The extraordinary impact of Michael Faraday on chemistry and related subjects
Sir
John Meurig
Thomas
ab
aDepartment of Materials Science and Metallurgy, University of Cambridge, 27 Charles Babbage Road, Cambridge, CB3 0FS, UK. E-mail: jmt2@cam.ac.uk
bUniversity Chemical Laboratories, Lensfield Road, Cambridge, CB2 1EW, UK
Abstract
Biographers of Michael Faraday, as well as many dictionaries of science, often describe him as a physicist, which he certainly was. But he was also an astonishingly effective chemist: in fact, he was the Fullerian Professor of Chemistry (at the Royal Institution, RI) from 1834 until the time of his death in August, 1867. To mark the sesquicentenary of his passing, this editorial, by one of his distant successors as Director and Fullerian Professor at the RI, focuses on Faraday's output and influence as a scientist.
Of the numerous major and relatively minor discoveries made by Faraday, arguably his most famous from a practical standpoint is that of electromagnetic induction, which he made on August 29, 1831. This enabled him to extract electricity by moving a magnet past a conductor, as demonstrated in Fig. 1, using the principles that Faraday uncovered that day in the quiet of his basement laboratory at the Royal Institution of Great Britain, in the heart of Mayfair, London.
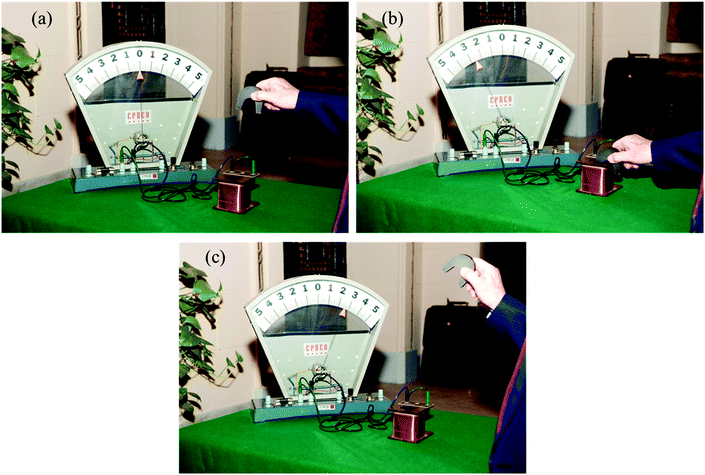 | ||
| Fig. 1 An illustration of the nature of electromagnetic induction, in which electricity is produced when conducting wires move past his imagined lines of force (see Fig. 2 and text). The energy that goes into the electrical current comes from the work done by moving the magnet. The current passes in one direction when the magnet is inserted into the coil and in the other when it is retracted. [I am grateful to Professors J. Ragai and H. Omar, American University of Cairo, for help with this illustration.] | ||
This discovery led to the dynamo, transformer and other electrical machines, and later the industrial-scale generation of energy, as well as the establishment of the discipline of electrical engineering. What is not widely appreciated is that the numerous modern instruments deployed in chemical, medical and adjacent subjects, such as NMR and ESR spectrometers, as well as Magnetic Resonance Imaging (MRI), also utilize electromagnetic induction in their mode of operation. This fact was demonstrated in 1986 by a principal founder of both NMR and MRI, E. R. Andrew,1 at a Friday Evening Discourse in the Royal Institution (RI), where Faraday lived and worked, and made almost all of his multiplicity of discoveries.
On the centenary of the discovery of electromagnetic induction, Lord Rutherford, writing in The Times, said:
“The more we study the work of Faraday with the perspective of time, the more we are impressed by his unrivalled genius as an experimenter and a natural philosopher. When we consider the magnitude and extent of his discoveries, and their influence on the progress of science and industry, there is no honour too great to pay to the memory of Michael Faraday – one of the greatest scientific discoverers of all time.”
Whilst many regard electromagnetic induction as Faraday's most celebrated discovery, others have argued equally for his introduction of the concept of “field” and for demonstrating that the plane of polarisation of a light beam was influenced by a magnetic field – the so-called Faraday effect. Over 60 years ago, Kondo, an eminent US physicist, writing in the Scientific American2 said that Faraday started the revolution which upset the long reign of Newton and rebuilt physics on new theoretical foundations. The concept of field, along with the Faraday effect, was to become a cornerstone of James Clerk Maxwell's electromagnetic theory, and also Einstein's General Theory of Relativity and present-day progress towards a deeper understanding of the nature of the physical world.
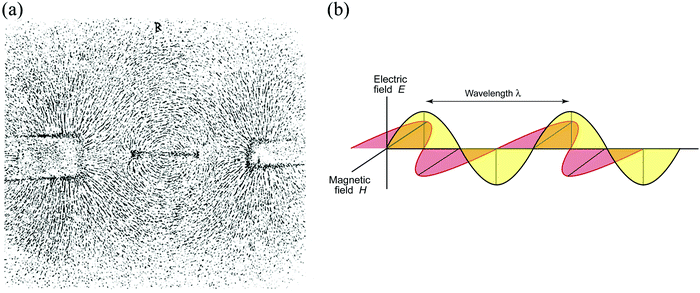
In explaining how electromagnetic induction occurred, Faraday resorted to a physical model: the familiar phenomenon of the way that iron filings on a sheet of paper arrange themselves in a pattern of lines about a magnet (Fig. 2a). Faraday proposed the idea that the space surrounding the magnet was filled with “lines of force”: the magnetic force was manifest as invisible lines. But Faraday did not stop there: he filled all space with lines of force. He further outlined the revolutionary idea that space was pervaded by various kinds of force: magnetic, electric, radiant, thermal, and gravitational. And following his work carried out in 1845: “On the Magnetization of Light and the Illumination of Magnetic Lines by Force”, published in the Royal Society, the opening paragraph had celestial resonance, Faraday declared:
“I have long held the opinion, almost amounting to conviction, in common, I believe with many other lovers of natural knowledge, that the various forms under which the forces of matter are made manifest have one common origin; or, in other words, are so directly related and mutually dependent, that they are convertible, as it were, one into another and possess equivalents of power in their action.”3
In his (still-valid) qualitative explanation of how electricity is generated in a conducting wire when it traverses the space around a magnet – by intersecting lines of force – Faraday aroused the interest of many mathematically-oriented physicists. One of them was James Clerk Maxwell, whose research fellowship thesis submitted to Trinity College, Cambridge in the early 1850s was entitled “Faraday's Lines of Force”. Maxwell, along with the work of others, notably Oliver Heaviside, went on to show, step-by-step, that Faraday's discoveries led to the nature of electromagnetic waves depicted in Fig. 2b.
It is because of the magnetic and electric components associated with electromagnetic waves that the transmission and reception of radio waves are possible. It makes feasible the construction and operation of the telephone, TV, the CD, email, the internet, WiFi, smart phones and Twitter. Little wonder that Einstein had on the wall of his study in Berlin in the early 1920s three portraits: Newton, Maxwell and Faraday, to remind him that Maxwell and Faraday, between them, were responsible for the greatest change in the intellectual picture of the nature of the physical world since Isaac Newton.4
The trajectory of Faraday's chemical studies
A brief summary of Faraday's principal contributions is given in Table 1. But there were many other significant advances made by him, starting in 1816, when he helped his mentor, Sir Humphry Davy5 invent the miner’s safety lamp. Shortly thereafter his skill as an analytical chemist was demonstrated in his first paper: “Analysis of the native caustic lime of Tuscany”. Within a few years he had become one of the leading analytical chemists in the land. He also prepared a number of interesting alloy steels; and some of the “rustless” platinum-containing sheets, that he fashioned into razors, are still extant.| • Colloidal methods. (Nanocrystalline materials) | (1857) |
| • Magnetochemistry and the magnetic properties of matter; paramagnetism (of oxygen); diamagnetism. Magneto-optics. Faraday effect (blood and haemoglobin). | (1845–1850) |
| • Electrochemistry and the electrical properties of matter. Laws of electrolysis. Equivalence of voltaic, static, animal and thermal electricity. Superionic conductors. | (1833–1836) |
| • Heterogeneous catalysis. Inhibition of activity by pre-adsorbed species. Selective adsorption by solids. | (1834) |
| • Improvements of the production of optical-grade glass. | (1825–1831) |
| • Liquefaction of gases (H2S, SO2, etc.). Critical temperature and continuity of state. | (1823 and 1845) |
| • Organic chemistry. Discovery of benzene and isobutene. Isomers. Photochemical preparations. | (1820–1826) |
| • Analytical chemistry. | (1813–1830) |
| • Preparation and properties of alloy steels. Metallography. | (1818–1824) |
| • Evolution of miner's safety lamp (with Davy). | (1816) |
During his active life, Faraday published numerous original papers on experimental aspects of chemistry and physics. And in 1858, a collection of them appeared: this was re-published during his bicentenary in 1991 by the original publishers, with a commentary by me from a contemporary perspective.6,7 The reader who consults this book is struck by Faraday's versatility, originality, and intellectual energy, the enormous sweep of his brush and his limpid style of writing. For the practitioner, the book is a gold mine strewn with glittering nuggets. Some of the experiments are ideally suited, even now, as class demonstrations for contemporary primary school children; others may be used, without modification, as practical exercises for pre-university or college students; whilst yet others identify themes that are currently at the frontier of modern chemical physics.
A monograph published to coincide with the bicentenary of Faraday's birth8 gave a chronological account of all the major scientific advances that Faraday made during his entire career, as well as some new concepts that he introduced.9
In the ensuing paragraphs I concentrate on the discoveries of greatest interest to chemists. In 1823, Faraday analysed the first recorded example of a gas hydrate, a material now termed a clathrate (from the Latin for a grating) because the guest molecule, chlorine, is enclosed in a cage formed by molecules of the host, in this case the crystallised water. (It is now known that as much carbon, as a methane clathrate in water, exists at the sea-bed as all the carbon in fossilized form!) In the same year (and later in 1845) he studied the liquefaction of gases, and succeeded in liquefying NH3, CO2, SO2, N2O, HCl, H2S, C2H4 (ethene) and C2N2. He also became the first to recognise the existence of the critical temperature, above which, no matter how high the pressure, liquefaction of a gas will not ensue.
In 1825 he discovered the organic substance which he called “bicarburet of carbon”, later named benzene. He did this by analysing the liquid left at the bottom of cylinders of gas delivered to the RI by his brother, who worked for the London Gas Company. This liquid possessed a strong aroma. It turned out to be benzene, the parent aromatic compound; and he soon produced it, by an independent method involving the thermal treatment of fish oil.10
Shortly thereafter Faraday discovered isobutene (2-methylpropene) and he commented that although it had the same empirical formula as 1-butene (already known to him), it was totally different. He had encountered the occurrence of molecular isomerism. In this organic chemical phase of his activity, Faraday also discovered tetrachloroethene and hexachloroethane, as well as the isomeric forms of naphthalene sulphonic acid.
His masterly textbook: “Chemical Manipulations”, published in 1827, running to over 640 pages, which went to three editions, was very well received. Over a hundred years later, Sir Robert Robinson, Nobel Laureate and one of the immortals of organic chemistry, wrote of it:
“This is a treatise on methods used in chemical work, all of which had been proved and many devised by himself, a book which may be profitably studied by the chemical student of today…A striking example is obtained when he gets little chemical action between the vapour of his benzene and chlorine until he subjects the mixture to sunlight, a type of reaction which forms the subject matter ofphotochemistry.”
In the mid-1820s, Faraday initiated two brilliantly successful educational ventures in the public understanding and popularisation of science, which continue to this day: the Friday Evening Discourses for lay audiences which the Prince Consort frequently attended and the Christmas Lectures for children, to which the Prince brought his young children at least once. Faraday gave the Christmas Lectures on nineteen occasions. His most famous series on “The Chemical History of a Candle”, first published in 1850, has become a classic translated into many languages; it is still recommended reading in the summer vacation for Japanese schoolchildren.
It was in the early 1830s that he turned to the study of electrochemistry, which yielded results of everlasting importance. In 1833 alone, he published four important papers on the identity of electricity derived from different sources. What he showed, in effect, was that the electricity of thunderstorms, the ‘galvanism’ of the frog's leg, the static charges stored in Leyden jars, and the current generated by a Voltaic pile, as well as that produced by a moving magnet in a nearby wire, are all synonymous.
Important as these facts are, of even greater value for the physical sciences was his discovery, around this time, of his Laws of Electrolysis. Richard Feynman, the eminent American theoretical physicist, once stated that when Faraday discovered his laws of electrolysis, it was arguably the most dramatic moment in the whole of science for they demonstrated, for the first time, that matter in its very nature is electrical.11 Faraday brought two great, hitherto separate fields (matter and electricity) together.
We recall that these laws describe in quantitative terms the relationship between the extent of chemical decomposition of a conducting substance and the amount of electricity that passes through it. His first law, in his own words, states “Chemical action or decomposing power is exactly proportional to the quantity of electricity which passes.” The second law, again in his own words, asserts “Electrochemical equivalents coincide and are the same with ordinary chemical equivalents.” In other words, the electrochemical equivalent of an element is proportional to its ordinary chemical equivalent. These laws brought order where there was hitherto confusion. They also pinpointed the relevant factors and, conversely, the irrelevant ones. It did not matter what concentration of solution the current passed through. The nature or the dimensions of the electrodes used were also of no consequence. The key factors were simply the quantity of electricity and the chemical equivalents.
As the German physiologist-turned-physicist, Hermann von Helmholtz, during the course of his Faraday prize lecture at the RI in 1881, pointed out: electricity must have a unitary structure, the maximum value of the unit being that which is sufficient to react with one univalent atom. All ions12 (the species spoken of first by Faraday), bear a charge, and this charge occurs in multiples of the electronic charge.13
The practical consequences of Faraday's work on electrolysis were major. They led to the industry of electroplating, which quickly took root in Russia, the UK and France. Also, Birmingham became a world centre for electrogilding and silvering, and it led to the gradual demise of the Sheffield silver plate industry. Faraday's work also led to the ultrasensitive method of chemical analysis now widely exploited as electroanalysis and to sensitive coulometry.
In the 1830s, Faraday also made several other significant advances of chemical interest:
• he discovered that certain solids like Ag2S, AgCl, and PbCl2 were what we now designate as superionic conductors. He uncovered also the phenomenon of fused-salt electrolytes, which now serve as vital components of fuel cells;
• he identified the fact that a platinum surface can catalyse the oxidation of hydrogen, an early example of heterogeneous catalysis, and, of equal importance, the phenomenon of inhibition (or poisoning) of a metal surface as a catalyst by prior adsorption of other molecules;
• the wettability of solids such as quartz, obsidian, topaz, calcite and mica (both cleaved and uncleaved) were first recorded by him;
• he established that an electrostatically charged solid always held its charge at its surface:14 this led him to discover the Faraday cage, which is still of great practical importance. It gives passengers in jet planes flying through lightning peace of mind. It also serves as a means of electronic security;
• his work at this time on dielectrics and specific inductive capacities was described by Debye, the Nobel prizewinner for his work on polar molecules, as “profound and prophetic”.15
• he carried out the first scientific research on mechanochemistry, which he described as “the influence of mechanical forces over chemical affinity” – for example, he showed that scratching the surface of a crystal of sodium carbonate decahydrate leads to efflorescence, whereas crystals of this material (in Faraday’s words) “preserved from external violence” do not effloresce.
In the mid-1840s, Faraday embarked on yet further important experiments. In 1845, he suspended a piece of heavy glass between the poles of his new (self-designed) ultra-powerful horseshoe electromagnet and discovered that, when he switched on the current, the glass tended to set itself perpendicular to the magnetic field in the horizontal plane. This was the beginning of his monumental work on magnetochemistry, a subject which originated with him. This is now an indispensable tool in the armoury of the physical and biological scientist, just as it is for the materials scientist.
Faraday worked with frenetic zeal in examining the magnetic properties of materials. He discovered that O2, unlike N2 and H2 or CO2, was paramagnetic,16 and his studies of blood were later to guide Linus Pauling in his classic 1936 work, and Perutz's later seminal studies, on the nature of haemoglobin before and after its reaction with O2.
In 1857 Faraday delivered his last Bakerian Lecture on Experimental Relations of Gold (and other Metals) on Light. This is of major relevance in modern colloid science, in nanoscience generally, and in nanotechnology. It is a vast repository of experiments with thin films, metal island films, aerosols, hydrosols and gels carried out with gold, silver, platinum, copper, tin, iron, lead, zinc, palladium, aluminium, rhodium, iridium, mercury and arsenic. In 2007, the physico-chemical significance of this seminal work was summarized and its relevance to modern chemistry emphasized.17 This study of matter in an ultramicroscopic state (nowadays termed nanoscience) paved the way for his colleague and admirer, John Tyndall (and later Lord Rayleigh), to explain the blueness of the sky, the opalescence of certain solutions and the colour of birds and butterflies. Also, following the investigations of Gustav Mie in the 1900s, the changing colours of Faraday's gold and silver sols can be interpreted in terms of plasmons, which are a lively topic of current research.
The very last of his experiments, to see if a magnetic field changed the quality of the orange light from a gas flame seeded with common salt, yielded nothing. Yet Faraday's intuition was sound enough for, nearly forty years later, Pieter Zeeman (Nobel Prize 1902 for magneto-optics) found the effect that now bears his name, and provided the first hints of what was to become the modern theory of atomic structure. If, through his successes, Faraday changed the world he knew, his failures, as often as not, pointed towards changes in a world he could not have foreseen.
There are yet other discoveries made by Faraday and these are still of relevance, e.g. the generation of electrical plasmas, and the influence of defects upon the reactivity of solids.
What was the essence of Faraday's genius?
It is important to recognise that Faraday (and his family) belonged to a small exclusive Christian sect, the Sandemanians, who believed in lay clergy and were opposed to the accumulation of wealth.18 He was a deeply religious man; and one of the texts in the Bible that he, himself, used as a preacher was:Romans 1:20 “The invisible things of him from the creation of the world are clearly seen, being understood by the things that are made, even his eternal power and Godhead.”
On the occasion of the bicentenary of Faraday's birth in 1991, the then Archbishop of York, John Habgood,19 a scientist by training, recalled the religious roots of Faraday's scientific commitment. A deep belief in the order and intelligibility of the world, a belief that “the invisible things” can indeed be “clearly seen” through “the things that are made. And for Faraday this belief that the world was created as an ordered whole provided the stimulus to go on seeking connections between things that did not at that time seem to have any clear connections: between electricity and magnetism; between electricity and chemistry; and ultimately as the most daring of them all, between electro-magnetism and gravity.”
We now return, finally, to focus on his staggeringly successful output. How could he have accomplished so much? To say he was an experimenter of genius is to risk exhibiting him as little more than a skilled manipulator; that he certainly was, but one for whom skill was the servant of imagination. His true genius lay in the ability to notice some oddity, to devise some experiments to test its significance and, with astonishing economy of effort, to discover how, if at all, his picture of the physical world must be modified. And though he never mastered anything beyond the elements of arithmetic, his mode of working was exemplary. As a researcher, discoverer and expositor he excelled because:
• he possessed unquenchable curiosity;
• he had a passion for clarity – in the conception, execution of his experiments and in describing them;
• to all questions that he posed, he believed there were answers;
• his choice of problems was astute – he raised important, fundamental questions;
• his tactics, strategy and economy of effort in answering questions were impeccable;
• he devised the best possible equipment, instruments and materials – his coulometers were more sensitive, his electromagnet more powerful, his glass specimens were of superior quality and heavier than those of his contemporaries;
• he demonstrated his discoveries (and those of others) to lay audiences and children, in memorable, dramatic ways;
• he popularized science to children and adults in graphic, gripping and eloquent terms.
In summary, we see that Faraday combined in a singular fashion supreme intellectual power, profound intuition, exceptional technical virtuosity and an almost timeless (Chekhovian) style of describing his work. To cap it all he was morally incorruptible.
Post script
In an accompanying article in this issue (DOI: 10.1039/c7cc90240a), the author describes many other discoveries by, and attributes of, Michael Faraday, whose famous 1861 lecture on the “beautiful, magnificent and valuable metal”, platinum, is discussed from a wider perspective.Acknowledgements
I dedicate this article to my friend, Roald Hoffmann, whose Friday Evening Discourse at the RI in November 1986 on Modern Chemistry is still ringing in my ears.I am grateful to the late Dr Irena McCabe who drew to my attention ref. 2, and to Professors M. F. Ashby, F. Gadala-Maria, K. D. M. Harris, K. Holmberg and A. Howie for their constructive comments.
References
- E. R. Andrew, Proc. R. Inst. G. B., 1987, 59, 279 Search PubMed . (Andrew invented magic-angle-spinning NMR in 1958).
- H. Kondo, Scientific American, 1953, p. 90 Search PubMed.
- Faraday was not alone in thinking about the “unity of the forces of nature” at that time. The distinguished lawyer and electrochemist, W. R. Grove, also felt likewise, as his classic book “The Correlation of Forces”, published in 1846, makes clear.
- Newton's laws of nature enable us to compute precisely the instants of sunrise and sunset; to predict also the trajectories of the planets, comets and space vehicles; to foretell the ebb and flow of the tides on all the shores of the seas and oceans of the earth. But they cannot guide us one iota in the use, interpretation and improvements of the multifarious modes of modern communication.
- J. M. Thomas, Sir Humphry Davy: Natural Philosopher, Discoverer, Inventor, Poet, Man of Action, Proc. Am. Philos. Soc., 2013, 157, 143 Search PubMed.
- And was republished in 1991 by Taylor and Francis, the original publishers, with an extended Foreword to the Bicentennial Edition by me.
- Faraday wrote a series of thirty papers for Phil. Trans. Roy. Soc., between 1832 and 1856 under the title of “Experimental Researches in Electricity”, each taking up where the previous left off. In 1859 he produced a book under this title. Later versions were to appear incorporating his subsequent articles in electricity.
- “Michael Faraday and the Royal Institution: The Genius of Man and Place”, by J. M. Thomas (1991), originally published by the Institute of Physics and Adam Hilger, now published by Taylor and Francis.
- Field theory, which modern day cosmologists and nuclear and particle theorists cannot do without, was first introduced by Faraday. The energy and force associated with a magnet does not reside inside the magnet itself, but in its surroundings. This ‘heretical’ view was rejected by many of his contemporary theorists. But not by Maxwell. Field theory is indispensable in interpreting the nature of gravitons and gluons – from the unimaginably large to the seemingly indescribably small.
- In 1991, as part of the Faraday bicentenary, Sir John Cadogan (President of the RSC) lectured at the RI on “Benzene since Faraday”. In it he disclosed that a nanogram of the benzene sample prepared by Faraday contained less than 0.1% of sulphur, thereby proving beyond doubt that Faraday's sample had not come from a fossil source.
- R. P. Feynman, Thoughts of a citizen-scientist, Basic Books, 2005, pp. 14–15 Search PubMed.
- It was William Whewell, the polymathic, Master of Trinity College, Cambridge, who suggested the word “ion” to Faraday.
- Nowadays, it is obvious that Faraday's laws of electrolysis mean that each atom can acquire or lose one or more units of electrical charge; not so for Faraday, who seriously doubted the existence of atoms.
- This led him to illustrate the effectiveness and the reality of a Faraday cage when he carried out his famous demonstration to an audience of the RI, when he sat inside a highly electrostatically charged wire cube of 12 × 12 × 12 feet.
- The Farad is the unit of capacitance. Faraday is the only scientist that has two universal units named in his honour, the Faraday being 9.6485 × 104 Coulombs, the charge of one mole of electrons.
- The paramagnetism of O2 had to await another eighty years after its discovery by Faraday before it could be explained quantum-mechanically.
- P. P. Edwards and J. M. Thomas, Angew. Chem., Int. Ed., 2007, 46, 5480 CrossRef CAS PubMed.
- C. A. Russell, Michael Faraday: Science and Faith, Oxford Univ. Press, 2001 Search PubMed.
- J. Habgood, The Archbishop of York's address in Westminster Abbey, 20 Sept 1991, reproduced in Proc. Royal Institution of GB, 1992, vol. 64, p. 1.
| This journal is © The Royal Society of Chemistry 2017 |