Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dissecting the chloride–nitrate anion transport assay†
Yufeng
Yang
ab,
Xin
Wu
 c,
Nathalie
Busschaert
c,
Nathalie
Busschaert
 a,
Hiroyuki
Furuta
a,
Hiroyuki
Furuta
 b and
Philip A.
Gale
b and
Philip A.
Gale
 *c
*c
aChemistry, University of Southampton, Southampton, SO17 1BJ, UK
bDepartment of Chemistry and Biochemistry, Kyushu University, Fukuoka, 819-0395, Japan
cSchool of Chemistry, The University of Sydney, NSW 2006, Australia. E-mail: philip.gale@sydney.edu.au
First published on 2nd August 2017
Abstract
A systematic study of chloride vs. nitrate selectivity across six anion transporters has revealed a good correlation between the selectivities of their anion binding and membrane transport properties. This work reveals the limitations of the chloride–nitrate exchange assay and shows how new approaches can be used to measure anion uniport.
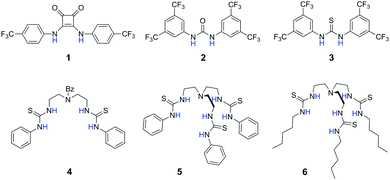
The design of synthetic anion receptors1 that can carry biological anions, most importantly chloride, across phospholipid bilayers has been an active area of research in supramolecular chemistry.2 These compounds have future therapeutic potential to replace the function of faulty anion channels in genetic diseases,3 or to disrupt the ionic and pH gradients in cancer cells.4 Efforts have been made to improve the potency of anion transporters, allowing them to function at low concentrations as required for therapeutic applications. Typically, chloride–nitrate exchange (antiport) assays have been used to evaluate the anion transport potency of the transporters.5 In this assay, it is assumed that nitrate transport is unlikely to be the rate-limiting process due to the anion's high lipophilicity, and therefore this assay is assumed to indicate the chloride transport activity of the transporters.6 However, in anion binding studies, chloride is almost always more strongly bound to hydrogen bond donor anion receptors than nitrate due to the higher charge density of Cl−.7 This could result in faster chloride transport than nitrate transport. The study of Cl−vs. NO3− selectivity in membrane transport and its correlation with binding selectivity is thus of fundamental importance in addressing the question whether anion transport selectivity is dominated by anion lipophilicity8 or anion affinity.9 This information is also practically useful to unravel the potential limitations of the chloride–nitrate exchange assay. Recently, we have developed membrane transport assays that could measure the rate of anion uniport mediated by anion transporters without the need for an anion exchange process to occur.10 We here make use of two complementary vesicle-based assays to determine anion transport selectivity, in particular Cl−/NO3− selectivity, of a library of hydrogen bond-based anion transporters 1–6 that contain increasing numbers of hydrogen bond donors (Fig. 1). By comparing these results with association constants for anion complexation determined in acetonitrile, we demonstrate for the first time a strong correlation between binding selectivity and transport selectivity across a series of structurally diverse anion transporters.
Compounds 1, 2, 3, 5, and 6 are examples of anion transporters previously studied by our group.9,10b,11 These compounds represent two distinct design approaches to highly effective anion transporters. Compounds 1–3 contain highly acidic NH groups, leading to high anion binding affinity and the ability to disperse the negative charge of the bound anion. Compounds 5 and 6 are without the electron-withdrawing CF3 groups but contain more hydrogen bond donors that are favourable for binding and transport because of multivalency12 and encapsulation.13 Their protonated cationic forms do not participate in anion transport as demonstrated previously.10b We synthesised a new receptor 4 as a dipodal control of the tripodal thiourea 5. The affinities of these receptors towards anions including Cl−, Br− and NO3− in acetonitrile were determined by UV-vis absorption titrations using tetrabutylammonium (TBA+) salts of the anions (see Table 1 for Cl− and NO3− binding data, and Table S1 in ESI† for Br− binding data). For all compounds, the anion binding selectivity trend was found to follow the anion charge density (Cl− > Br− > NO3−). Despite the same anion binding selectivity sequence, the actual extent of Cl−/NO3− binding selectivity, i.e. the Sb(Cl−/NO3−) value, did show significant variation among different scaffolds. The two tripodal compounds 5 and 6 have very high (>1500-fold) binding selectivities for Cl− over NO3−, whereas the other ureas and thioureas have a selectivity value of less than 150. A similar trend was found for the Br−/NO3− binding selectivity (Table S1, ESI†). Compared with the dipodal thiourea 4, the additional thiourea arm in 5 dramatically enhances the binding of Cl− and Br− but does not improve NO3− binding as much. These binding results give a hint that the tripodal compounds could behave differently in anion transport selectivity compared with other compounds.
| Comp. | Anion binding in acetonitrilea | Anion transport in lipid bilayers | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ISE exchange assayb | HPTS assaye | Osmotic assayh | ||||||||
| K a(Cl−)/M−1 | K a(NO3−)/M−1 | S b(Cl−/NO3−) | ECISE50(Cl−–NO3− exchange)/mol% | ECHPTS50(Cl−)/mol% | ECHPTS50(NO3−)/mol% | S HPTSt(Cl−/NO3−) | ECOs50(Cl−)/mol% | ECOs50(NO3−)/mol% | S Ost(Cl−/NO3−) | |
| a Association constants determined by UV-vis titration in acetonitrile at 298 K, using tetrabutylammonium anion salts. Errors were found to be <10%. Cl−/NO3− binding selectivity Sb(Cl−/NO3−) = Ka(Cl−)/Ka(NO3−). b Cl−–NO3− exchange assay schematically shown in Fig. 2a, using POPC LUVs with a mean diameter of ∼200 nm. EC50 calculated at 270 s. c Reported in ref. 9. d Reported in ref. 11a. e H+–Cl− cotransport assay schematically shown in Fig. 2b, using POPC LUVs with a mean diameter of ∼200 nm. EC50 calculated at 200 s. Cl−/NO3− transport selectivity in this assay: SHPTSt(Cl−/NO3−) = ECHPTS50(NO3−)/ECHPTS50(Cl−). f Determined in the absence of proton channel gramicidin D. The addition of gramicidin D did not affect the overall transport rate because these compounds are by themselves good H+/OH− transporters. See ref. 10b. g Determined in the presence of gramicidin D to remove the need of anion transporters to facilitate H+/OH− transport. Without gramicidin, H+/OH− transport facilitated by these compounds is slow and would rate-limit the overall transport process. See ref. 10b. h K+–Cl− cotransport assay schematically shown in Fig. 2c, using POPC LUVs with a mean diameter of ∼400 nm. EC50 calculated at 600 s. Cl−/NO3− transport selectivity in this assay: SOst(Cl−/NO3−) = ECOs50(NO3−)/ECOs50(Cl−). | ||||||||||
| 1 | 8.2 × 105 | 2.5 × 103 | 330 | 0.060c | 0.0089f | 0.0073f | 0.82 | 0.093 | 0.078 | 0.85 |
| 2 | 1.8 × 104 | 1.1 × 103 | 17 | 0.30c | 0.043f | 0.0052f | 0.12 | 0.27 | 0.069 | 0.26 |
| 3 | 2.3 × 104 | 6.0 × 102 | 38 | 0.16c | 0.013f | 0.0014f | 0.11 | 0.12 | 0.031 | 0.26 |
| 4 | 3.0 × 104 | 2.1 × 102 | 140 | >5 | 0.49g | 0.045g | 0.091 | 3.3 | 0.92 | 0.28 |
| 5 | 1.7 × 106 | 2.7 × 102 | 6200 | 0.31d | 0.0044g | 0.036g | 8.1 | 0.055 | 0.56 | 10 |
| 6 | 8.3 × 105 | 4.9 × 102 | 1700 | 0.11 | 0.0034g | 0.010g | 3.0 | 0.037 | 0.18 | 4.9 |
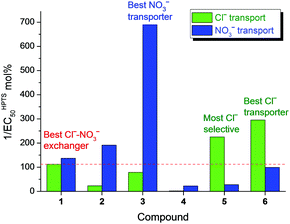
All compounds have been initially subject to a traditional Cl−–NO3− exchange assay (Fig. 2a). Briefly, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) large unilamellar vesicles (LUVs) were loaded with NaCl (490 mM) buffered at pH 7.2, and suspended in NaNO3 (490 mM) buffered at pH 7.2. The anion transporter was added to the vesicle suspension as a DMSO solution. Chloride efflux (due to Cl−–NO3− exchange induced by the anion transporters) was monitored via appearance of Cl− in the external solution, measured by a chloride ion-selective electrode (ISE). The activity of a transporter was quantified by an effective concentration of the transporter to reach 50% of ion transport (EC50 value) at 270 s. In this assay, the simple squaramide 1 was the champion while the two tripodal thioureas 5 and 6 significantly fell behind (Table 1).
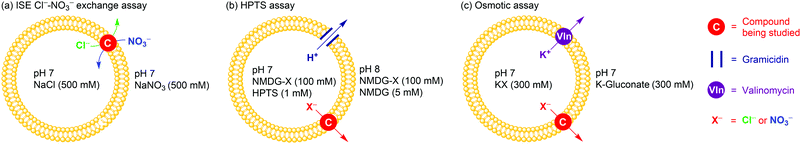 | ||
| Fig. 2 Schematic representation of three membrane transport assays used. See ESI,† for Experimental details. | ||
As the above-mentioned exchange assay provides no information on Cl−/NO3− selectivity, we conducted two additional assays to directly measure anion selectivity. In the first assay (an HPTS assay, Fig. 2b), POPC LUVs with a mean diameter of 200 nm were loaded with and suspended in a solution of the N-methyl-D-glucamine salt of the anion of interest (NMDG-X, 100 mM, X− = Cl− or NO3−) buffered at pH 7.0 with HEPES. The external pH was brought to ∼8 by addition of a base pulse (5 mM of N-methyl-D-glucamine), and then the transporter-induced dissipation of the pH gradient across vesicle membranes was measured using an intravesicular fluorescent pH indicator 8-hydroxypyrene-1,3,6-trisulfonic acid (HPTS). The overall process leading to pH gradient dissipation is H+–X− symport or OH−–X− antiport, which may be rate-limited by H+ or OH− transport. However the need for an anion transporter to facilitate H+ or OH− transport can be eliminated by using the proton channel gramicidin,10b allowing this assay to reveal the ability of an anion transporter to facilitate X− uniport (Fig. 2b).14 In the second assay (osmotic assay), larger POPC LUVs (mean diameter 400 nm) were loaded with a buffered KX (300 mM) solution and suspended in a buffered K+ gluconate (300 mM) solution. A combination of valinomycin (for transporting K+) and an anion transporter give overall K+–X− cotransport (Fig. 2c), leading to water efflux to balance the osmotic difference and the resultant shrinkage of vesicles, which can be monitored by following the light scattering of vesicles using a fluorometer.15 This provides a method of quantifying the rate of anion uniport facilitated by anion transporters.14 For all compounds, we determined the EC50 value at 200 s in the HPTS assay and at 600 s in the osmotic assay. In both assays, the Cl−/NO3− transport selectivity St(Cl−/NO3−) was quantified by the ratio between EC50 of NO3− transport and EC50 of Cl− transport, where an St(Cl−/NO3−) value > 1 indicates Cl− selectivity (Table 1).
The results from both HPTS and osmotic assays demonstrate that NO3− transport proceeds faster than Cl− transport for monopodal transporters 1–3 and dipodal transporter 4. This is consistent with the higher lipophilicity of NO3− than Cl− while contrary to the Cl− > NO3− binding selectivity of the compounds in acetonitrile. The results suggest that in these cases transport selectivity is governed by the ease of anion dehydration. In contrast to 1–4, tripodal compounds 5 and 6 transported Cl− faster than NO3−, with 5 showing an 8-fold Cl−/NO3− selectivity in the HPTS assay and a 10-fold selectivity in the osmotic assay. Therefore, 5 and 6 are unusual examples of anion transporters that can overrule the normally observed NO3− > Cl− lipophilicity bias. This cannot be interpreted as a general “anti-Hofmeister” selectivity as 5 and 6 transported the more lipophilic anion Br− faster than Cl− (Fig. S50 in ESI†). It is interesting to note that the transport selectivity St(Cl−/NO3−) in general shows a good correlation with the binding selectivity Sb(Cl−/NO3−) (Fig. 3 and Fig. S52 in ESI†). Both selectivities follow the trend of 5 > 6 > 1 > 2–4. Chloride > nitrate selectivity is only observed for 5 and 6 that have the largest binding preference for Cl− over NO3− among the library. Squaramide 1, despite being a NO3−-selective transporter, has a significantly larger St(Cl−/NO3−) value compared with 2–4, consistent with 1 being the third most Cl− > NO3− selective binder. The correlation, however, is not perfect with compounds 2–4. This may be rationalised by the idea that the rate of ionophore-catalysed ion transport depends not only on the ion binding affinity (which determines the amount of ion–ionophore complex with respect to the free ionophore) but also on the ability of the complex to translocate across lipid bilayers.16
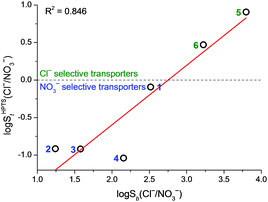 | ||
Fig. 3 Correlation between transport selectivity log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SHPTSt(Cl−/NO3−) and binding selectivity log SHPTSt(Cl−/NO3−) and binding selectivity log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sb(Cl−/NO3−). Compound numbers are shown next to the data points. Sb(Cl−/NO3−). Compound numbers are shown next to the data points. | ||
It is interesting to compare the activity determined in the three membrane transport assays. The data from HPTS and osmotic assays agreed well with each other, with the EC50 value in the osmotic assay consistently being about an order of magnitude higher than that in the HPTS assay for the same compound transporting the same anion.17 Both assays have revealed the Cl− transport activity in the sequence of 6 > 5 > 1 > 3 > 2 > 4, whereas NO3− transport activity in the sequence of 3 > 2 > 1 > 6 > 5 > 4. The Cl−–NO3− exchange assay showed the activity sequence of 1 > 6 > 3 > 2 ≈ 5 > 4, which reflected the activity of transporting the “slower” anion (Cl− in the cases of 1–4, and NO3− in the cases of 5 and 6). By converting the EC50 values to normalised activities, a reasonable agreement between the three transport assays can be observed (Table S2 in ESI†). It is evident that the Cl−–NO3− exchange assay gives a fair assessment of Cl− transport activity of compounds 1–4 because the overall process is rate-limited by Cl− transport in these cases. However, the Cl− transport activity of 5 and 6 is clearly underestimated in the exchange assay. Both compounds are in fact better Cl− transporters than the best Cl−–NO3− exchanger 1. Compound 1 turned out to be neither the best Cl− transporter nor the best NO3− transporter (Fig. 4).
For a better understanding of the Cl− > NO3− selectivity of the tripodal scaffold, we performed PM6-optimisation of the structures of the Cl− and NO3− complexes of 5. Fig. 5 (see Fig. S53 in ESI† for ball-and-stick models) shows that the spherical anion Cl− fits well and is well-encapsulated inside the cavity of the tripodal scaffold, whereas binding of the planar anion NO3− forced the scaffold to adopt a more open conformation leaving a significant part of the bound NO3− exposed to the solvent. Encapsulation of the anion is crucial for effective membrane transport as demonstrated by previous work by Davis13 and us,10b and therefore the poor encapsulation of NO3− could contributes to the Cl− > NO3− transport selectivity of the tripodal compounds.
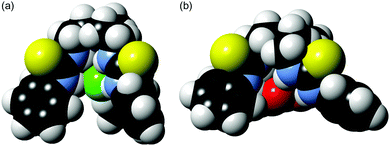 | ||
| Fig. 5 PM6-optimised structures of Cl− (a) and NO3− (b) complexes of 5 shown in space-filling models. | ||
In summary, we have examined the chloride vs. nitrate selectivity of representative hydrogen bond-based anion transporters in both binding and transport. A strong correlation between transport selectivity and binding selectivity has been found. Only the tripodal transporters 5 and 6 that have a large Cl−/NO3− binding preference can overcome the Hofmeister lipophilicity bias to transport Cl− selectively over NO3−. We have compared the anion transport potency determined from different assays, showing that the commonly used Cl−–NO3− exchange assay is valid in most cases for evaluating Cl− transport activity but sometimes gives an underestimation in the case of a poor NO3− transporter. Importantly, we have also demonstrated that compound 5 functions as a highly Cl−-selective transporter showing a ∼10-fold Cl− over NO3− selectivity in membrane transport, because of its complementary fit for Cl−. We believe that the structure-selectivity and binding-transport relationships demonstrated here, and the different assays provided in this work will provide valuable tools for future development of highly potent and selective anion transporters for biomedical applications.
PAG thanks the University of Sydney and the ARC (DP170100118) for funding and the JSPS for an Invitation Fellowship to the University of Kyushu. We thank the Kyushu University Leading Program for “Molecular System for Devices” from MEXT Japan (Y. Yang).
Notes and references
- P. A. Gale, E. N. W. Howe and X. Wu, Chem., 2016, 1, 351–422 CAS.
- (a) P. A. Gale, J. T. Davis and R. Quesada, Chem. Soc. Rev., 2017, 46, 2497–2519 RSC; (b) S. Benz, M. Macchione, Q. Verolet, J. Mareda, N. Sakai and S. Matile, J. Am. Chem. Soc., 2016, 138, 9093–9096 CrossRef CAS PubMed; (c) A. Vargas Jentzsch, A. Hennig, J. Mareda and S. Matile, Acc. Chem. Res., 2013, 46, 2791–2800 CrossRef CAS PubMed; (d) J. T. Davis, O. Okunola and R. Quesada, Chem. Soc. Rev., 2010, 39, 3843–3862 RSC.
- H. Li, H. Valkenier, L. W. Judd, P. R. Brotherhood, S. Hussain, J. A. Cooper, O. Jurček, H. A. Sparkes, D. N. Sheppard and A. P. Davis, Nat. Chem., 2016, 8, 24–32 CrossRef CAS PubMed.
- (a) N. Busschaert, S.-H. Park, K.-H. Baek, Y. P. Choi, J. Park, E. N. W. Howe, J. R. Hiscock, L. E. Karagiannidis, I. Marques, V. Félix, W. Namkung, J. L. Sessler, P. A. Gale and I. Shin, Nat. Chem., 2017, 9, 667–675 CrossRef CAS PubMed; (b) A. M. Rodilla, L. Korrodi-Gregório, E. Hernando, P. Manuel-Manresa, R. Quesada, R. Pérez-Tomás and V. Soto-Cerrato, Biochem. Pharmacol., 2017, 126, 23–33 CrossRef CAS PubMed; (c) V. Soto-Cerrato, P. Manuel-Manresa, E. Hernando, S. Calabuig-Fariñas, A. Martínez-Romero, V. Fernández-Dueñas, K. Sahlholm, T. Knöpfel, M. García-Valverde, A. M. Rodilla, E. Jantus-Lewintre, R. Farràs, F. Ciruela, R. Pérez-Tomás and R. Quesada, J. Am. Chem. Soc., 2015, 137, 15892–15898 CrossRef CAS PubMed; (d) S.-K. Ko, S. K. Kim, A. Share, V. M. Lynch, J. Park, W. Namkung, W. Van Rossom, N. Busschaert, P. A. Gale, J. L. Sessler and I. Shin, Nat. Chem., 2014, 6, 885–892 CrossRef CAS PubMed.
- (a) M. Olivari, R. Montis, S. N. Berry, L. E. Karagiannidis, S. J. Coles, P. N. Horton, L. K. Mapp, P. A. Gale and C. Caltagirone, Dalton Trans., 2016, 45, 11892–11897 RSC; (b) C. Lang, X. Zhang, Q. Luo, Z. Dong, J. Xu and J. Liu, Eur. J. Org. Chem., 2015, 6458–6465 CrossRef CAS; (c) Y. R. Choi, G. C. Kim, H.-G. Jeon, J. Park, W. Namkung and K.-S. Jeong, Chem. Commun., 2014, 50, 15305–15308 RSC; (d) B. A. McNally, A. V. Koulov, B. D. Smith, J.-B. Joos and A. P. Davis, Chem. Commun., 2005, 1087–1089 RSC.
- H. Valkenier and A. P. Davis, Acc. Chem. Res., 2013, 46, 2898–2909 CrossRef CAS PubMed.
- (a) B. A. McNally, A. V. Koulov, T. N. Lambert, B. D. Smith, J.-B. Joos, A. L. Sisson, J. P. Clare, V. Sgarlata, L. W. Judd, G. Magro and A. P. Davis, Chem. – Eur. J., 2008, 14, 9599–9606 CrossRef CAS PubMed; (b) S. Nishizawa, P. Bühlmann, M. Iwao and Y. Umezawa, Tetrahedron Lett., 1995, 36, 6483–6486 CrossRef CAS.
- (a) N. Busschaert, S. J. Bradberry, M. Wenzel, C. J. E. Haynes, J. R. Hiscock, I. L. Kirby, L. E. Karagiannidis, S. J. Moore, N. J. Wells, J. Herniman, G. J. Langley, P. N. Horton, M. E. Light, I. Marques, P. J. Costa, V. Felix, J. G. Frey and P. A. Gale, Chem. Sci., 2013, 4, 3036–3045 RSC; (b) S. K. Berezin and J. T. Davis, J. Am. Chem. Soc., 2009, 131, 2458–2459 CrossRef CAS PubMed.
- N. Busschaert, I. L. Kirby, S. Young, S. J. Coles, P. N. Horton, M. E. Light and P. A. Gale, Angew. Chem., Int. Ed., 2012, 51, 4426–4430 CrossRef CAS PubMed.
- (a) X. Wu and P. A. Gale, J. Am. Chem. Soc., 2016, 138, 16508–16514 CrossRef CAS PubMed; (b) X. Wu, L. W. Judd, E. N. W. Howe, A. M. Withecombe, V. Soto-Cerrato, H. Li, N. Busschaert, H. Valkenier, R. Pérez-Tomás, D. N. Sheppard, Y.-B. Jiang, A. P. Davis and P. A. Gale, Chem, 2016, 1, 127–146 CrossRef CAS.
- (a) N. Busschaert, M. Wenzel, M. E. Light, P. Iglesias-Hernández, R. Pérez-Tomás and P. A. Gale, J. Am. Chem. Soc., 2011, 133, 14136–14148 CrossRef CAS PubMed; (b) I. Marques, A. R. Colaco, P. J. Costa, N. Busschaert, P. A. Gale and V. Felix, Soft Matter, 2014, 10, 3608–3621 RSC.
- C. Fasting, C. A. Schalley, M. Weber, O. Seitz, S. Hecht, B. Koksch, J. Dernedde, C. Graf, E.-W. Knapp and R. Haag, Angew. Chem., Int. Ed., 2012, 51, 10472–10498 CrossRef CAS PubMed.
- L. W. Judd and A. P. Davis, Chem. Commun., 2010, 46, 2227–2229 RSC.
- N.B. there are transporters that are incapable of (or have a poor activity in) mediating anion uniport. Examples include prodigiosin and an analogue of 5 with cyano substituents. In those cases, the HPTS assay showed no gramicidin-induced enhancement of transport rate and the valinomycin-coupled assay showed little or no activity. See ref. 10b.
- R. B. Stockbridge, H.-H. Lim, R. Otten, C. Williams, T. Shane, Z. Weinberg and C. Miller, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 15289–15294 CrossRef CAS PubMed.
- P. Läuger, Science, 1972, 178, 24–30 Search PubMed.
- The difference in EC50 between the two assays is mainly due to the fact that the HPTS assay measures a much smaller flux of X− (5 mM) compared with the osmotic assay (300 mM) and also in part due to the different vesicle sizes. As a result, the transport process in the HPTS assay completes more quickly than in the osmotic assay. For a discussion of transport rate dependence on vesicle size, see H. Valkenier, N. L. Mora, A. Kros and A. P. Davis, Angew. Chem., Int. Ed., 2015, 54, 2137–2141 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Compound synthesis, anion association constant determination, membrane transport data and other experimental details. See DOI: 10.1039/c7cc04912a |
| This journal is © The Royal Society of Chemistry 2017 |