Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rhodium-catalyzed odorless synthesis of diaryl sulfides from borylarenes and S-aryl thiosulfonates†
Kazuya
Kanemoto
a,
Yasuyuki
Sugimura
b,
Shigeomi
Shimizu
b,
Suguru
Yoshida
 *a and
Takamitsu
Hosoya
*a and
Takamitsu
Hosoya
 *a
*a
aLaboratory of Chemical Bioscience, Institute of Biomaterials and Bioengineering, Tokyo Medical and Dental University (TMDU), 2-3-10 Kanda-Surugadai, Chiyoda-ku, Tokyo 101-0062, Japan. E-mail: s-yoshida.cb@tmd.ac.jp; thosoya.cb@tmd.ac.jp
bDepartment of Pathological Cell Biology, Medical Research Institute, Tokyo Medical and Dental University (TMDU), 1-5-45 Yushima, Bunkyo-ku, Tokyo 113-8510, Japan
First published on 18th September 2017
Abstract
Various diaryl sulfides, including heteroaryl- and nitrogen-containing sulfides, have been efficiently prepared by rhodium-catalyzed odorless deborylative arylthiolation of organoborons with S-aryl thiosulfonates. The ready availability of starting materials and further transformation of sulfides have rendered a diverse range of organosulfur compounds easily accessible.
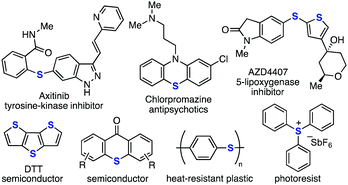
Aromatic sulfides represent a privileged class of compounds that exhibits broad applications in various research fields such as medicinal chemistry1,2 and materials science3 (Fig. 1). Thio group-guided transformations as well as the ipso-substitution reactions via the C–S bond cleavage of thioarenes have enhanced the utility of aromatic sulfides as synthetic intermediates.4 Although recent developments in the transition-metal catalyzed C–S bond forming reactions have improved the accessibility of aromatic sulfides,5,6 the types of sulfides that can be prepared are still limited and a novel method that enables the synthesis of a wide range of sulfides is still required.
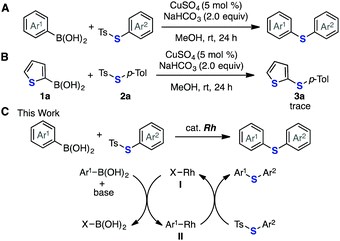
In this context, we previously developed a facile synthetic route to aryl and alkenyl sulfides via the copper-catalyzed deborylthiolation of organoborons with thiosulfonates (Fig. 2A).6h The use of readily available thiosulfonates7,8 as the source of the thio group rendered the entire transformation free of unpleasant odor and diverse sulfides easily accessible. However, while examining the substrate scope and limitation of this reaction, we found that the reaction between 2-thienylboronic acid (1a) and S-p-tolyl p-toluenethiosulfonate (2a) to prepare 2-thienyl 4-tolyl sulfide (3a) did not proceed under the standard copper-catalyzed conditions (Fig. 2B). This reaction possibly failed because of the deactivation of the catalyst, which is related to the strong coordination of the sulfur atom in 1a to copper. Furthermore, thiolation of 1a was not easy to achieve efficiently by the conventional catalytic thiolation methods using an easily available reagent, including thiol.6d,f,g,9 To address this issue, we focused on a rhodium catalyst because rhodium is analogous to copper in terms of catalytic reactivity,10 such as in 1,4-addition reactions, and several sulfides have been successfully prepared using a rhodium catalyst.11 Based on this idea, we designed a catalytic cycle for the reaction between arylboronic acid and S-aryl thiosulfonate to afford the desired diaryl sulfide (Fig. 2C). This cycle involved the formal substitution reaction of a thiosulfonate with an arylrhodium intermediate II, which is generated from a rhodium catalyst I and arylboronic acid in the presence of a base. Herein, we report that a rhodium catalyst efficiently promoted the deborylthiolation of borylarenes with S-aryl thiosulfonates, including heteroaryl substrates such as 1a, which enabled the synthesis of various diaryl sulfides.
After the catalysts were extensively screened, several rhodium catalysts were found to promote the deborylative p-tolylthiolation of 1a with 2a (Table 1, entries 4–8). In particular, the desired sulfide 3a was obtained efficiently when the reaction was conducted in methanol at 50 °C or room temperature using a rhodium catalyst coordinated with cyclooctadiene (5–10 mol% of Rh) in the presence of potassium phosphate (entries 5 and 8–10). Besides potassium phosphate, weaker bases, such as potassium carbonate and cesium fluoride, were also suitable (entries 11 and 12). The reactions using a rhodium catalyst coordinated with cyclooctene, 1,5-hexadiene, norbornadiene, or triphenylphosphine gave poor results (entries 2–4 and 13). These results suggested that cyclooctadiene with a suitable coordination ability to rhodium is crucial to efficiently achieve this transformation. In addition, an iridium catalyst was less effective for this transformation (entry 14). Similar to the copper-catalyzed reaction, the entire experimental process was free from unpleasant organosulfurous odor.
| Entry | Catalyst (mol%) | Base | Yielda (%) |
|---|---|---|---|
| a Yields were determined by HPLC analysis. b Isolated yield shown in parentheses. c Reaction was conducted at room temperature. | |||
| 1 | [Rh(OCOCF3)2]2 (5) | K3PO4 | Trace |
| 2 | [RhCl(coe)2]2 (5) | K3PO4 | Trace |
| 3 | [Rh(Cl)(1,5-hexadiene)]2 (5) | K3PO4 | Trace |
| 4 | [RhCl(nbd)]2 (5) | K3PO4 | 36 |
| 5 | [RhCl(cod)]2 (5) | K3PO4 | 70 |
| 6 | Rh(cod)2OTf (10) | K3PO4 | 27 |
| 7 | [Rh(cod)(MeCN)2]BF4 (10) | K3PO4 | 34 |
| 8 | [Rh(OH)(cod)]2 (5) | K3PO4 | 71 (69)b |
| 9 | [Rh(OH)(cod)]2 (2.5) | K3PO4 | 43 |
| 10c | [Rh(OH)(cod)]2 (5) | K3PO4 | 62 |
| 11 | [Rh(OH)(cod)]2 (5) | K2CO3 | 55 |
| 12 | [Rh(OH)(cod)]2 (5) | CsF | 65 |
| 13 | Rh(PPh3)3Cl (10) | K3PO4 | Trace |
| 14 | [IrCl(cod)]2 (5) | K3PO4 | 38 |
A wide range of arylboronic acids were efficiently deborylthiolated with thiosulfonate 2a under the optimized conditions using 2.5 or 5.0 mol% of [Rh(OH)(cod)]2 with potassium phosphate or potassium carbonate (Fig. 3). Various phenylboronic acids with either an electron-rich or electron-deficient substituent, such as methoxy, hydroxy, dimethylamino, bromo, and methoxycarbonyl groups, were efficiently thiolated to afford diaryl sulfides 3b–f, leaving these groups untouched. The deborylthiolation of a phenylboronic acid bearing a bulky group at the ortho position, such as 2-(pivaloylamino)phenylboronic acid, also occurred, affording 2-thioaniline derivative 3g. Notably, substrates containing a group with an acidic proton, such as hydroxy and amide groups, tolerated the reaction. Moreover, the deborylthiolation of heteroaromatic substrates, such as 2-benzofuranyl- and 3-thienylboronic acids, smoothly proceeded to afford sulfides 3h and 3i in excellent yields. Besides the aromatic substrates, an alkenyl substrate such as β-styrylboronic acid also participated in this reaction to quantitatively afford alkenyl sulfide 3j.
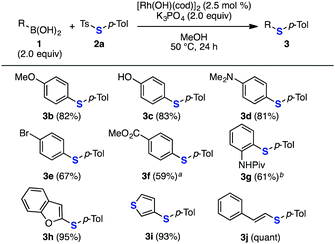 | ||
| Fig. 3 Thiolation of various boronic acids with thiosulfonate 2a. Isolated yields are shown. a K2CO3 was used instead of K3PO4. b 5 mol% of [Rh(OH)(cod)]2 was used. | ||
Various S-aryl thiosulfonates were also used to synthesize diaryl sulfides, as demonstrated in the reaction with 3-thienylboronic acid (1i) (Fig. 4). Thiosulfonates bearing an electron-donating or electron-withdrawing group successfully participated in this transformation to afford diaryl sulfides 3k–s. Notably, the reactions with thiosulfonates bearing an unprotected amino or carbamate group, which were unfavorable substrates under copper-catalyzed conditions, also smoothly proceeded to afford diaryl sulfides 3n and 3o in high yields.
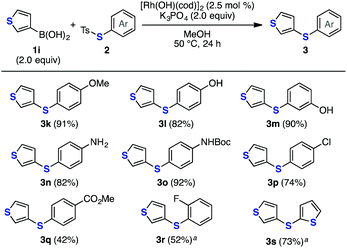 | ||
| Fig. 4 Thiolation of arylboronic acid 1i with various S-aryl thiosulfonates. Isolated yields are shown. a 5 mol% of [Rh(OH)(cod)]2 was used. | ||
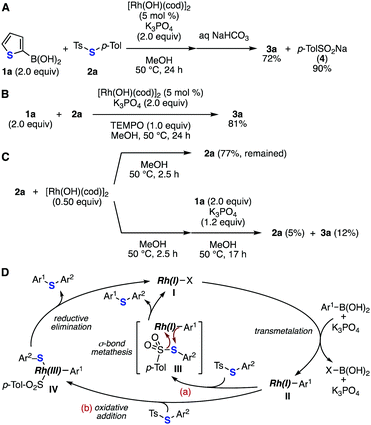
To gain insights into the mechanism of the rhodium-catalyzed deborylthiolation, we conducted several control experiments (Fig. 5). From a comprehensive survey of the products formed in the reaction between 1a and 2a, the formation of a significant amount of sodium p-toluenesulfinate (4) along with sulfide 3a was confirmed after the treatment with an aqueous solution of sodium bicarbonate (Fig. 5A).12 The addition of 2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPO) did not inhibit the formation of sulfide 3a, suggesting that radical intermediates were not involved in the reaction mechanism (Fig. 5B). The treatment of thiosulfonate 2a with an equimolar amount of rhodium salt at 50 °C for 2.5 h led to the partial consumption (23%) of 2a, and the subsequent addition of arylboronic acid 1a and potassium phosphate to this mixture afforded only a small amount of sulfide 3a (12% yield) (Fig. 5C). This result suggested that arylboronic acid and a base also play a significant role in generating an active catalytic species. Based on these results, the reaction was expected to start with transmetalation between the rhodium catalyst I and arylboronic acid in the presence of a base (Fig. 5D). Subsequent σ-bond metathesis of the arylrhodium intermediate II with thiosulfonate (path a) or the oxidative addition of thiosulfonate to the arylrhodium intermediate II, followed by reductive elimination (path b), afforded diaryl sulfide with the regeneration of rhodium sulfinate.
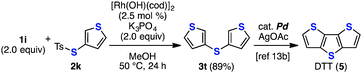
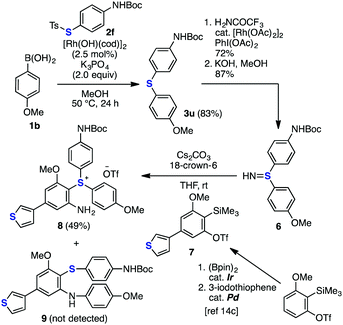
The improvement in the reaction efficiency for the deborylthiolation of heteroatom-containing substrates rendered the synthesis of diverse sulfur-containing molecules easily achievable (Schemes 1–3). For example, the formal synthesis of dithienothiophene (DTT, 5)13 was achieved via the rhodium-catalyzed coupling between 3-thienylboronic acid (1i) and 3-thienyl thiosulfonate 2k, affording di(3-thienyl)sulfide (3t), which has been reported as the precursor of DTT (5)13b (Scheme 1). Furthermore, in combination with our aryne chemistry,14 the short synthesis of a highly functionalized triaryl sulfonium salt 8 was accomplished (Scheme 2). The rhodium-catalyzed thiolation of p-anisylboronic acid (1b) with thiosulfonate 2f afforded the corresponding diaryl sulfide 3u in high yield. Subsequently, 3u was transformed to sulfilimine 6 based on a reported method.15 The generation of 3-methoxy-5-(3-thienyl)benzyne from the precursor 714c in the presence of sulfilimine 6 by the treatment of the mixture with cesium carbonate and 18-crown-6 at room temperature14a unexpectedly afforded multisubstituted triaryl sulfonium salt 8 as the major product. The formation of 2-sulfanylaniline derivative 9, which was assumed to be obtained via the direct thioamination of aryne and subsequent migratory N-arylation,14c was not observed.
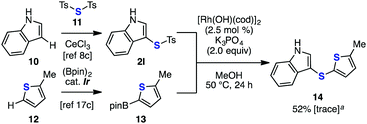 | ||
| Scheme 3 Formal C–H arylthiolation of indole. a Yield of 14 when the reaction was performed under copper-catalyzed conditions (CuSO4 (5 mol%), NaHCO3 (2.0 equiv.), MeOH, rt, 24 h) in brackets. | ||
The utility of the rhodium-catalyzed deborylthiolation reaction was further demonstrated in the synthesis of diheteroaryl sulfide 14 from simple starting materials, which were easily prepared using the recently emerging C–H functionalization chemistry16,17 (Scheme 3). According to the reported method,8c thiosulfonate 2l was prepared by the cerium trichloride-mediated electrophilic p-toluenesulfonylthiolation of indole (10) using di(p-toluenesulfonyl)sulfide (11) that proceeded in a regioselective manner at the C3-position. Meanwhile, the C5-borylated 2-methylthiophene 13 was prepared regioselectively by the iridium-catalyzed C–H borylation of 2-methylthiophene (12) according to the reported method.17c The rhodium-catalyzed coupling between 2l and 13 successfully proceeded to afford diaryl sulfide 14. In contrast, an attempt of the same transformation under copper-catalyzed conditions resulted in the formation of only a trace amount of the desired product 14. These results clearly highlighted the advantage of rhodium-catalyzed conditions for synthesizing various di(hetero)aryl sulfides.
In summary, we have developed an odorless synthetic route to diaryl sulfides by the rhodium-catalyzed deborylthiolation of borylarenes using thiosulfonates. Because of the mild reaction conditions, a wide range of substrates could be used, which significantly expanded the scope of the diaryl sulfides that could be synthesized, including heteroaryl- and nitrogen-containing compounds. Further studies of this reaction in terms of the application to the synthesis of bioactive compounds and the detailed mechanism are currently underway.
This work was supported by the Platform Project for Supporting Drug Discovery and Life Science Research funded by AMED; P-CREATE from AMED; JSPS KAKENHI Grant Numbers 15H03118 (B; T. H.), 16H01133 (Middle Molecular Strategy; T. H.), 26350971 (C; S. Y.); Naito Foundation (S. Y.).
Conflicts of interest
There are no conflicts to declare.Notes and references
- For selected reviews of bioactive sulfur-containing compounds, see: (a) K. Pluta, B. Morak-Młodawska and M. Jeleń, Eur. J. Med. Chem., 2011, 46, 3179 CrossRef CAS PubMed; (b) E. A. Ilardi, E. Vitaku and J. T. Njardarson, J. Med. Chem., 2014, 57, 2832 CrossRef CAS PubMed.
- Recent examples of our studies on sulfur-containing bioactive compounds, see: (a) Y. Ogawa, Y. Nonaka, T. Goto, E. Ohnishi, T. Hiramatsu, I. Kii, M. Yoshida, T. Ikura, H. Onogi, H. Shibuya, T. Hosoya, N. Ito and M. Hagiwara, Nat. Commun., 2010, 1, 86 Search PubMed; (b) I. Kii, Y. Sumida, T. Goto, R. Sonamoto, Y. Okuno, S. Yoshida, T. Kato-Sumida, Y. Koike, M. Abe, Y. Nonaka, T. Ikura, N. Ito, H. Shibuya, T. Hosoya and M. Hagiwara, Nat. Commun., 2016, 7, 11391 CrossRef CAS PubMed.
- For selected reviews of sulfur-containing compounds in materials science, see: (a) A. S. Rahate, K. R. Nemade and S. A. Waghuley, Rev. Chem. Eng., 2013, 29, 471 CAS; (b) S. Dadashi-Silab, C. Aydogan and Y. Yagci, Polym. Chem., 2015, 6, 6595 RSC.
- For selected examples, see: (a) L. Wang, W. He and Z. Yu, Chem. Soc. Rev., 2013, 42, 599 RSC; (b) S. G. Modha, V. P. Mehta and E. V. Van der Eycken, Chem. Soc. Rev., 2013, 42, 5042 RSC; (c) F. Pan and Z.-J. Shi, ACS Catal., 2014, 4, 280 CrossRef CAS; (d) T. Sugahara, K. Murakami, H. Yorimitsu and A. Osuka, Angew. Chem., Int. Ed., 2014, 53, 9329 CrossRef CAS PubMed; (e) Y. Uetake, T. Niwa and T. Hosoya, Org. Lett., 2016, 18, 2758 CrossRef CAS PubMed; (f) M. Bhanuchandra, A. Baralle, S. Otsuka, K. Nogi, H. Yorimitsu and A. Osuka, Org. Lett., 2016, 18, 2966 CrossRef CAS PubMed; (g) Z. Lian, B. N. Bhawal, P. Yu and B. Morandi, Science, 2017, 356, 1059 CrossRef CAS PubMed; (h) H. Saito, K. Nogi and H. Yorimitsu, Chem. Lett., 2017, 46, 1122 CrossRef.
- For reviews, see: (a) T. Kondo and T. Mitsudo, Chem. Rev., 2000, 100, 3205 CrossRef CAS PubMed; (b) S. V. Ley and A. W. Thomas, Angew. Chem., Int. Ed., 2003, 42, 5400 CrossRef CAS PubMed; (c) E. M. Beccalli, G. Broggini, M. Martinelli and S. Sottocornola, Chem. Rev., 2007, 107, 5318 CrossRef CAS PubMed; (d) J. F. Hartwig, Acc. Chem. Res., 2008, 41, 1534 CrossRef CAS PubMed; (e) I. P. Beletskaya and V. P. Ananikov, Chem. Rev., 2011, 111, 1596 CrossRef CAS PubMed; (f) C. Liu, H. Zhang, W. Shi and A. Lei, Chem. Rev., 2011, 111, 1780 CrossRef CAS PubMed; (g) C.-F. Lee, Y.-C. Liu and S. S. Badsara, Chem. – Asian J., 2014, 9, 706 CrossRef CAS PubMed; (h) M. Arisawa, Tetrahedron Lett., 2014, 55, 3391 CrossRef CAS.
- For selected examples of deborylthiolations, see: (a) C. Savarin, J. Srogl and L. S. Liebeskind, Org. Lett., 2002, 4, 4309 CrossRef CAS PubMed; (b) N. Taniguchi, J. Org. Chem., 2007, 72, 1241 CrossRef CAS PubMed; (c) L. Wang, W.-Y. Zhou, S.-C. Chen, M.-Y. He and Q. Chen, Synlett, 2011, 3041 CrossRef CAS; (d) H.-J. Xu, Y.-Q. Zhao, T. Feng and Y.-S. Feng, J. Org. Chem., 2012, 77, 2878 CrossRef CAS PubMed; (e) J.-T. Yu, H. Guo, Y. Yi, H. Fei and Y. Jiang, Adv. Synth. Catal., 2014, 356, 749 CrossRef CAS; (f) R. Singh, B. K. Allam, N. Singh, K. Kumari, S. K. Singh and K. N. Singh, Adv. Synth. Catal., 2015, 357, 1181 CrossRef CAS; (g) Z. Qiao, N. Ge and X. Jiang, Chem. Commun., 2015, 51, 10295 RSC; (h) S. Yoshida, Y. Sugimura, Y. Hazama, Y. Nishiyama, T. Yano, S. Shimizu and T. Hosoya, Chem. Commun., 2015, 51, 16613 RSC; (i) J. Li, C. Li, S. Yang, Y. An, W. Wu and H. Jiang, J. Org. Chem., 2016, 81, 7771 CrossRef CAS PubMed; (j) Z. Qiao and X. Jiang, Org. Biomol. Chem., 2017, 15, 1942 RSC.
- (a) L. Field and T. F. Parsons, J. Org. Chem., 1965, 30, 657 CrossRef CAS; (b) G. Liang, J. Chen, J. Chen, W. Li, J. Chen and H. Wu, Tetrahedron Lett., 2012, 53, 6768 CrossRef CAS; (c) N. Taniguchi, J. Org. Chem., 2015, 80, 1764 CrossRef CAS PubMed; (d) Y. Zheng, F.-L. Qing, Y. Huang and X.-H. Xu, Adv. Synth. Catal., 2016, 358, 3477 CrossRef CAS.
- (a) T. Hanamoto, K. Korekoda, K. Nakata, K. Handa, Y. Koga and M. Kondo, J. Fluorine Chem., 2002, 118, 99 CrossRef CAS; (b) F. Kopp and P. Knochel, Org. Lett., 2007, 9, 1639 CrossRef CAS PubMed; (c) C. C. Silveira, S. R. Mendes, J. R. Soares, F. N. Victoria, D. M. Martinez and L. Savegnago, Tetrahedron Lett., 2013, 54, 4926 CrossRef CAS; (d) S. Yoshida, K. Uchida and T. Hosoya, Chem. Lett., 2014, 43, 116 CrossRef CAS.
- For Pd-catalyzed thiolation of 1a using phenylsulfenyl chloride, see: P. Gogoi, M. Kalita and P. Barman, Synlett, 2014, 25, 866 CrossRef CAS.
- (a) T. Hayashi and K. Yamasaki, Chem. Rev., 2003, 103, 2829 CrossRef CAS PubMed; (b) A. Alexakis, J. E. Bäckvall, N. Krause, O. Pàmies and M. Diéguez, Chem. Rev., 2008, 108, 2796 CrossRef CAS PubMed; (c) N. Matsuda, K. Hirano, T. Satoh and M. Miura, Angew. Chem., Int. Ed., 2012, 51, 3642 CrossRef CAS PubMed; (d) T. Yasuhisa, K. Hirano and M. Miura, Chem. Lett., 2017, 46, 463 CrossRef CAS.
- (a) K. Ajiki, M. Hirano and K. Tanaka, Org. Lett., 2005, 7, 4193 CrossRef CAS PubMed; (b) M. Arisawa, T. Suzuki, T. Ishikawa and M. Yamaguchi, J. Am. Chem. Soc., 2008, 130, 12214 CrossRef CAS PubMed; (c) C.-S. Lai, H.-L. Kao, Y.-J. Wang and C.-F. Lee, Tetrahedron Lett., 2012, 53, 4365 CrossRef CAS; (d) S. D. Timpa, C. J. Pell and O. V. Ozerov, J. Am. Chem. Soc., 2014, 136, 14772 CrossRef CAS PubMed.
- See the ESI† for details.
- (a) J. Frey, S. Proemmel, M. A. Armitage and A. B. Holmes, Org. Synth., 2006, 83, 209 CrossRef CAS; (b) H. Kaida, T. Satoh, K. Hirano and M. Miura, Chem. Lett., 2015, 44, 1125 CrossRef CAS.
- (a) S. Yoshida, Y. Hazama, Y. Sumida, T. Yano and T. Hosoya, Molecules, 2015, 20, 10131 CrossRef CAS PubMed; (b) S. Yoshida, K. Shimomori, T. Nonaka and T. Hosoya, Chem. Lett., 2015, 44, 1324 CrossRef CAS; (c) S. Yoshida, T. Yano, Y. Misawa, Y. Sugimura, K. Igawa, S. Shimizu, K. Tomooka and T. Hosoya, J. Am. Chem. Soc., 2015, 137, 14071 CrossRef CAS PubMed; (d) S. Yoshida, H. Nakajima, K. Uchida, T. Yano, M. Kondo, T. Matsushita and T. Hosoya, Chem. Lett., 2017, 46, 77 CrossRef CAS.
- H. Okamura and C. Bolm, Org. Lett., 2004, 6, 1305 CrossRef CAS PubMed.
- For selected reviews, see: (a) L. McMurray, F. O’Hara and M. J. Gaunt, Chem. Soc. Rev., 2011, 40, 1885 RSC; (b) J. Yamaguchi, A. D. Yamaguchi and K. Itami, Angew. Chem., Int. Ed., 2012, 51, 8960 CrossRef CAS PubMed; (c) J. F. Hartwig, J. Am. Chem. Soc., 2016, 138, 2 CrossRef CAS PubMed.
- (a) C. N. Iverson and M. R. Smith, III, J. Am. Chem. Soc., 1999, 121, 7696 CrossRef CAS; (b) T. Ishiyama, J. Takagi, K. Ishida, N. Miyaura, N. R. Anastasi and J. F. Hartwig, J. Am. Chem. Soc., 2002, 124, 390 CrossRef CAS PubMed; (c) T. Ishiyama, J. Takagi, Y. Yonekawa, J. F. Hartwig and N. Miyaura, Adv. Synth. Catal., 2003, 345, 1103 CrossRef CAS; (d) I. A. I. Mkhalid, J. H. Barnard, T. B. Marder, J. M. Murphy and J. F. Hartwig, Chem. Rev., 2010, 110, 890 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization for new compounds including NMR spectra. See DOI: 10.1039/c7cc05868c |
| This journal is © The Royal Society of Chemistry 2017 |