, BE) contributed a collection of work on the assembly of dehydrobenzo[12]annulene (DBA) derivatives at the interface between chiral alcohol solvents and graphite substrates (DOI: 10.1039/C7FD00103G). Varying degrees of solvent dependent chiral induction were observed in the clockwise (CW) and counterclockwise (CCW) nanoporous system. A detailed discussion on the possible mechanistic rationalization for these observations followed a comprehensive experimental overview. This work demonstrates the active role of the solvent in determining the assembly outcomes. Nevertheless, conclusive evidence into the mechanism of chiral induction remains elusive.
The last two contributions in this session represent the application of liquid–solid interfacial principles to resolve challenges in the larger scientific field. Sebastian Schwaminger (Technical University of Munich, DE) presented work that described the synthesis of magnetite nanoparticles and their binding affinity toward glutamic-acid based homo-peptides (DOI: 10.1039/C7FD00105C). Surface composition and structural characterization of the nanoparticles was elucidated with zeta potential measurements and transmission electron microscopy (TEM) imaging. XPS, Raman, and infrared (IR) spectroscopy aided a deeper understanding of the surface coordination of the peptides to the nanoparticle surfaces. The work represents a broader theme of utilizing nanoparticle surfaces to develop practical assay and separations technologies.
The final paper of the session was presented by Deepak Dwivedi (Curtin University, Australia). This contribution (DOI: 10.1039/C7FD00092H) described applied work at liquid–solid interfaces detecting screw dislocation defects inside of oil pipeline materials, namely 1030 carbon steel. Under saturated CO2 conditions that mimic the native environment of pipelines, films are generated on these carbon steel substrates by sodium thiosulfate, Na2S2O3, a commonly used corrosion inhibitor. Field-emission scanning electron microscopy images (FESEM) and focused-ion beam (FIB) milling studies provide insight into the hierarchical arrangement of the molecular assembly that creates the spiral dislocations and propagations (Fig. 3). Mass spectroscopy information supplements the structural characterization. This contribution highlights how underlying fundamentals, such as hierarchical molecular assembly, are found within highly applied studies on oil transportation pipelines. The mitigation of such detrimental screw defects has practical utility in reducing the cost of vital oil pipeline transportation systems.
 | ||
| Fig. 3 FIB SEM images of screw dislocation spirals in films synthesized on a carbon steel substrate using 0.1 M sodium thiosulphate in CO2-saturated brine at room temperature, (a) showing hierarchical morphology with a dislocation loop for film and (b) exhibiting the same region after ion milling using FIB (reproduced from DOI: 10.1039/C7FD00092H with permission from the Royal Society of Chemistry). | ||
Session 3: Preparing macromolecular systems on surfaces
After lunch, the first speaker Lifeng Chi (Soochow University, China), opened the discussion by describing the development of an innovative pathway for the fabrication of graphene nanoribbons (GNRs) on Cu(111). In the last few years, this type of material has attracted significant interest for potential applications in advanced carbon-based electronic devices. In this paper (DOI: 10.1039/C7FD00129K), STM measurements highlighted long-range ordered monolayers of a perylene dianhydride derivative deposited on a Cu(111) surface at room temperature. After annealing at higher temperatures, a surface-catalyzed decarboxylation produced extended copper-perylene chains which undergo a C–C coupling reaction to generate GNRs (Fig. 4). | ||
| Fig. 4 STM micrographs of 5-AGNRs at different resolution after annealing at 630 K acquired on a Cu(111) surface (reproduced from DOI: 10.1039/C7FD00129K with permission from the Royal Society of Chemistry). | ||
Ahmad Jabbarzadeh (University of Sydney, Australia) presented the next paper, analyzing the effect of surface roughness on the crystallization process of polymeric nanodroplets (DOI: 10.1039/C7FD00071E). In this paper, the crystallization process of a linear unsaturated polymer was explored when interacting with surfaces having different roughness features. Four cases were analyzed: a polymeric nanodroplet in an isolated state (no surface effect); a drop partially, or wholly wetting the rough surface; and a drop on a smooth surface. Molecular dynamic simulations demonstrated that rough features inhibit crystal growth, with crystallinity becoming smaller as the surface roughness increases. The smooth surface induced the highest degree of crystallinity and fastest crystal growth, whereas polymer drops in an isolated state presented the slowest crystallization and the smallest crystal growth. Overall, this work demonstrated the importance that surface features can play on the kinetics of crystallization processes.
Markus Lackinger (Technical University of Munich, Germany) concluded the morning session by revisiting the well-known self-assembly of trimesic acid (TMA) monolayers on graphite surfaces (DOI: 10.1039/C7FD00113D). STM experiments and theoretical calculations on the moiré periodicity were analyzed alongside the geometric relationships between TMA and graphite to expose the origin of the incommensurable differences between the two lattice structures. Consequently, adsorbate lattice parameters with pico-metric precision were derived. Broadly, this work represents a highly-precise method for characterizing molecular adlayers through its periodic relationship with well-known substrate lattice information.
After the afternoon tea break, James D. Batteas (Texas A&M University, USA) detailed charge transport properties of thiol-derivative porphyrin clusters self-organized within a dodecanethiol monolayer on an Au(111) surface (DOI: 10.1039/C7FD00118E). By performing AFM, STM, and STS measurements, the authors described how prolonged self-assembly times, create changes in the morphology of mixed monolayer films affecting charge transport and conductance properties. On the first day, charge transfer is comparable with that of a single molecule. After three days, cluster conductance measurements indicated slow aggregation generating a bias-induced switching. Stochastic switching behaviour was observed after five days, showing current fluctuation and spread conductance onset assigned to a more close-packed organization. This work has emphasized the use of simple molecular entities for the fabrication of electro-active self-assembled nanomaterials, opening opportunities to create supramolecular devices with tunable electronic properties.
The next paper presented by Julien Gautrot (Queen Mary University of London, UK) focused on the role of the mechanical and physical properties of polydimethylsiloxane (PDMS) substrates on directing cell adhesion and motility on surfaces (DOI: 10.1039/C7FD00091J). In this investigation, an epidermal cell line (HaCaT) demonstrated that rather than bulk mechanical properties, a cell's behavior is highly sensitive to nanoscale stimuli acting at the liquid–solid interface between protein and substrate. These findings can improve the understanding of mechanisms and driving forces that regulate cell adhesion and phenotypic differentiation at interfaces, leading to novel supports for stem cell technologies, immunology and regenerative medicine.
This session concluded with Han Zuilhof (Wageningen University and Research, The Netherlands), who reported work on self-complementary pyrimidine-based derivatives on aluminum surfaces under specific external stimuli. A detailed kinetic analysis revealed the dynamic nature of the hydrogen bonds when exposed to different solvents and binding-site competitors (DOI: 10.1039/C7FD00068E). This work showed how the binding energy of a hydrogen bond motif measured on a surface is comparable in strength to the same system in solution. The proposed approach provided an elegant example of a supramolecular “ON/OFF” switch on a surface with potential applications in bio-sensing technology.
Session 4: Probing properties of molecules-based interfaces
The next morning, Angelika Kühnle (Johannes Gutenberg Universität Mainz, Germany) was the first speaker. Professor Kühnle's work detailed the importance of long-range repulsion forces in driving self-assembly of simple molecules on a surface (DOI: 10.1039/C7FD00089H). Several benzoic acid derivatives were chosen for their ability to form molecular stripes when deposited on the (10.4) cleavage plane of calcite. Deviations of stripe-to-stripe distance distribution from the ordinary random geometric placement (Fig. 5) were observed in statistically relevant AFM measurements. The work explored the parameters that might be affecting the appearance of such a phenomenon, e.g. high temperatures or kinetic effects. Overall, this work provided strong evidence for long-range electrostatic repulsions and their role in surface self-assembly processes. | ||
| Fig. 5 (a) Model of calcite (10.4) cleavage plane. (b) Structure of one of the benzoic acid derivatives studied (3-HBA). (c) Experimental stripe distance distribution (blue bars) and theoretical random geometric distribution (grey bars) evaluated from AFM micrographs (inset) of molecular stripes of 3-HBA on calcite (10.4) (reproduced from DOI: 10.1039/C7FD00089H with permission from the Royal Society of Chemistry). | ||
The next presentation was given by Professor Karl-Heinz Ernst (EMPA, Switzerland). His contribution focused on the characterization of a 2D self-assembly of bowl-shaped fragments of fullerenes on a Cu(111) surface (DOI: 10.1039/C7FD00109F). A corannulene core modified at the rim with additional aromatic rings acquired a particular bowl-shape that peculiarly affects its deposition on surfaces. STM measurements and AMBER force field calculations confirmed that the adsorbate is standing on its edge with the additional benzo group at the rim strongly interacting with the surface. Due to their particular shape and the different chemical and physical properties of concave and convex sides, these molecules represent an intriguing carbon-based material for advanced organic electronics.
The discussion continued with the next paper presented by Neil Robinson (University of Cambridge, United Kingdom). The topic of this talk focused on the development of an original tool for probing solvent dynamics in mesoporous catalytic materials using high field 1H NMR spin–lattice relaxation (DOI: 10.1039/C7FD00098G). It is well-known that adsorption interactions at the substrate–surface interface and transport properties through the active sites of a catalyst can deeply affect the performance of a given catalytic system. In this paper, the authors examine the mobility of methanol as a prototypical solvent within different oxide supports (γ-alumina, θ-alumina, silica and anatase-titania). Results obtained from T1 spin relaxation measurements showed that upon surface passivation with hydroxyl groups, methanol mobility increased when compared with a less hydrophilic surface and unrestricted bulk solvent. Upon further investigation, this method could be extended to broader cases of functionalized mesoporous materials.
Federico Rosei (INRS-EMT, Canada) gave the next talk of the session, discussing an extensive analysis of the role of halogens in Ullmann polymerization on a Cu(110) surface (DOI: 10.1039/C7FD00099E). In this paper, the authors adopted several aryl-halide building blocks to analyze the halogen dependence of both dehalogenation and C–C coupling steps. Combination of STM measurements, fast-X-ray photoelectron and near edge X-ray absorption fine structure spectroscopies, revealed different activation energies and temperature dependence of the dehalogenation process, highlighting the diverse reactivity of aryl-halides which determined the geometry and orientation of the final polymer chains on surface.
The last session of the conference concluded with a talk from Marco Sacchi (University of Surrey, United Kingdom). This work examined the dynamic behavior of benzene, chosen as a prototypical molecule, when adsorbed on a Cu(111) substrate (DOI: 10.1039/C7FD00095B). In this paper, the authors adopted a combination of helium spin echo spectroscopy and DFT calculations for measuring the diffusion rate of benzene on the metallic substrate on a picosecond timescale, assessing a jump-diffusion process with low barrier energy and a preferential benzene adsorption for the hollow sites of Cu(111). The need to achieve a better understanding of molecular motion and of the energy barriers involved in such processes has become essential in order to predict and design supramolecular systems with desired properties. To this end, this paper gave relevant insights into the complex field of diffusion dynamics of molecules on surfaces.
Poster session
In addition to the papers presented during the meeting, the Faraday Discussion also included the presentation and discussion of scientific posters. The meeting’s wide range of topics diversified during the poster session, creating a cross-disciplinary landscape that covered supramolecular self-assembly, electrochemical grafting, photo-active systems, and on-surface synthesis.Among the topics debated in the poster session, surface confined self-assembly and on-surface synthesis were two popular approaches. Davidson et al.18 showed that the formation of open porous halogen-bounded supramolecular networks is not favored by tridentate halogen bonding molecules, using a combination of theoretical and complementary experimental methods. Li et al. investigated the covalent functionalization of graphitic surfaces, demonstrating a highly-local modification in the presence of dry films of n-pentacontane. Jones et al.19 studied the interplay between chain–chain and Coulombic interactions on self-assembled structures of ionic liquids adsorbed on Au(100). Goodeal et al. and Nowicka-Dylag et al. exploited Schiff bases to synthesize 2D organic frameworks. Judd et al.20 investigated the formation of covalent bonds and intermediate metal–organic structures on Ag(111) and Ag(110), elucidating the effect of the metal substrate on the reaction’s fate. Nalbach et al.21 showed the self-assembly behavior of benzopurpurin on calcite surfaces using in situ atomic force microscopy. Humphreys et al.22 investigated diketopyrrolopyrrole chromophores combining STM and single crystal X-ray diffraction. Bilbao et al.23 synthesized and characterized hydrogen-bonded supramolecular macrocycles at the liquid–solid interface. Samperi et al. investigated the gelation process on gemini imidazolium-based amphiphilic molecules, showing the effect of solvent composition on the morphology of the fibers.
Molecular manipulation at the nanoscale was also a recurrent topic of discussion within this meeting. Mali et al. demonstrated that the electric field between the STM tip and a highly oriented pyrolytic graphite (HOPG) substrate can define mixing or phase separation. Hirsch et al.24 focused on the electrochemical reduction of diazonium cations on surfaces, showing confinement effects within nanocorrals as well as perturbation effects on the physisorbed assemblies. Seibel et al.25 explored the use of electrochemical grafting of chiral molecules on graphite. Hu et al.26 used STM tip nano-shaving for confined-space self-assembly of dehydrobenzo[12]annulene on HOPG.
Plasmonic and photoactive surfaces also had a relevant space in the discussion. Kawazumi et al. investigated the adsorption of surfactant molecules on gold nanoparticles using terahertz spectroscopy. Diaz-Fernandez et al.27 presented a photo-thermal surface, based on anisotropic metal nanoparticles, with effective antimicrobial activity at the liquid–solid interface. Bhattacharyya et al.28 synthesized and characterized Fe/Ti layered double hydroxide materials displaying photocatalytic activity with visible light.
During the conference dinner, the Chairs presented the winners of the poster awards: Yi Hu (KU Leuven, BE) and Niall Goodeal (University College London, UK).
Concluding remarks
The cross-disciplinary debate which took place at the Faraday Discussion identified several key areas defining the future of this field. The need of theoretical and experimental methods to address disordered systems at surfaces and interfaces will provide new clues on the interplay between chaotic and ordered regimes. New tools to probe and interpret dynamic and out-of-equilibrium assemblies will contribute to overcoming our current limitations and poor knowledge of transient and kinetically-trapped states at surfaces. Although classical surface science methods (e.g. STM, XPS, AFM) are still playing (and will play in the nearby future) a fundamental role, examples of translational approaches from other disciplines (e.g. liquid phase NMR, THz spectroscopy, XRD) are providing new insight into the structure and behavior of surface confined molecular structures. As the complexity of the systems increases, from single molecules on metal surfaces to multi-component bio-interfaces controlling the response of 3D living systems, surface science methods need to evolve and diversify, transducing and adapting knowledge based design rules that have revolutionized bulk materials chemistry to demonstrate a similar breakthrough in the challenging and largely unexplored territory of lower dimensional systems.Acknowledgements
M. S. thanks GSK, the EPSRC, and the University of Nottingham for funding. B. E. H. thanks the FWO for postdoctoral funding. Y. D. F. thanks the EPSRC and BBSRC for funding.Notes and references
- E. M. Tuite and F. Puntoriero, Chem. Commun., 2016, 52, 4410–4417 RSC.
- E. Gellings, S. Faez and L. Piatkowski, Chem. Commun., 2016, 52, 2213–2219 RSC.
- N. Fischer, H. G. Manyar and A. Roldan, Chem. Commun., 2016, 52, 8335–8341 RSC.
- M. Ortega Lorenzo, C. J. Baddeley, C. Muryn and R. Raval, Nature, 2000, 404, 376–379 CrossRef PubMed.
- M. O. Lorenzo, S. Haq, T. Bertrams, P. Murray, R. Raval and C. J. Baddeley, J. Phys. Chem. B, 1999, 103, 10661–10669 CrossRef CAS.
- S. Haq, N. Liu, V. Humblot, A. P. J. Jansen and R. Raval, Nat. Chem., 2009, 1, 409–414 CrossRef CAS PubMed.
- G. R. Darling, M. Forster, C. Lin, N. Liu, R. Raval and A. Hodgson, Phys. Chem. Chem. Phys., 2017, 19, 7617–7623 RSC.
- M. Forster, M. S. Dyer, M. Persson and R. Raval, J. Am. Chem. Soc., 2011, 133, 15992–16000 CrossRef CAS PubMed.
- A. G. Mark, M. Forster and R. Raval, ChemPhysChem, 2011, 12, 1474–1480 CrossRef CAS PubMed.
- M. Forster and R. Raval, Chem. Commun., 2016, 52, 14075–14084 RSC.
- S. Haq, F. Hanke, M. S. Dyer, M. Persson, P. Iavicoli, D. B. Amabilino and R. Raval, J. Am. Chem. Soc., 2011, 133, 12031–12039 CrossRef CAS PubMed.
- F. Hanke, S. Haq, R. Raval and M. Persson, ACS Nano, 2011, 5, 9093–9103 CrossRef CAS PubMed.
- S. Haq, F. Hanke, J. Sharp, M. Persson, D. B. Amabilino and R. Raval, ACS Nano, 2014, 8, 8856–8870 CrossRef CAS PubMed.
- S. Haq, B. Wit, H. Sang, A. Floris, Y. Wang, J. Wang, L. Pérez-García, L. Kantorovitch, D. B. Amabilino and R. Raval, Angew. Chem., Int. Ed., 2015, 54, 7101–7105 CrossRef CAS PubMed.
- D. Skomski, C. D. Tempas, K. A. Smith and S. L. Tait, J. Am. Chem. Soc., 2014, 136, 9862–9865 CrossRef CAS PubMed.
- C. Méthivier, H. Cruguel, D. Costa, C.-M. Pradier and V. Humblot, Langmuir, 2016, 32, 13759–13763 CrossRef PubMed.
- H. Frisch, E.-C. Fritz, F. Stricker, L. Schmüser, D. Spitzer, T. Weidner, B. J. Ravoo and P. Besenius, Angew. Chem., Int. Ed., 2016, 55, 7242–7246 CrossRef CAS PubMed.
- A. Y. Brewer, M. Sacchi, J. E. Parker, C. L. Truscott, S. J. Jenkins and S. M. Clarke, Phys. Chem. Chem. Phys., 2014, 16, 19608–19617 RSC.
- R. Foulston, S. Gangopadhyay, C. Chiutu, P. Moriarty and R. G. Jones, Phys. Chem. Chem. Phys., 2012, 14, 6054–6066 RSC.
- A. Saywell, J. Schwarz, S. Hecht and L. Grill, Angew. Chem., Int. Ed., 2012, 51, 5096–5100 CrossRef CAS PubMed.
- M. Nalbach, S. Klassen, R. Bechstein and A. Kühnle, Langmuir, 2016, 32, 9975–9981 CrossRef CAS PubMed.
- F. Pop, W. Lewis and D. B. Amabilino, CrystEngComm, 2016, 18, 8933–8943 RSC.
- N. Bilbao, I. Destoop, S. De Feyter and D. González-Rodríguez, Angew. Chem., Int. Ed., 2016, 55, 659–663 CrossRef CAS PubMed.
- L. Verstraete, B. E. Hirsch, J. Greenwood and S. De Feyter, Chem. Commun., 2017, 53, 4207–4210 RSC.
- A. M. Bragança, J. Greenwood, O. Ivasenko, T. H. Phan, K. Müllen and S. De Feyter, Chem. Sci., 2016, 7, 7028–7033 RSC.
- L. Verstraete, J. Greenwood, B. E. Hirsch and S. De Feyter, ACS Nano, 2016, 10, 10706–10715 CrossRef CAS PubMed.
- P. Pallavicini, B. Bassi, G. Chirico, M. Collini, G. Dacarro, E. Fratini, P. Grisoli, M. Patrini, L. Sironi, A. Taglietti, M. Moritz, I. Sorzabal-Bellido, A. Susarrey-Arce, E. Latter, A. J. Beckett, I. A. Prior, R. Raval and Y. A. Diaz Fernandez, Sci. Rep., 2017, 7, 5259 CrossRef PubMed.
- P. R. Chowdhury and K. G. Bhattacharyya, RSC Adv., 2016, 6, 112016–112034 RSC.
| This journal is © The Royal Society of Chemistry 2017 |
